BODIPY-Based Nanomaterials—Sensing and Biomedical Applications
Abstract
:1. Introduction
1.1. Nanotechnology and Nanomaterials in Medicine
1.2. Metallic Nanoparticles in Medicine
1.3. Brief Characterization of Selected Nanoparticles
- Some nanoparticles, such as gold and TiO2, can generate ROS species when irradiated with light at the proper wavelength.
- Silver and zinc oxide nanoparticles reveal antibacterial activity.
- The most interesting feature of quantum dots is their fluorescence activity.
- The emission of light of shorter wavelength after low-energy light excitation is an attribute of upconverted nanoparticles.
- MOFs can be used as potential drug delivery systems due to their large surface area and high porosity.
1.4. Nanoparticles Involved in the Photodynamic Therapy
- In PDT, the nanoparticles can be used as passive drug carriers or active co-photosensitizers.
- To the most frequently studied nanoparticles for PDT belong gold nanoparticles, quantum dots, upconverted nanoparticles, and titanium dioxide nanoparticles.
- To the advantages of combining nanoparticles and photosensitizers in PDT belong increased nanocarrier surface, better solubility of PS, and better permeability of nanoparticles due to the EPR effect and dual mechanism of action.
1.5. BODIPY Dyes
- Photosensitizers based on BODIPY dyes can be considered for photodynamic therapy and antimicrobial treatment as well as for the treatment of skin surface lesions.
- BODIPY dyes reveal sensing properties towards the determination of heavy metal ions.
2. Metallic Nanoparticles@BODIPY
2.1. Sensing Applications
2.1.1. Magnetite Nanoparticles
2.1.2. Gold Nanoparticles
2.1.3. Other Metallic Nanoparticles
- Different strategies were used for conjugation of BODIPY dyes to metallic nanoparticles through direct coordination or through indirect attachment via silica or thiolate linkers.
- BODIPY/metallic nanoparticles sensors can act as fluorescent probes.
- BODIPY dyes in conjugation with gold and magnetic nanoparticles were mostly used to determine heavy metal ions in aqueous solutions.
- Upconverted nanoparticles surface functionalized with BODIPY dyes can be potentially used as pH sensors.
2.2. Cancer Diagnosis and Therapy
2.2.1. Gold Nanoparticles
2.2.2. Magnetic Nanoparticles
2.2.3. Zirconium and Metal-Organic Framework Nanoparticles
2.2.4. Other Metallic Nanoparticles
- Gold nanoparticles/BODIPY conjugates were applied in in vitro studies as Raman or fluorescent sensors or co-photosensitizers.
- Fluorescent probes were obtained by combining magnetic nanoparticles and BODIPY dyes.
- Zirconium-based metal-organic frameworks functionalized with BODIPY dyes were used as photosensitizers in in vitro photocytotoxicity studies and revealed high photoactivity.
- A high antibacterial effect was achieved with the use of Ag nanoparticles/BODIPY dyes in photodynamic inactivation in vitro tests.
2.3. The Relationship of the Chemical Structure on Physicochemical Properties and Activity of BODIPY
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caldwell, M.A.; Jeyasingh, R.G.D.; Wong, H.-S.P.; Milliron, D.J. Nanoscale phase change memory materials. Nanoscale 2012, 4, 4382. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Rajeshkumar, S.; Venkat Kumar, S. A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. Effic. Technol. 2017, 3, 516–527. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef] [Green Version]
- Arvizo, R.; Bhattacharya, R.; Mukherjee, P. Gold nanoparticles: Opportunities and challenges in nanomedicine. Expert Opin. Drug Deliv. 2010, 7, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Yong, K.-T.; Roy, I.; Dinh, X.-Q.; Yu, X.; Luan, F. A review on functionalized gold nanoparticles for biosensing applications. Plasmonics 2011, 6, 491–506. [Google Scholar] [CrossRef]
- Schröfel, A.; Kratošová, G.; Šafařík, I.; Šafaříková, M.; Raška, I.; Shor, L.M. Applications of biosynthesized metallic nanoparticles—A review. Acta Biomater. 2014, 10, 4023–4042. [Google Scholar] [CrossRef] [PubMed]
- Boisseau, P.; Loubaton, B. Nanomedicine, nanotechnology in medicine. C. R. Phys. 2011, 12, 620–636. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Miyoshi, H.; Nakamura, M. Nanomedicine for drug delivery and imaging: A promising avenue for cancer therapy and diagnosis using targeted functional nanoparticles. Int. J. Cancer 2007, 120, 2527–2537. [Google Scholar] [CrossRef]
- Rauscher, H.; Rasmussen, K.; Sokull-Klüttgen, B. Regulatory aspects of nanomaterials in the EU. Chem. Ing. Tech. 2017, 89, 224–231. [Google Scholar] [CrossRef]
- Tuantranont, A. (Ed.) Applications of Nanomaterials in Sensors and Diagnostics; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.N. Chemical, physical, and biological coordination: An interplay between materials and enzymes as potential platforms for immobilization. Coord. Chem. Rev. 2019, 388, 1–23. [Google Scholar] [CrossRef]
- Stark, W.J.; Stoessel, P.R.; Wohlleben, W.; Hafner, A. Industrial applications of nanoparticles. Chem. Soc. Rev. 2015, 44, 5793–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, T.A.J.; Souza, L.R.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011, 13, 2638. [Google Scholar] [CrossRef]
- Mahendiran, C.; Ganesan, R.; Gedanken, A. Sonoelectrochemical synthesis of metallic aluminum nanoparticles. Eur. J. Inorg. Chem. 2009, 2009, 2050–2053. [Google Scholar] [CrossRef]
- Parekh, G.; Shi, Y.; Zheng, J.; Zhang, X.; Leporatti, S. Nano-carriers for targeted delivery and biomedical imaging enhancement. Ther. Deliv. 2018, 9, 451–468. [Google Scholar] [CrossRef]
- Klębowski, B.; Depciuch, J.; Parlińska-Wojtan, M.; Baran, J. Applications of noble metal-based nanoparticles in medicine. Int. J. Mol. Sci. 2018, 19, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.Z.; Akhter, S.; Jain, G.K.; Rahman, M.; Pathan, S.A.; Ahmad, F.J.; Khar, R.K. Metallic nanoparticles: Technology overview & drug delivery applications in oncology. Expert Opin. Drug Deliv. 2010, 7, 927–942. [Google Scholar] [CrossRef]
- Thakor, A.S.; Gambhir, S.S. Nanooncology: The future of cancer diagnosis and therapy. CA Cancer J. Clin. 2013, 63, 395–418. [Google Scholar] [CrossRef]
- Kumari, P.; Ghosh, B.; Biswas, S. Nanocarriers for cancer-targeted drug delivery. J. Drug Target. 2016, 24, 179–191. [Google Scholar] [CrossRef]
- Zhao, P.; Li, N.; Astruc, D. State of the art in gold nanoparticle synthesis. Coord. Chem. Rev. 2013, 257, 638–665. [Google Scholar] [CrossRef]
- Peng, J.; Liang, X. Progress in research on gold nanoparticles in cancer management. Medicine 2019, 98, e15311. [Google Scholar] [CrossRef]
- Beik, J.; Khateri, M.; Khosravi, Z.; Kamrava, S.K.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Gold nanoparticles in combinatorial cancer therapy strategies. Coord. Chem. Rev. 2019, 387, 299–324. [Google Scholar] [CrossRef]
- Amendola, V.; Pilot, R.; Frasconi, M.; Maragò, O.M.; Iatì, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Güzel, E.; Atmaca, G.Y.; Kuznetsov, A.E.; Turkkol, A.; Bilgin, M.D.; Erdoğmuş, A. Ultrasound versus light: Exploring photophysicochemical and sonochemical properties of phthalocyanine-based therapeutics, theoretical study, and in vitro evaluations. ACS Appl. Bio Mater. 2022, 5, 1139. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, M.; Czarczynska-Goslinska, B.; Ziental, D.; Michalak, M.; Güzel, E.; Sobotta, L. Excited state and reactive oxygen species against cancer and pathogens: A review on sonodynamic and sono-photodynamic therapy. ChemMedChem 2022, 17, e202200185. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amini, S.M. Preparation of antimicrobial metallic nanoparticles with bioactive compounds. Mater. Sci. Eng. C 2019, 103, 109809. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [Green Version]
- Chelminiak, D.; Ziegler-Borowska, M.; Kaczmarek, H. Polymer coated magnetite nanoparticles for biomedical application. Part I. Preparation of nanoparticles Fe3O4 coated by polysaccharides. Polimery 2015, 60, 12–17. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Juzenas, P.; Chen, W.; Sun, Y.-P.; Coelho, M.A.N.; Generalov, R.; Generalova, N.; Christensen, I.L. Quantum dots and nanoparticles for photodynamic and radiation therapies of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1600–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zottel, A.; Paska, A.V.; Jovčevska, I. Nanotechnology meets oncology: Nanomaterials in brain cancer research, diagnosis and therapy. Materials 2019, 12, 1588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium dioxide nanoparticles: A review of current toxicological data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef] [Green Version]
- Bassyouni, M.; Abdel-Aziz, M.H.; Zoromba, M.S.; Abdel-Hamid, S.M.S.; Drioli, E. A review of polymeric nanocomposite membranes for water purification. J. Ind. Eng. Chem. 2019, 73, 19–46. [Google Scholar] [CrossRef]
- Master, A.; Livingston, M.; Gupta, A.S. Photodynamic nanomedicine in the treatment of solid tumors: Perspectives and challenges. J. Control. Release 2013, 168, 88–102. [Google Scholar] [CrossRef] [Green Version]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in photodynamic therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.; Hariharan, R.; Suganthi, A.; Ashokkumar, M.; Rajarajan, M.; Pitchumani, K. Synergistic photodynamic action of ZnO nanomaterials encapsulated meso-tetra (4-sulfonatophenyl) porphyrin. Powder Technol. 2013, 237, 497–505. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [Green Version]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal–organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 9, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, H.; Zhao, Q.; Zhu, R.; Yang, Y.; Jia, Q.; Bian, B.; Zhuo, L. Glucose-sensitive colorimetric sensor based on peroxidase mimics activity of porphyrin-Fe3O4 nanocomposites. Mater. Sci. Eng. C 2014, 41, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, M.; Han, S.; Lai, Z.; Yang, J.; Tan, C.; Ma, Q.; Lu, Q.; Chen, J.; Zhang, X.; et al. Growth of Au Nanoparticles on 2D Metalloporphyrinic Metal-organic framework nanosheets used as biomimetic catalysts for cascade reactions. Adv. Mater. 2017, 29, 1700102. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Lim, C.-K.; Heo, J.; Shin, S.; Jeong, K.; Seo, Y.H.; Jang, W.-D.; Park, C.R.; Park, S.Y.; Kim, S.; Kwon, I.C. Nanophotosensitizers toward advanced photodynamic therapy of Cancer. Cancer Lett. 2013, 334, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Rasheed, T.; Raza, A.; Bilal, M.; Yahya, R.; Yar, M.; Iqbal, H.M.N. Photodynamic-based therapeutic modalities to fight against cancer—A review from synergistic viewpoint. J. Drug Deliv. Sci. Technol. 2019, 51, 70–82. [Google Scholar] [CrossRef]
- Spadavecchia, J.; Méthivier, C.; Landoulsi, J.; Pradier, C.-M. Interaction of Zn II porphyrin with TiO2 nanoparticles: From mechanism to synthesis of hybrid nanomaterials. ChemPhysChem 2013, 14, 2462–2469. [Google Scholar] [CrossRef]
- Fan, Z.; Fan, L.; Shuang, S.; Dong, C. Highly sensitive photoelectrochemical sensing of bisphenol A based on zinc phthalocyanine/TiO2 nanorod arrays. Talanta 2018, 189, 16–23. [Google Scholar] [CrossRef]
- Achilli, E.; Flores, C.Y.; Temprana, C.F.; Alonso, S.d.; Radrizzani, M.; Grasselli, M. Enhanced gold nanoparticle-tumor cell recognition by albumin multilayer coating. OpenNano 2021, 6, 100033. [Google Scholar] [CrossRef]
- Li, Z.; Li, S.; Lv, H.; Shen, J.; He, X.; Peng, B. BODIPY-based rapid response fluorescence probe for sensing and bioimaging endogenous superoxide anion in living cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 269, 120766. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Zhang, X.; Yang, P.; Zhao, J.; Zhang, W.; Feng, N.; Yang, W.; Tang, J. Defect self-assembly of metal-organic framework triggers ferroptosis to overcome resistance. Bioact. Mater. 2021, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Laoui, S.; Bag, S.; Dantiste, O.; Frenette, M.; Hatamimoslehabadi, M.; Bellinger-Buckley, S.; Tseng, J.-C.; Rochford, J.; Yelleswarapu, C. BODIPY Derivatives as Molecular Photoacoustic Contrast Agents; Achilefu, S., Raghavachari, R., Eds.; SPIE: San Francisco, CA, USA, 2014; p. 895609. [Google Scholar] [CrossRef]
- Kubheka, G.; Uddin, I.; Amuhaya, E.; Mack, J.; Nyokong, T. Synthesis and photophysicochemical properties of BODIPY dye functionalized gold nanorods for use in antimicrobial photodynamic therapy. J. Porphyr. Phthalocyanines 2016, 20, 1016–1024. [Google Scholar] [CrossRef]
- Atukorale, P.U.; Guven, Z.P.; Bekdemir, A.; Carney, R.P.; van Lehn, R.C.; Yun, D.S.; Silva, P.H.J.; Demurtas, D.; Yang, Y.-S.; Alexander-Katz, A.; et al. Structure–property relationships of amphiphilic nanoparticles that penetrate or fuse lipid membranes. Bioconjug. Chem. 2018, 29, 1131–1140. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Guo, P.; Qian, X. A dual channel chemodosimeter for Hg2+ and Ag+ using a 1,3-dithiane modified BODIPY. New J. Chem. 2012, 36, 1621. [Google Scholar] [CrossRef]
- Bilgic, A.; Cimen, A. Two novel BODIPY-functional magnetite fluorescent nano-sensors for detecting of Cr(VI) ions in aqueous solutions. J. Fluoresc. 2020, 30, 867–881. [Google Scholar] [CrossRef]
- Salis, F.; Descalzo, A.B.; Benito-Peña, E.; Moreno-Bondi, M.C.; Orellana, G. Highly fluorescent magnetic nanobeads with a remarkable stokes shift as labels for enhanced detection in immunoassays. Small 2018, 14, 1703810. [Google Scholar] [CrossRef]
- Lee, H.Y.; Bae, D.R.; Park, J.C.; Song, H.; Han, W.S.; Jung, J.H. A Selective fluoroionophore based on BODIPY-functionalized magnetic silica nanoparticles: Removal of Pb2+ from human blood. Angew. Chem. 2009, 48, 1239–1243. [Google Scholar] [CrossRef]
- Son, H.; Lee, H.Y.; Lim, J.M.; Kang, D.; Han, W.S.; Lee, S.S.; Jung, J.H. A Highly sensitive and selective turn-on fluorogenic and chromogenic sensor based on BODIPY-functionalized magnetic nanoparticles for detecting lead in living cells. Chemistry 2010, 16, 11549–11553. [Google Scholar] [CrossRef]
- Bilgic, A.; Cimen, A. A highly sensitive and selective ON-OFF fluorescent sensor based on functionalized magnetite nanoparticles for detection of Cr(VI) metal ions in the aqueous medium. J. Mol. Liq. 2020, 312, 113398. [Google Scholar] [CrossRef]
- Bilgic, A.; Cimen, A. Synthesis, characterisation, adsorption studies and comparison of superparamagnetic iron oxide nanoparticles (SPION) with three different amine groups functionalised with BODIPY for the removal of Cr(VI) metal ions from aqueous solutions. Int. J. Environ. Anal. Chem. 2021, 101, 1–26. [Google Scholar] [CrossRef]
- Lee, H.Y.; Son, H.; Lim, J.M.; Oh, J.; Kang, D.; Han, W.S.; Jung, J.H. BODIPY-functionalized gold nanoparticles as a selective fluoro-chromogenic chemosensor for imaging Cu2+ in living cells. Analyst 2010, 135, 2022. [Google Scholar] [CrossRef]
- Son, H.; Lee, J.H.; Kim, Y.-R.; Lee, I.S.; Han, S.; Liu, X.; Jaworski, J.; Jung, J.H. A BODIPY-functionalized bimetallic probe for sensitive and selective color-fluorometric chemosensing of Hg2+. Analyst 2012, 137, 3914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, S.-H.; Wu, M.-C.; Wu, S.-P. A turn-on fluorescent sensor for cysteine based on BODIPY functionalized Au nanoparticles and its application in living cell imaging. Sens. Actuators B Chem. 2015, 221, 1366–1371. [Google Scholar] [CrossRef]
- Radunz, S.; Andresen, E.; Würth, C.; Koerdt, A.; Tschiche, H.R.; Resch-Genger, U. Simple self-referenced luminescent pH sensors based on upconversion nanocrystals and pH-sensitive fluorescent BODIPY dyes. Anal. Chem. 2019, 91, 7756–7764. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Wang, X.; Gao, W.; Liu, Z.; Chen, Z.; Cheng, G.; Cao, W.; Sui, M.; Tang, B. CeO2 nanowire-BODIPY-adenosine triphosphate fluorescent sensing platform for highly specific and sensitive detection of arsenate. Anal. Chem. 2018, 90, 14507–14513. [Google Scholar] [CrossRef] [PubMed]
- Adarsh, N.; Ramya, A.N.; Maiti, K.K.; Ramaiah, D. Unveiling NIR aza-boron-dipyrromethene (BODIPY) dyes as raman probes: Surface-enhanced raman scattering (SERS)-guided selective detection and imaging of human cancer cells. Chem. Eur. J. 2017, 23, 14286–14291. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Ghosh, P.; Pagliuca, C.; Zhu, Z.-J.; Menichetti, S.; Rotello, V.M. Entrapment of hydrophobic drugs in nanoparticle monolayers with efficient release into cancer cells. J. Am. Chem. Soc. 2009, 131, 1360–1361. [Google Scholar] [CrossRef] [Green Version]
- Lucarini, M.; Franchi, P.; Pedulli, G.F.; Gentilini, C.; Polizzi, S.; Pengo, P.; Scrimin, P.; Pasquato, L. Effect of core size on the partition of organic solutes in the monolayer of water-soluble nanoparticles: An ESR investigation. J. Am. Chem. Soc. 2005, 127, 16384–16385. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Yadav, P.; Shanavas, A.; Thurakkal, S.; Joseph, J.; Neelakandan, P.P. A three-component supramolecular nanocomposite as a heavy-atom-free photosensitizer. Chem. Commun. 2019, 55, 5623–5626. [Google Scholar] [CrossRef]
- Kainz, Q.M.; Schätz, A.; Zöpfl, A.; Stark, W.J.; Reiser, O. Combined covalent and noncovalent functionalization of nanomagnetic carbon surfaces with dendrimers and BODIPY fluorescent dye. Chem. Mater. 2011, 23, 3606–3613. [Google Scholar] [CrossRef]
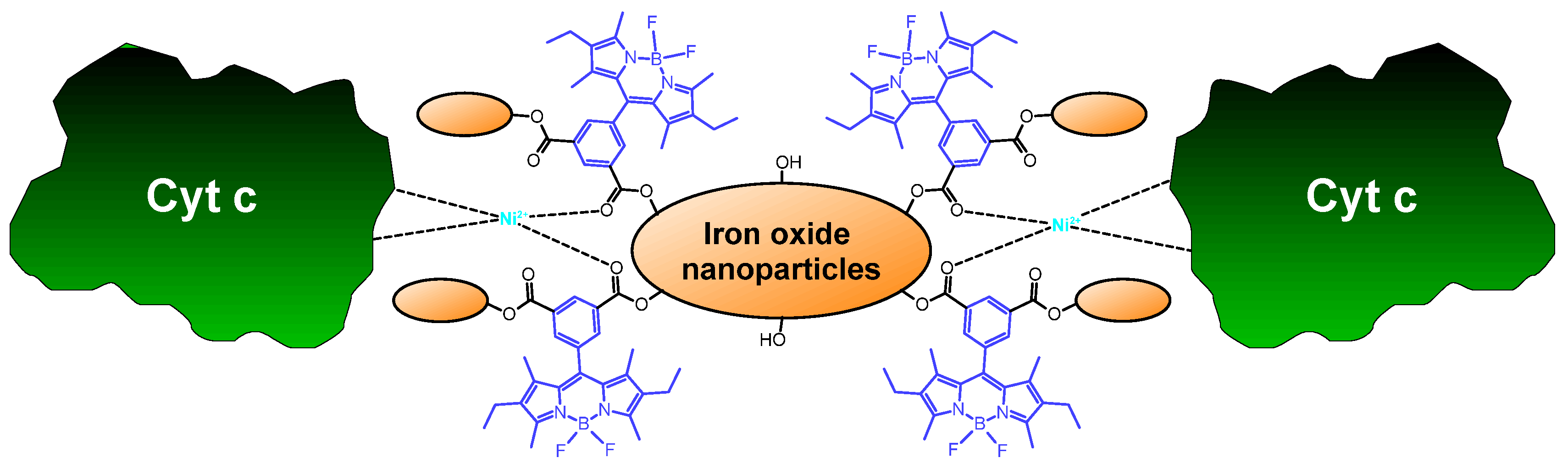
- Maltas, E.; Kursunlu, A.N.; Arslan, G.; Ozmen, M. A new BODIPY/nanoparticle/Ni affinity system for binding of cytochrome c. Appl. Surf. Sci. 2015, 349, 811–816. [Google Scholar] [CrossRef]
- Oh, J.S.; You, Y.; Park, K.C.; Gupta, G.; Kang, D.-K.; Lee, C.Y. Toward an efficient photosensitizer for photodynamic therapy: Incorporating BODIPY into porphyrinic nanoscale MOFs through the solvent-assisted ligand incorporation. Dye. Pigment 2019, 170, 107576. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Li, Z.; Xie, Z. BODIPY-containing nanoscale metal–organic frameworks for photodynamic therapy. Chem. Commun. 2016, 52, 5402–5405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, L.; Ma, C.; Wang, W.; Ding, J.; Liu, S.; Zhang, X.; Xie, Z. BODIPY-containing nanoscale metal–organic frameworks as contrast agents for computed tomography. J. Mater. Chem. B 2017, 5, 2330–2336. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F. Crystallized frameworks with giant pores: Are there limits to the possible? Acc. Chem. Res. 2005, 38, 217–225. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic modifications of iron-carboxylate nanoscale metal–organic frameworks for imaging and drug delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdemir, T.; Lu, Y.-C.; Kolemen, S.; Tanriverdi-Ecik, E.; Akkaya, E.U. Generation of singlet oxygen by persistent luminescent nanoparticle-photosensitizer conjugates: A proof of principle for photodynamic therapy without light. ChemPhotoChem 2017, 1, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Abdukayum, A.; Chen, J.-T.; Zhao, Q.; Yan, X.-P. Functional near infrared-emitting Cr3+/Pr3+ Co-doped zinc gallogermanate persistent luminescent nanoparticles with superlong afterglow for in vivo targeted bioimaging. J. Am. Chem. Soc. 2013, 135, 14125–14133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Z.; Yu, Z.; Zhong, W.; Jiang, H.; Zhao, Q.; Li, F.; Zhang, X.; Wang, D. Photosensitizer–AgNP composite with an ability to selectively recognize pathogen and enhanced photodynamic efficiency. New J. Chem. 2017, 41, 12371–12374. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, M.; Niu, N.; Ren, J.; Yang, N.; Yu, C. Aza-BODIPY probe-decorated mesoporous black TiO2 nanoplatform for the highly efficient synergistic phototherapy. ACS Appl. Mater. Interfaces 2020, 12, 41071–41078. [Google Scholar] [CrossRef] [PubMed]



















| BODIPY Core | Metallic Nanoparticle | Type of Connection | Application | Ref. |
|---|---|---|---|---|
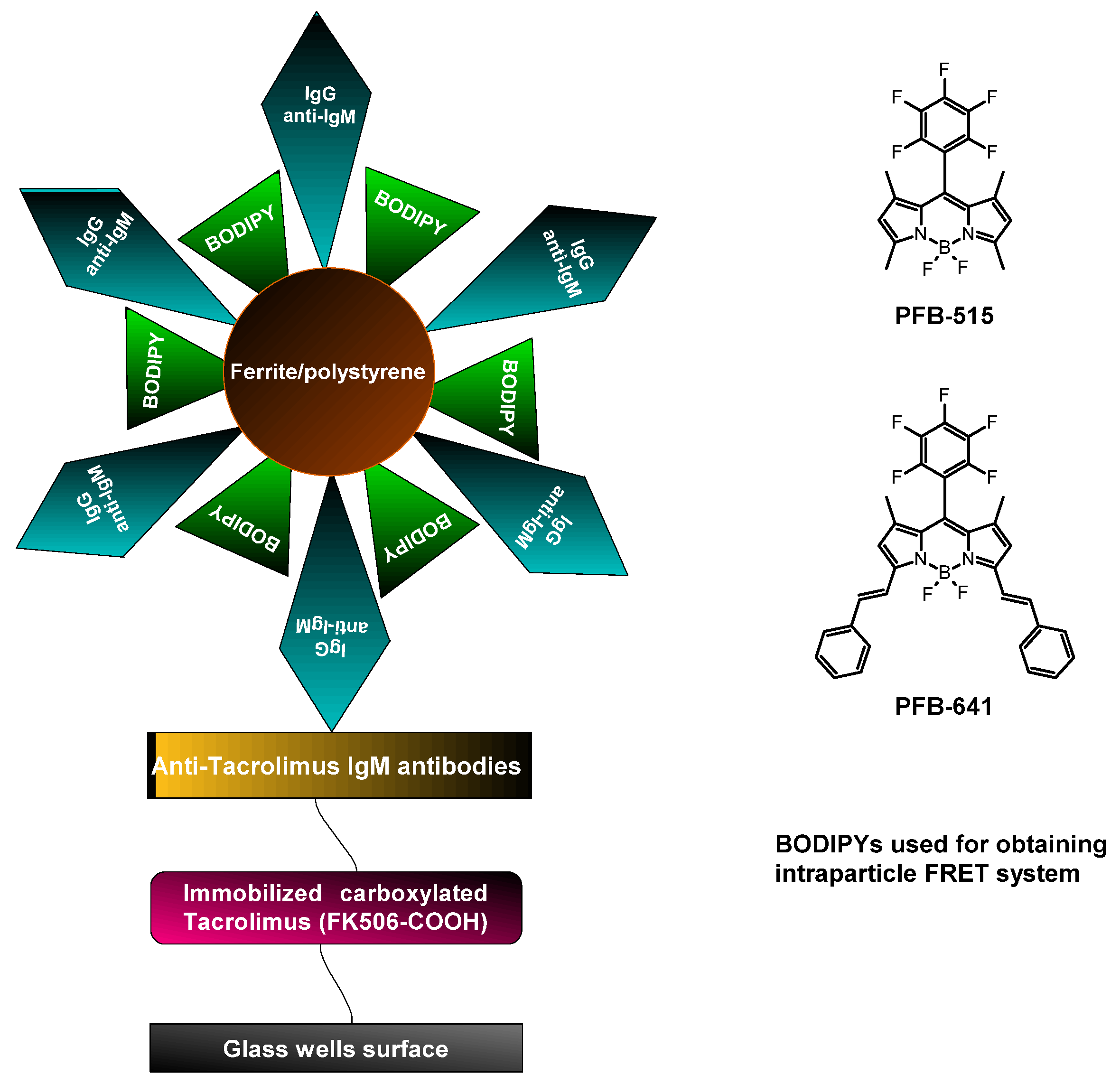
| Pentafluorophenyl-substituted BODIPY Styryl-substituted BODIPY | Ferrite/polystyrene | Physical deposition/adsorption | Determination of tacrolimus | [59] |
| Meso-phenyl-substituted BODIPY | Ni/silica | Covalent attachment via amide bonding | Pb2+ sensing | [60] |
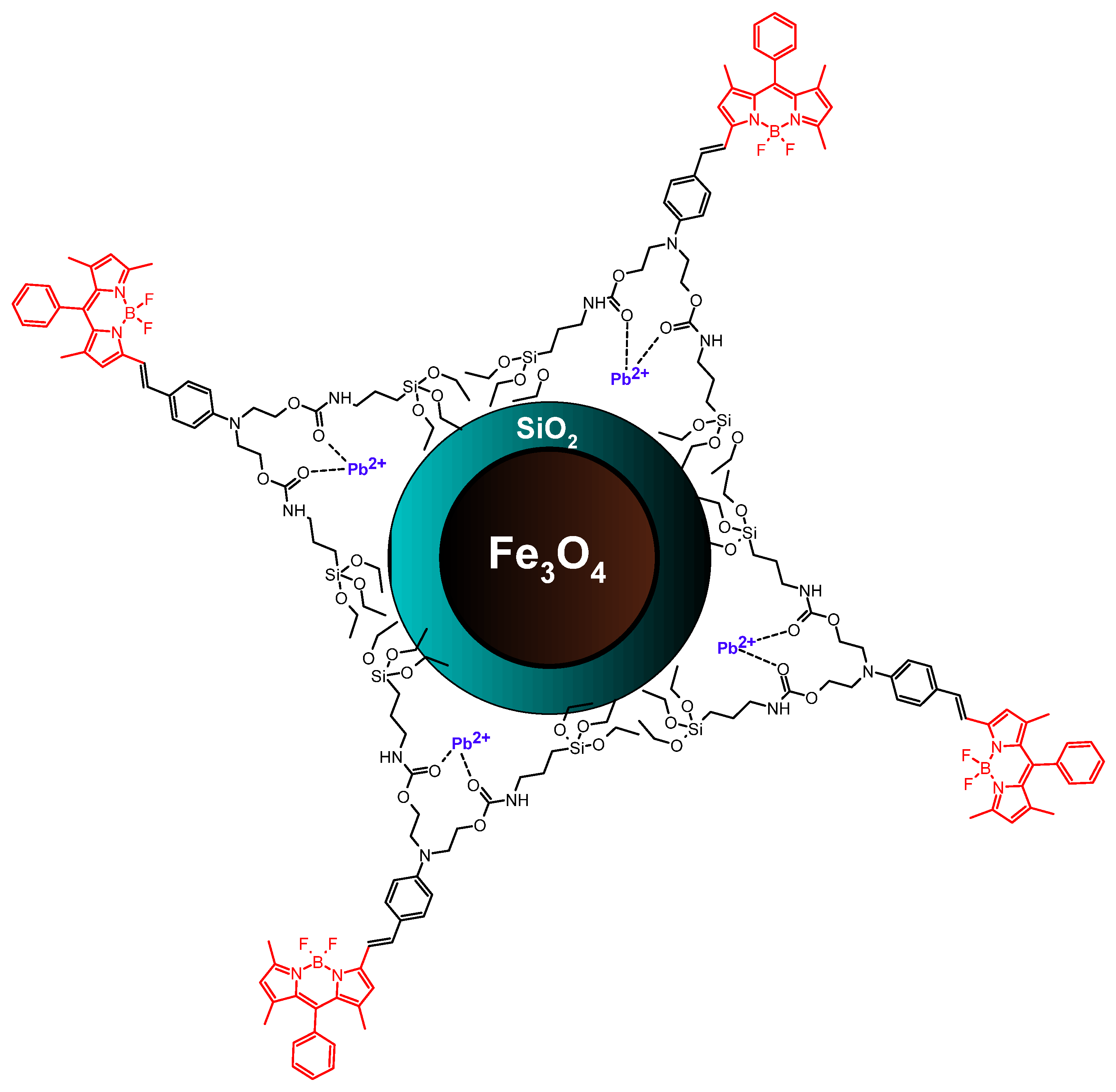
| Meso-phenyl- and styryl-substituted BODIPY | Fe3O4/silica | Covalent attachment via amide bonding | Pb2+ sensing | [61] |
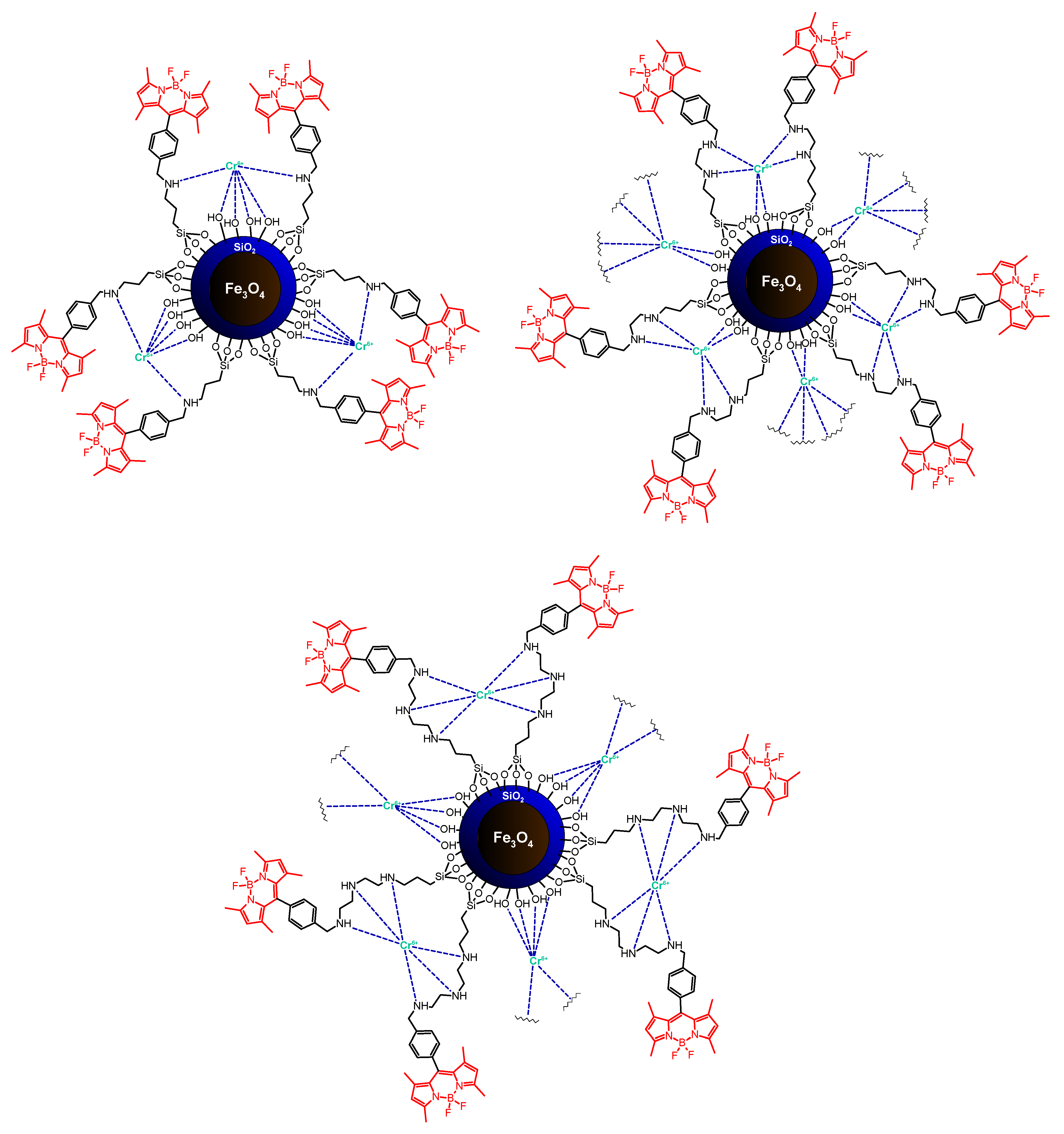
| Meso-phenyl-substituted BODIPY | Fe3O4/silica | Covalent attachment via amine bonding | Cr6+ sensing | [62] |
| Meso-phenyl- and styryl-substituted BODIPY | Au nanoparticles | Covalent attachment via ester bonding | Cu2+ sensing | [64] |
| Meso-phenyl- and styryl-substituted BODIPY | Au nanoparticles | Covalent attachment via thienyl bonding | Hg2+ sensing | [65] |
| Meso-pyridyl-substituted BODIPY | Au nanoparticles | Electrostatic interactions | Aminoacids sensing (cysteine) | [66] |
| Meso-bromohydroxyphenyl-substituted BODIPY | NaYF4:Yb3+, Tm3+ nanoparticles | Physical deposition/adsorption | ratiometric pH-nanosensor | [67] |
| Unsubstituted BODIPY | CeO2 nanowires | Covalent attachment via amide bonding | Arsenates sensing | [68] |
| Aromatic amino aza-BODIPY | Au nanoparticles | Electrostatic interactions | Nanoprobe for cancer cells | [69] |
| Meso-methyl-substituted BODIPY | Au nanoparticles | Physical deposition/adsorption | Cancer diagnosis | [70] |
| Meso-methyl-substituted BODIPY | Au nanoparticles | Physical deposition/adsorption | Photodynamic therapy | [72] |
| Ethyl-substituted BODIPY | Carbon-coated Co nanoparticles | Covalent attachment via alkyne-azide conjugation (triazole) | Cancer diagnosis | [73] |
| Meso-phenyl- and ethyl-substituted BODIPY | Iron oxide nanoparticles (SPIONs) | Covalent attachment via ester bonding | Ni2+ complexation | [74] |
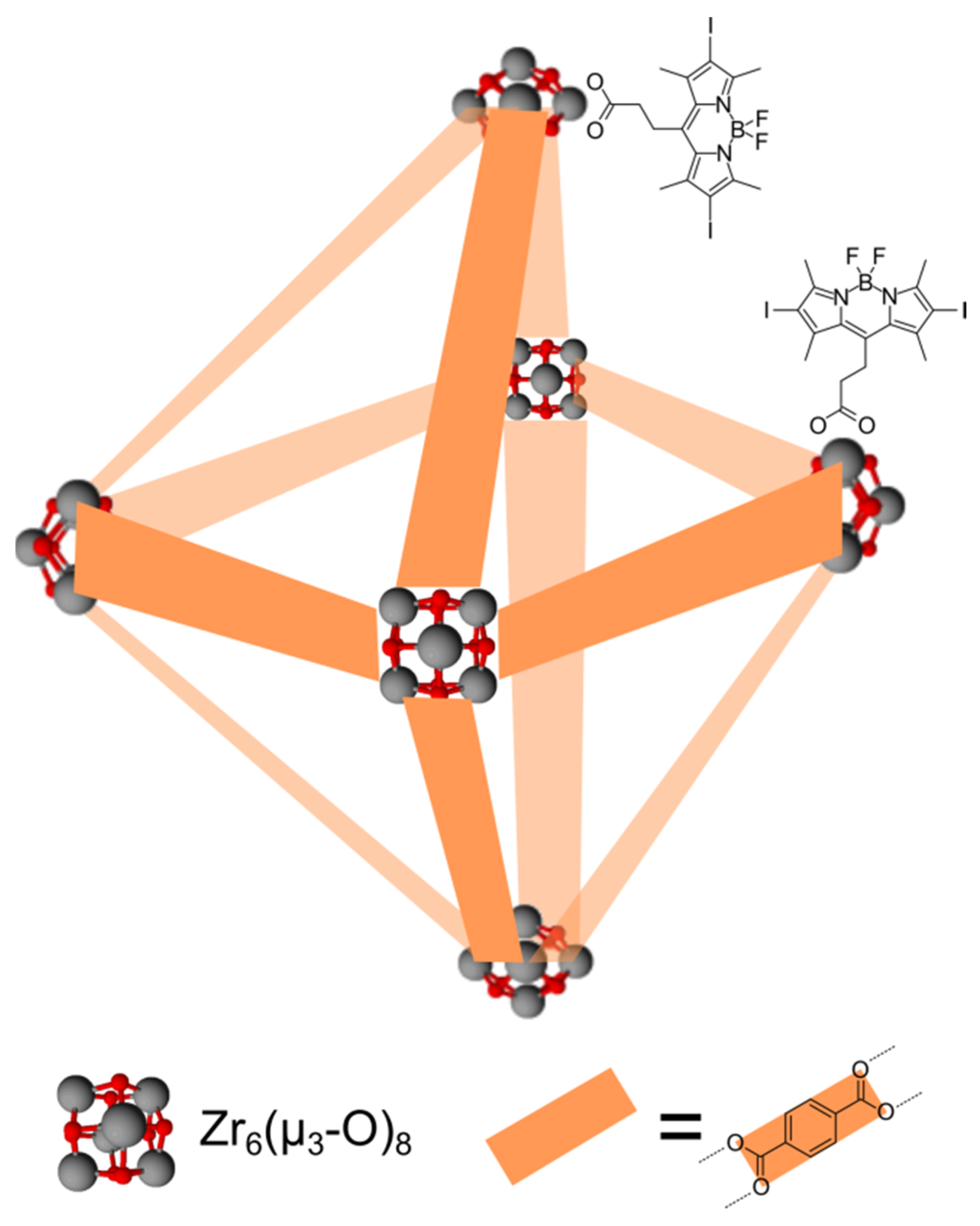
| Meso-phenyl- and iodide-substituted BODIPY | Zirconium hydroxide nanoparticles (MOFs) | Covalent attachment via ester bonding | Photodynamic therapy | [75] |
| Meso-alkyl- and iodide-substituted BODIPY | Zirconium hydroxide nanoparticles (MOFs) | Covalent attachment via ester bonding | Photodynamic therapy | [76,77] |
| Meso-ethyl-substituted BODIPY | Silica-coated Fe-based MOFs | Physical deposition/adsorption | Photodynamic therapy | [79] |
| Meso-phenyl-, iodide-, and styryl-substituted BODIPY | Zinc-germanogallate-based nanoparticles | Covalent attachment via ether bonding | Photodynamic therapy | [80,81] |
| Meso-phenyl- and iodide-substituted BODIPY | Silver nanoparticles | Covalent attachment via ester bonding | Photodynamic inactivation of bacteria | [82] |
| Aromatic aza-BODIPY | Titanium oxide nanoparticles | Physical deposition/adsorption | Photodynamic therapy | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koczorowski, T.; Glowacka-Sobotta, A.; Sysak, S.; Mlynarczyk, D.T.; Lesyk, R.; Goslinski, T.; Sobotta, L. BODIPY-Based Nanomaterials—Sensing and Biomedical Applications. Appl. Sci. 2022, 12, 7815. https://doi.org/10.3390/app12157815
Koczorowski T, Glowacka-Sobotta A, Sysak S, Mlynarczyk DT, Lesyk R, Goslinski T, Sobotta L. BODIPY-Based Nanomaterials—Sensing and Biomedical Applications. Applied Sciences. 2022; 12(15):7815. https://doi.org/10.3390/app12157815
Chicago/Turabian StyleKoczorowski, Tomasz, Arleta Glowacka-Sobotta, Stepan Sysak, Dariusz T. Mlynarczyk, Roman Lesyk, Tomasz Goslinski, and Lukasz Sobotta. 2022. "BODIPY-Based Nanomaterials—Sensing and Biomedical Applications" Applied Sciences 12, no. 15: 7815. https://doi.org/10.3390/app12157815









