A Novel Natural Active Coagulant Agent Extracted from the Sugarcane Bagasse for Wastewater Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material and Reagent
2.2. Preparation and Extraction of Saccharum officinarum
2.3. Physical Properties Characterization
2.4. Chemical Analysis
2.5. Morphological Characterization
2.6. Preparation of Synthetic Wastewater
2.7. Turbidity Estimation of the Treated Wastewater
2.8. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Characteristics of Bagasse
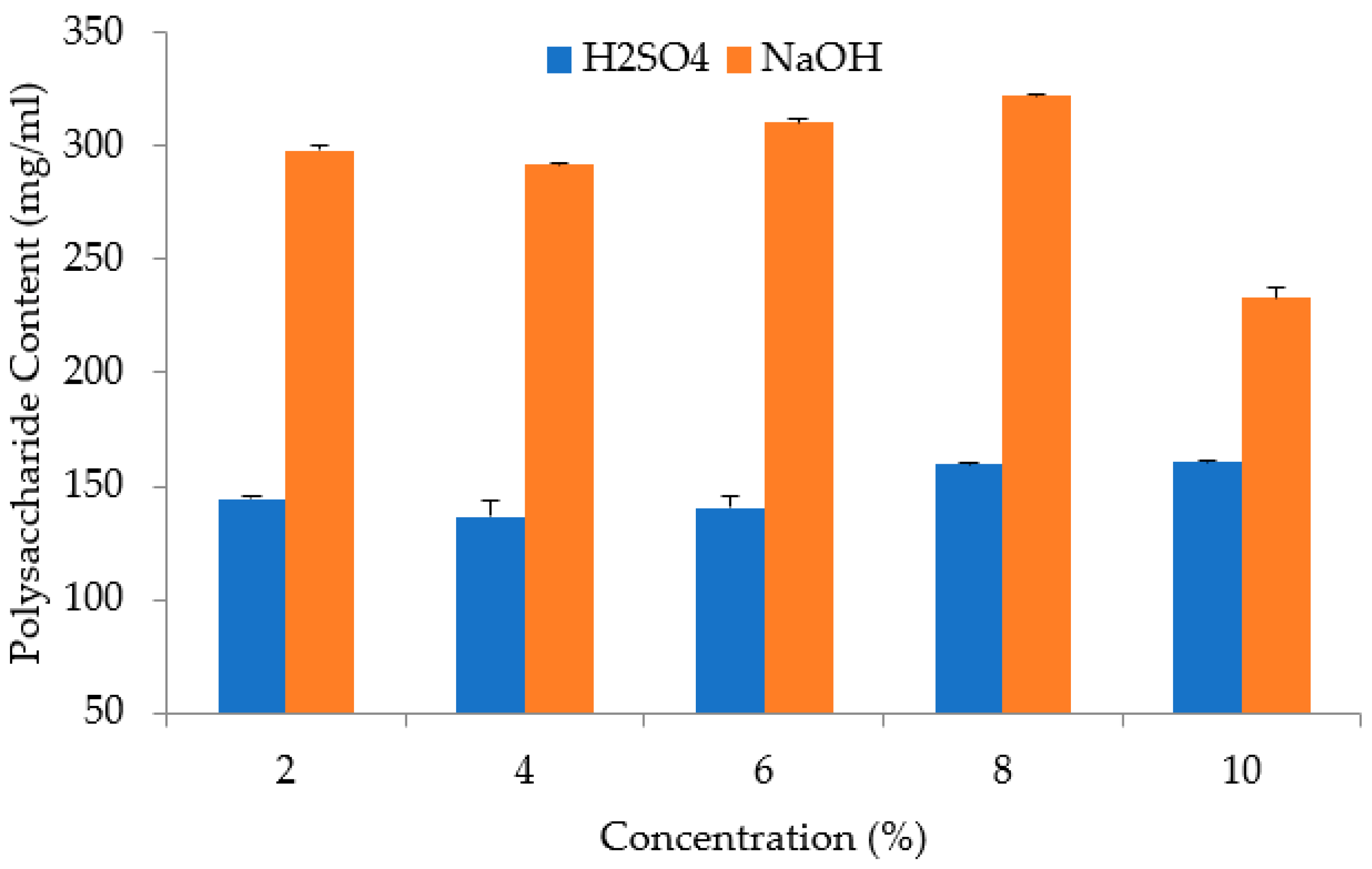
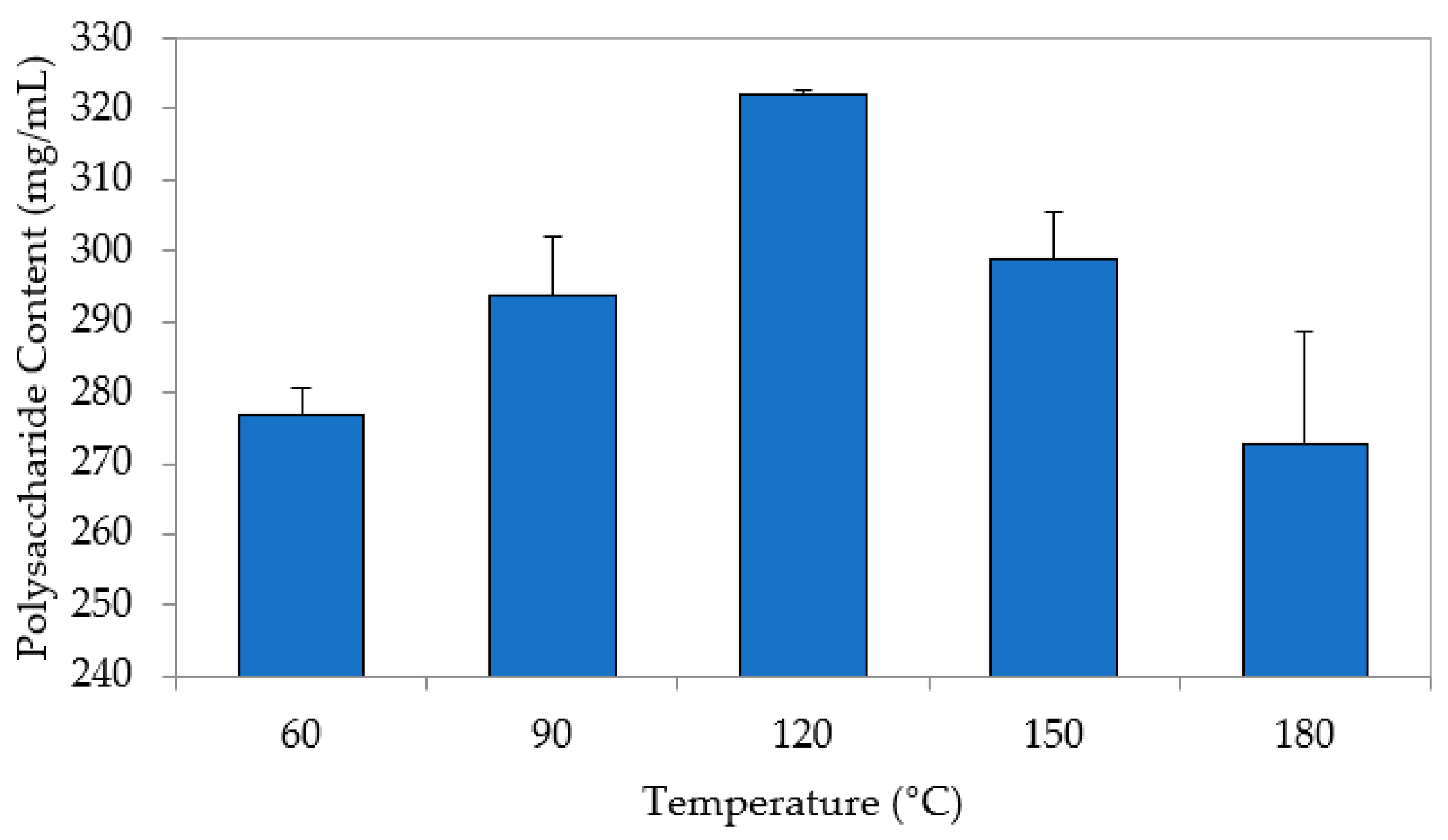
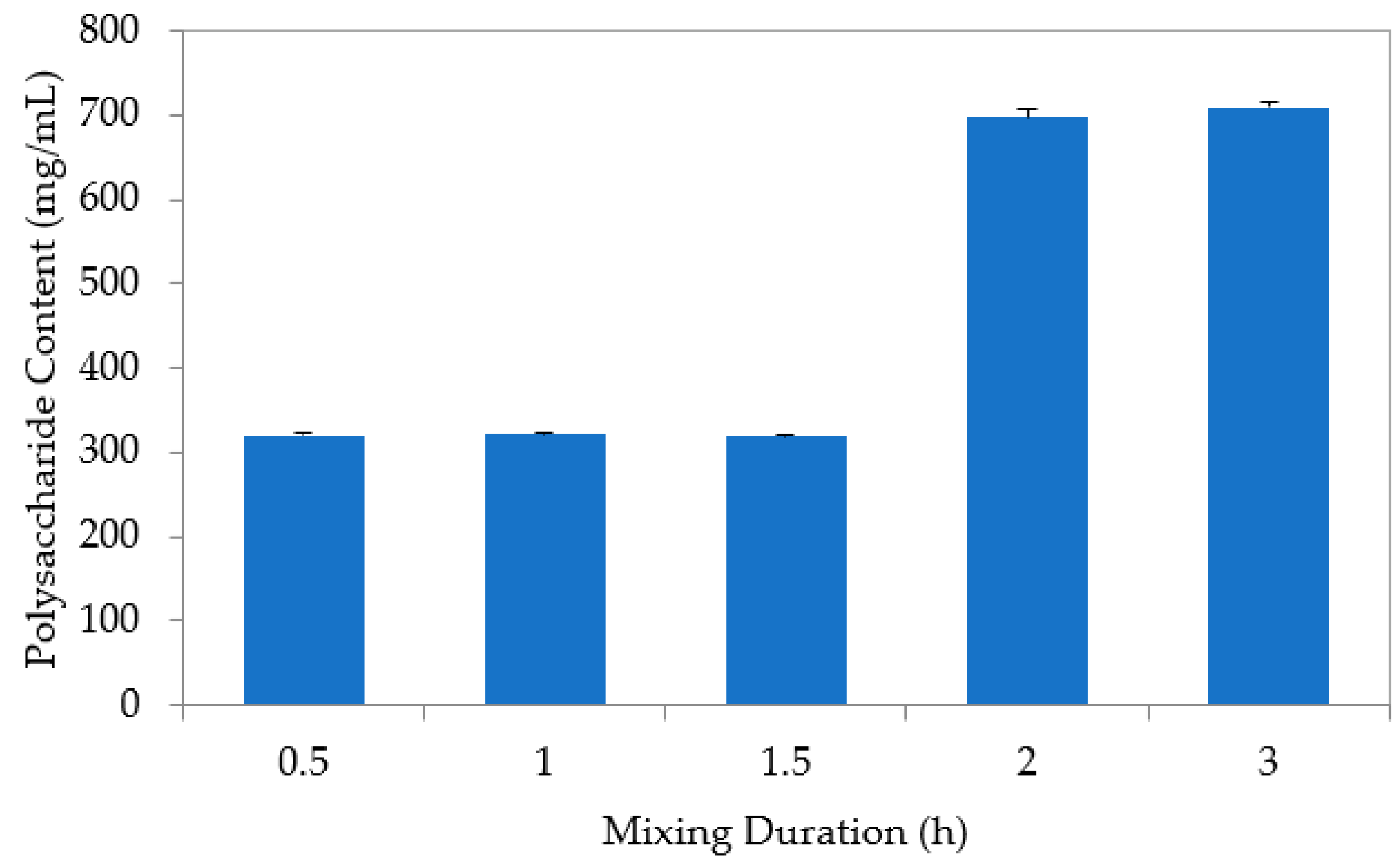
3.2. Extraction Process of Saccharum officinarum
3.3. Molecular Characterization of Stem from Saccharum officinarum
3.4. Surface Morphology of Saccharum officinarum
3.5. Coagulation Process of Bagasse as Natural Coagulant
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yaqob, A.A.; Parveen, T.; Umar, K.; Ibrahim, M.N.M. Role of nanomaterials in the treatment of wastewater: A review. Water 2020, 12, 495. [Google Scholar] [CrossRef] [Green Version]
- Yaqob, A.A.; Ibrahim, M.N.M.; Ahmad, A.; Reddy, A.V.B. Toxicology and environmental application of carbon nanocomposite. Green Energy Technol. 2021, 1–18. [Google Scholar]
- Amran, A.H.; Zaidi, N.S.; Muda, K.; Bahrodin, M.B.; Loan, L.W. Deshelled Carica papaya seeds as natural coagulant for improvement quality of river water. Sains Malays. 2021, 50, 1521–1529. [Google Scholar] [CrossRef]
- Zaidi, N.S.; Muda, K.; Abdul Rahman, M.A.; Sgawi, M.S.; Amran, A.H. Effectiveness of local waste materials as organic-based coagulant in treating water. IOP Conf. Ser. Mater. Sci. Eng. 2019, 636, 012007. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Abdullah, S.R.S.; Imron, M.F.; Said, N.S.M.; Ismail, N.I.; Hasan, H.A.; Othman, A.R.; Purwanti, I.F. Challenges and opportunities of biocoagulant/bioflocculant application for drinking water and wastewater treatment and its potential for sludge recovery. Int. J. Environ. Res. Public Health 2020, 17, 9312. [Google Scholar] [CrossRef] [PubMed]
- Loh, Z.Z.; Zaidi, N.S.; Syafiuddin, A.; Yong, E.L.; Boopathy, R.; Kueh, A.B.H.; Prastyo, D.D. Shifting from conventional to organic filter media in wastewater biofiltration treatment: A review. Appl. Sci. 2021, 11, 8650. [Google Scholar] [CrossRef]
- Jagaba, A.H.; Abdul Latiff, A.Z.; Umaru, I.; Abu Bakar, S. Treatment of palm oil mill effluent (POME) by coagulation flocculation using different natural and chemical coagulants: A review. IOSR J. Mech. Civ. Eng. 2016, 13, 67–75. [Google Scholar]
- Abiyu, A.; Yan, D.; Girma, A.; Song, X.; Wang, H. Wastewater treatment potential of Moringa stenopetala over Moringa oleifera as a natural coagulant, antimicrobial aganet and heavy metal removals. Cogent Environ. Sci. 2018, 4, 1433507. [Google Scholar] [CrossRef]
- Amran, A.H.; Bahrodin, M.B.; Zaidi, N.S.; Muda, K.; Aris, A.; Umor, N.A.; Mohd Amin, M.F.; Syafiuddin, A. Turbid water treatment using deshelled Carica papaya seed: Analysis via factorial design. Biointerface Res. Appl. Chem. 2022, 6, 7787–7795. [Google Scholar]
- Bahrodin, M.B.; Zaidi, N.S.; Hussein, N.; Sillanpää, M.; Prasetyo, D.D.; Syafiuddin, A. Recent advances on coagulation-based treatment of wastewater: Transition from chemical to natural coagulant. Curr. Pollut. Rep. 2021, 7, 379–391. [Google Scholar] [CrossRef]
- Choudhary, M.; Ray, M.B.; Neogi, S. Evaluation of the potential application of cactus (Opuntia ficus-indica) as a bio-coagulant for pre-treatment of oil sands process-affected water. Sep. Purif. Technol. 2019, 209, 714–724. [Google Scholar] [CrossRef]
- Shan, T.C.; Matar, M.A.; Makky, E.A.; Ali, E.N. The use of Moringa oleifera seed as a natural coagulant for wastewater treatment and heavy metals removal. Appl. Water Sci. 2017, 7, 1369–1376. [Google Scholar] [CrossRef]
- Maican, E.; Coz, A.; Ferdeş, M. Continuous Pretreatment Process for Bioethanol Production. In Proceedings of the 4th International Conference on Thermal Equipment, Renewable Energy and Rural Development, Vidraru, Romania, 4–6 June 2015; University Politehnica of Bucharest: București, Romania, 2015; Volume 35, p. 287. [Google Scholar]
- Uppal, N.; Pappu, A.; Gowri, V.K.S.; Thakur, V.K. Cellulosic fibres-based epoxy composites: From bioresource to a circular economy. Ind. Crops Prod. 2021, 182, 114895. [Google Scholar] [CrossRef]
- Tahir, H.; Sultan, M.; Akhtar, N.; Hameed, U.; Abid, T. Application of natural and modified sugar cane bagasse for the removal of dye from aqueous solution. J. Saudi Chem. Soc. 2016, 20, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Rudresh Gowda, D.R.; Suhag, K.V.; Poojashree, V.K.; Roopa, A.B.; Manjunath, H.S. Experimental study on treating waste water using natural adsorbents. Int. J. Innov. Res. Sci. Eng. Technol. 2019, 8, 5390–5396. [Google Scholar]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.J. Protein determination—Method matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, S.S. Food Analysis Laboratory Manual Revised Edition; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Kaewpradap, A.; Yoksenakul, W.; Jugjai, S. Effects of Moisture Content in Simulated Bagasse by Equilibrium Analysis. In Proceedings of the 4th TSME International Conference of Mechanical Engineering, Chonburi, Thailand, 16–18 October 2013. [Google Scholar]
- Alwi, H.; Idris, J.; Musa, M.; Ku Hamid, K.H. A preliminary study of banana stem juice as a plant-based coagulant for treatment of spent coolant wastewater. J. Chem. 2013, 2013, 165057. [Google Scholar] [CrossRef]
- Deng, L.; Manthey, F.A. Laboratory-scale milling of whole-durum flour quality: Effect of mill configuration and seed conditioning. J. Sci. Food Agric. 2017, 10, 3141–3150. [Google Scholar] [CrossRef]
- Shahimi, N.S.W.; Zaidi, N.S.; Bahrodin, M.B.; Amran, A.H. Utilization of Fruit Wastes (Jackfruit and mango seeds and banana trunk) as natural coagulants in treating municipal wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1144, 012049. [Google Scholar] [CrossRef]
- Kumar, V.; Othman, N.; Asharuddin, S. Applications of natural coagulants to treat wastewater—A review. MATEC Web Conf. 2017, 103, 06016. [Google Scholar] [CrossRef] [Green Version]
- Leang, Y.H.; Saw, H.Y. Proximate and functional properties of sugarcane bagasse. Agro. Food Ind. Hi Technol. 2011, 22, 5–8. [Google Scholar]
- Karim, R.; Ferdous, N.; Roy, N.; Jahan, M.G.S.; Sarkar, A.K.; Shovon, M.S. A study on nutritional components of the leaf and stem: A study on nutritional components of the leaf and stem of Alocasia Indica L. J. Adv. Appl. Sci. Technol. 2015, 2, 46–54. [Google Scholar]
- Singh, R.; Shukla, A.; Tiwari, S.; Srivastava, M. A review on delignification of lignocellulosic biomass for enhancement of ethanol production potential. Renew. Sustain. Energy Rev. 2014, 32, 713–728. [Google Scholar] [CrossRef]
- Wiedenhoeft, A.C.; Miller, R.B. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M. Pretreatment of wheat straw leads to structural changes and improved enzymatic hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Barman, D.N.; Kang, T.H.; Kim, M.K.; Kim, J.; Kim, H.; Yun, H.D. Effect of dilute alkali on structural features and enzymatic hydrolysis of barley straw (Hordeum vulgare) at boiling temperature with low residence time. J. Microbiol. Biotechnol. 2012, 22, 1681–1691. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Luo, L.; Zheng, L. Lignins: Biosynthesis and biological functions in plants. Int. J. Mol. Sci. 2018, 19, 335. [Google Scholar] [CrossRef] [Green Version]
- Del Río, J.C.; Rencoret, J.; Prinsen, P.; Martínez, Á.T.; Ralph, J.; Gutiérrez, A. Structural characterization of wheat straw lignin as revealed by analytical pyrolysis, 2D-NMR, and reductive cleavage methods. J. Agric. Food Chem. 2012, 60, 5922–5935. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Cheng, Y.; Zhang, W.; Jiang, J.; Lei, F. Characterization of lignins from sugarcane bagasse pretreated with green liquor combined with ethanol and hydrogen peroxide. Bioresources 2016, 11, 3191–3203. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Howard, B.H. Impact of thermal pretreatment temperatures on woody biomass chemical composition, physical properties and microstructure. Energies 2018, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, M.; Johansen, K.S.; Meyer, A.S. Low temperature lignocellulose pretreatment: Effects and interactions of pretreatment pH are critical for maximizing enzymatic monosaccharide yields from wheat straw. Biotechnol. Biofuels 2011, 4, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Chen, K.; Wang, K.; Yang, H.; Chen, J. The effects of time and temperature in hydrothermal pretreatment on the enzymatic efficiency of wheat straw. Bioresources 2018, 13, 5193–5203. [Google Scholar]
- Ma, Z.; Kasipandi, S.; Wen, Z.; Yu, L.; Cui, K.; Chen, H.; Li, Y. Highly efficient fractionation of corn stover into lignin monomers and cellulose-rich pulp over H2WO4. Appl. Catal. B Environ. 2021, 284, 119731. [Google Scholar] [CrossRef]
- Mohan, P.R.; Kumar, B.V.; Vijaya, O.; Reddy, S. Optimization of pretreatment conditions for increased cellulose conversion of sugarcane bagasse using peracetic acid employing central composite design. Songklanakarin J. Sci. Technol. 2013, 35, 177–185. [Google Scholar]
- Ong, V.Z.; Wu, T.Y.; Chu, K.K.L.; Sun, W.Y.; Shak, K.P.Y. A combined pretreatment with ultrasound-assisted alkaline solution and aqueous deep eutectic solvent for enhancing delignification and enzymatic hydrolysis from oil palm fronds. Ind. Crops Prod. 2021, 160, 112974. [Google Scholar] [CrossRef]
- Amran, A.H.; Zaidi, N.S.; Syafiuddin, A.; Zhan, L.Z.; Bahrodin, M.B.; Mehmood, M.A.; Boopathy, R. Potential of Carica papaya seed-derived bio-coagulant to remove turbidity from polluted water assessed through experimental and modeling-based study. Appl. Sci. 2021, 11, 5715. [Google Scholar] [CrossRef]
- Dong, S.J.; Zhang, B.X.; Gao, Y.F.; Hu, X.M. An efficient process for pretreatment of lignocelluloses in functional ionic liquids. Int. J. Polym. Sci. 2015, 2015, 978983. [Google Scholar] [CrossRef] [Green Version]
- Maceda, A.; Soto-Hernández, M.; Peña-Valdivia, C.B.; Trejo, C.; Terrazas, T. Characterization of lignocellulose of Opuntia (Cactaceae) species using FTIR spectroscopy: Possible candidates for renewable raw material. Biomass Convers. Bioref. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Shak, K.P.Y.; Wu, T.Y. Coagulation-flocculation treatment of high-strength agro-industrial wastewater using natural Cassia obtusifolia seed gum: Treatment efficiencies and flocs characterization. Chem. Eng. J. 2014, 256, 293–305. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Centhil, V.M.; Kameshwari, R.; Palaniyappan, M.; Kalaivani, V.D.; Pavithra, K.G. Experimental study on parameter estimation and mechanism for the removal of turbidity from groundwater and synthetic water using Moringa oleifera seed powder. Desalin. Water Treat. 2016, 57, 5488–5497. [Google Scholar] [CrossRef]
- Itodo, A.U.; Wuanaa, R.A.; Wombo, P.N. On the characterization, use and wastewater detoxification potential of pyrolysed Moringa oleifera pods and shells part: A sorbent preparation and characterization. Chem. Methodol. 2018, 2, 204–222. [Google Scholar]
- Cheng, S.Y.; Show, P.L.; Juan, J.C.; Ling, T.C.; Lau, B.F.; Lai, S.H.; Ng, E.P. Sustainable landfill leachate treatment: Optimize use of guar gum as natural coagulant and floc characterization. Environ. Res. 2020, 188, 109737. [Google Scholar] [CrossRef] [PubMed]
- Choong Lek, B.L.; Peter, A.P.; Qi Chong, K.H.; Ragu, P.; Sethu, V.; Selvarajoo, A.; Arumugasamy, S.K. Treatment of palm oil mill effluent (POME) using chickpea (Cicer arietinum) as a natural coagulant and flocculant: Evaluation, process optimization and characterization of chickpea powder. J. Environ. Chem. Eng. 2018, 6, 6243–6255. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Alves, V.N.; Rezende, H.C.; Almeida, I.L.S.; De Assunção, R.M.N.; Tarley, C.R.T.; Segatelli, M.G.; Coelho, N.M.M. Characterization and use of Moringa oleifera seeds as biosorbent for removing metal ions from aqueous effluents. Water Sci. Technol. 2010, 62, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Ben Salem-Fnayou, A.; Zemni, H.; Nefzaoui, A.; Ghorbel, A. Micromorphology of cactus-pear (Opuntia ficus-indica (L.) mill) cladodes based on scanning microscopies. Micron 2014, 56, 68–72. [Google Scholar] [CrossRef]
- Donohoe, B.S.; Vinzant, T.B.; Elander, R.T.; Pallapolu, V.R.; Lee, Y.Y.; Garlock, R.J.; Balan, V.; Dale, B.E.; Kim, Y.; Mosier, N.S.; et al. Surface and ultrastructural characterization of raw and pretreated switchgrass. Bioresour. Technol. 2011, 102, 11097–11104. [Google Scholar] [CrossRef]
- Karp, E.M.; Resch, M.G.; Donohoe, B.S.; Ciesielski, P.N.; O’Brien, M.H.; Nill, J.E.; Mittal, A.; Biddy, M.J.; Beckham, G.T. Alkaline pretreatment of switchgrass. ACS Sustain. Chem. Eng. 2015, 3, 1479–1491. [Google Scholar] [CrossRef]
- Rochana, A.; Dhalika, T.; Budiman, A.; Kamil, K.A. Nutritional value of a banana stem (Musa paradisiaca val) of anaerobic fermentation product supplemented with nitrogen, sulphur and phosphorus sources. Pak. J. Nutr. 2017, 16, 738–742. [Google Scholar] [CrossRef] [Green Version]
- Paroha, S. Sugarcane Bagasse as Dietary Fibre. Ind. J. Pure App. Biosci. 2020, 8, 590–597. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Panico, A.; Pirozzi, F. Use of acorn leaves as a natural coagulant in a drinking water treatment plant. Water 2018, 11, 57. [Google Scholar] [CrossRef] [Green Version]
- Smoczynski, L.; Kalinowski, S.; Cretescu, I.; Smoczynski, M.; Ratnaweera, H.; Trifescu, M.; Kosobucka, M. Study of sludge particles formed during coagulation of synthetic and municipal wastewater for increasing the sludge dewatering efficiency. Water 2019, 11, 101. [Google Scholar] [CrossRef] [Green Version]
- Aderibigbe, D.O.; Giwa, A.A.; Bello, I.A. Characterization and treatment of wastewater from food processing industry: A review. Imam J. Appl. Sci. 2018, 2, 27–36. [Google Scholar]
- Abidin, Z.Z.; Ismail, N.; Yunus, R.; Ahamad, I.S.; Idris, A. A preliminary study on Jatropha curcas as coagulant in wastewater treatmen. Environ. Technol. 2011, 32, 971–977. [Google Scholar] [CrossRef]
- Momeni, M.M.; Kahforoushan, D.; Abbasi, F.; Ghanbarian, S. Using Chitosan/CHPATC as coagulant to remove color and turbidity of industrial wastewater: Optimization through RSM design. J. Environ. Manag. 2018, 211, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Ting, W.C.; Loh, Z.Z.; Bahrodin, M.B.; Awang, N.A.; Kadier, A. Assessment and optimization of a natural coagulant (Musa paradisiaca) peels for domestic wastewater treatment. Environ. Toxicol. Manag. 2022, 2, 7–13. [Google Scholar]




| Chemical Reagent | Specification | Analysis |
|---|---|---|
| Bovine Serum Albumin (BSA) | Vivantis | Protein |
| Potassium sodium tartrate | AR QREC | |
| Sodium carbonate | AR QREC | |
| Sodium hydroxide | MERCK | |
| Copper (II) sulphate pentahydrate | AR QREC | |
| Folin reagent | Sigma Aldrich | |
| Glucose solution | Sigma Aldrich | Polysaccharide |
| Phenols | AR QREC | |
| Sulphuric acid | AR QREC | |
| High-range COD digestion reagent | HACH | COD |
| Kaolin | AR QREC | Synthetic wastewater |
| Sucrose | MERCK | |
| Ammonium chloride | AR QREC | |
| Potassium dihydrogen phosphate | AR QREC |
| Operating Conditions | Unit | Variation Value |
|---|---|---|
| Concentration | % | 2, 4, 6, 8, 10 |
| Temperature | °C | 60, 90, 120, 150, 180 |
| Duration | h | 0.5, 1.0, 1.5, 2.0, 3.0 |
| Composition | Unit | Saccharum officinarum (Bagasse) |
|---|---|---|
| Moisture content | % | 32.7 ± 3.8 |
| Yield | % | 18.5 ± 5.6 |
| Protein content | mg/mL | 0.9 ± 0.2 |
| Polysaccharide content | mg/mL | 74.5 ± 2.4 |
| Oil content | mg/L | 14.4 ± 1.1 |
| Ferric content | ppm | 0.1 ± 0.003 |
| Aluminium content | ppm | 0.3 ± 0.02 |
| Wavenumber Range (cm−1) | Peak Wavenumber (cm−1) | Group | Indicator |
|---|---|---|---|
| Raw Bagasse | |||
| 3500–3200 | 3338.1 | O-H stretching | Intramolecular hydrogen bonds for cellulose |
| ~1730 | 1728.7 | C-O stretching | Acetyl and ester linkage in lignin and hemicellulose |
| ~1369 | 1371.8 | C-H stretching | Ionic carboxylic groups that usually present in cellulose. |
| 1300–950 | 1243.4 | C-O stretching | Stretching of hemicellulose and lignin |
| 1160.3 | |||
| 1034.8 | |||
| Extracted Bagasse | |||
| 3500–3200 | 3339.0 | O-H stretching | Intramolecular hydrogen bonds for cellulose |
| 1649–1620 | 1638.0 | C=C stretching | Aromatic ring present in lignin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahrodin, M.B.; Zaidi, N.S.; Kadier, A.; Hussein, N.; Syafiuddin, A.; Boopathy, R. A Novel Natural Active Coagulant Agent Extracted from the Sugarcane Bagasse for Wastewater Treatment. Appl. Sci. 2022, 12, 7972. https://doi.org/10.3390/app12167972
Bahrodin MB, Zaidi NS, Kadier A, Hussein N, Syafiuddin A, Boopathy R. A Novel Natural Active Coagulant Agent Extracted from the Sugarcane Bagasse for Wastewater Treatment. Applied Sciences. 2022; 12(16):7972. https://doi.org/10.3390/app12167972
Chicago/Turabian StyleBahrodin, Muhammad Burhanuddin, Nur Syamimi Zaidi, Abudukeremu Kadier, Norelyza Hussein, Achmad Syafiuddin, and Raj Boopathy. 2022. "A Novel Natural Active Coagulant Agent Extracted from the Sugarcane Bagasse for Wastewater Treatment" Applied Sciences 12, no. 16: 7972. https://doi.org/10.3390/app12167972
APA StyleBahrodin, M. B., Zaidi, N. S., Kadier, A., Hussein, N., Syafiuddin, A., & Boopathy, R. (2022). A Novel Natural Active Coagulant Agent Extracted from the Sugarcane Bagasse for Wastewater Treatment. Applied Sciences, 12(16), 7972. https://doi.org/10.3390/app12167972







