Carrot Pomace Characterization for Application in Cereal-Based Products
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Properties
2.3. Functional Properties of Carrot Pomace Powder
2.3.1. Hydration Capacity (HC)
2.3.2. Water Absorption Capacity (WAC)
2.3.3. Oil Absorption Capacity (OAC)
2.3.4. Swelling Capacity (SC)
2.3.5. Water Retention Capacity (WRC)
2.3.6. Foaming Capacity (FC) and Foaming Stability (FS)
2.3.7. Bulk Density (BD)
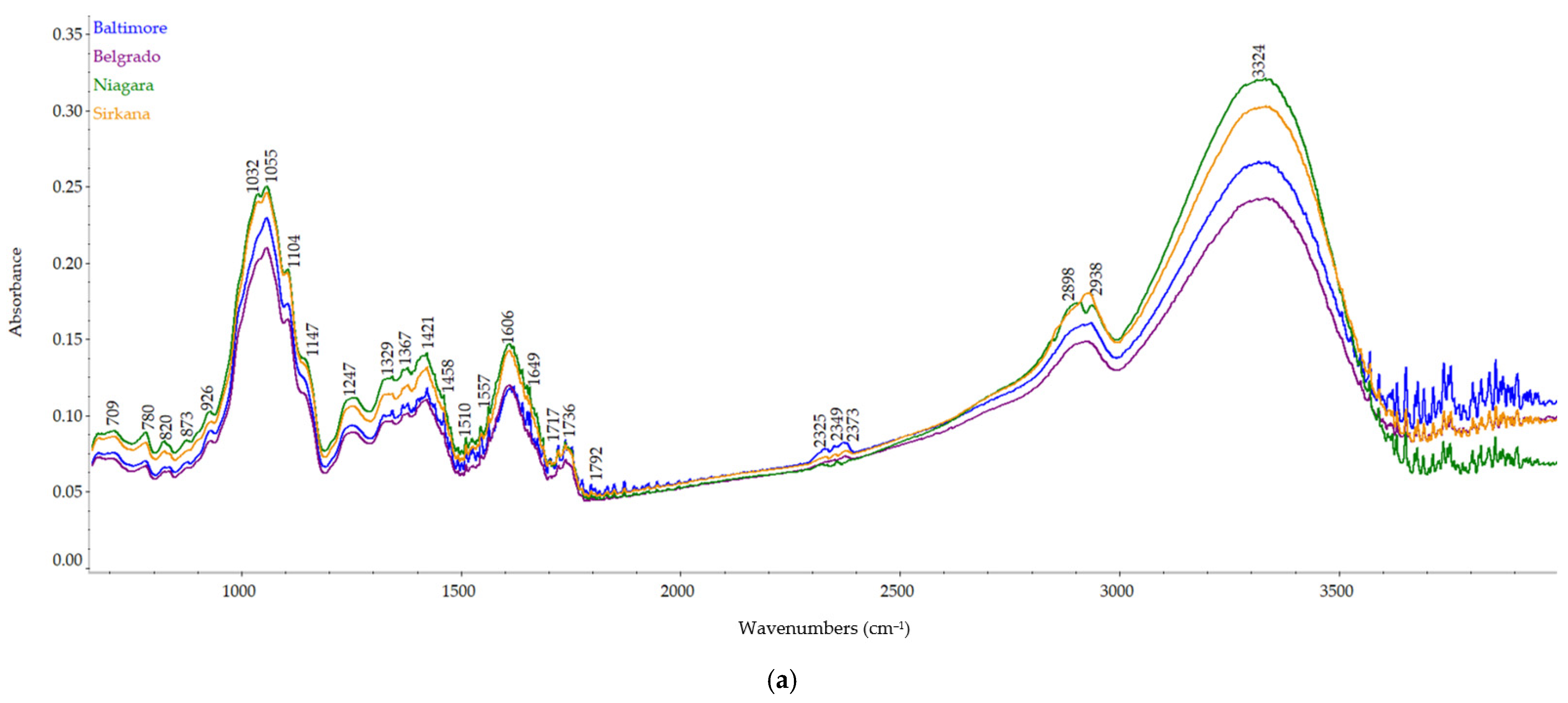
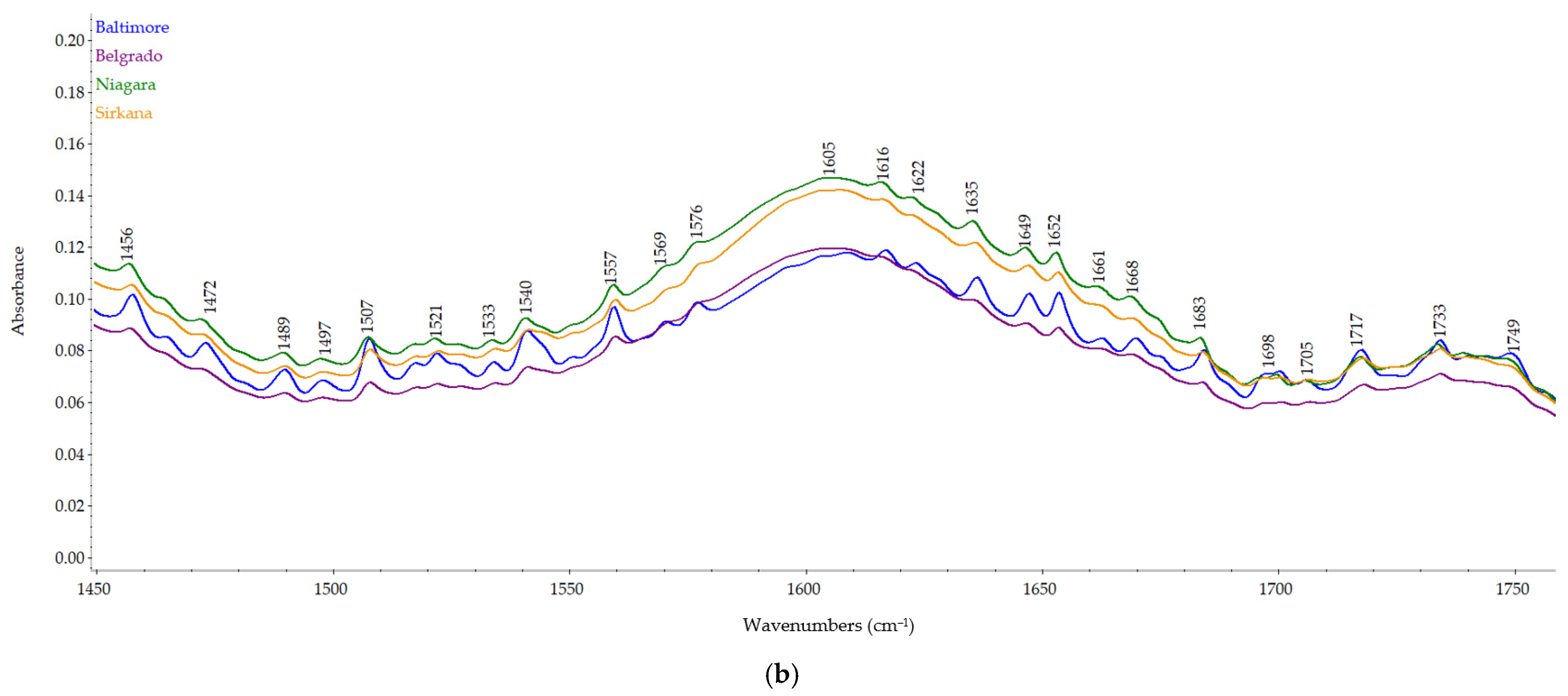
2.4. FT-IR Spectra
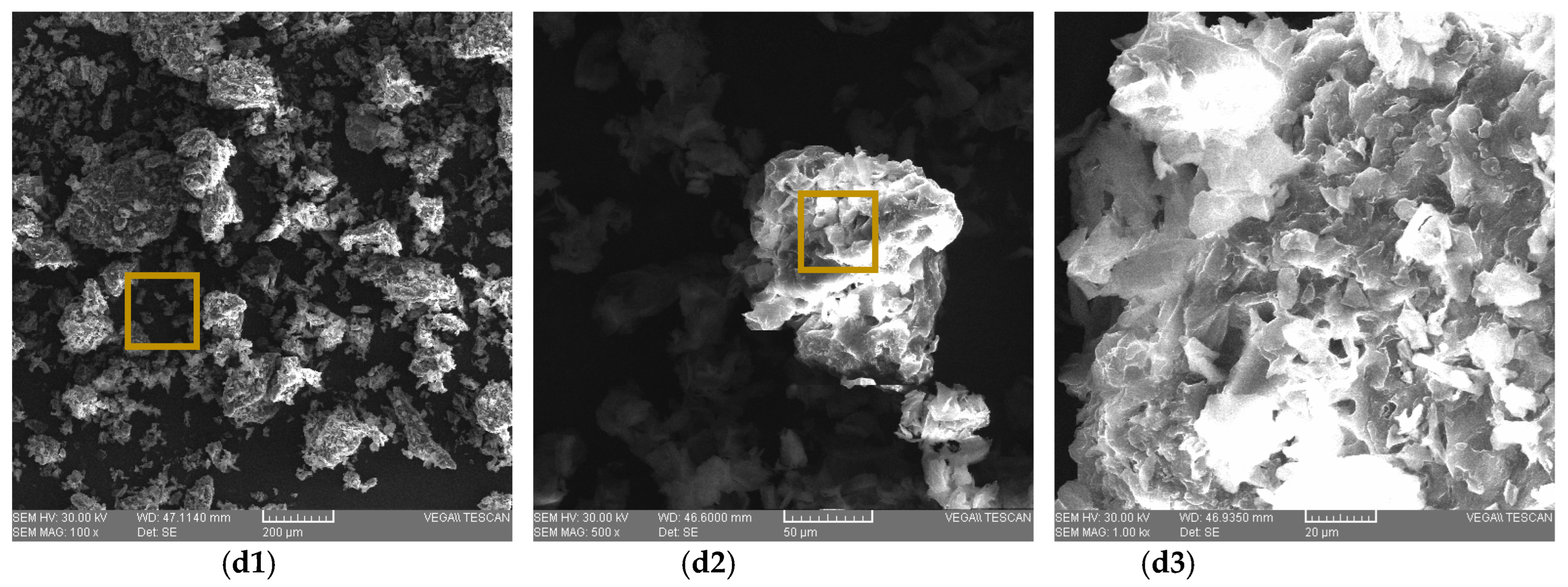
2.5. SEM Micrographs
2.6. Carrot Pomace Powder Color
2.7. Statistics
3. Results
3.1. Carrot Pomace Powder of Different Varieties Chemical Properties
3.2. Carrot Pomace Powder of Different Varieties Functional Properties and Color
3.3. Carrot Pomace Powder of Different Varieties Micrographs
3.4. Carrot Pomace Powder of Different Varieties FT-IR Spectra
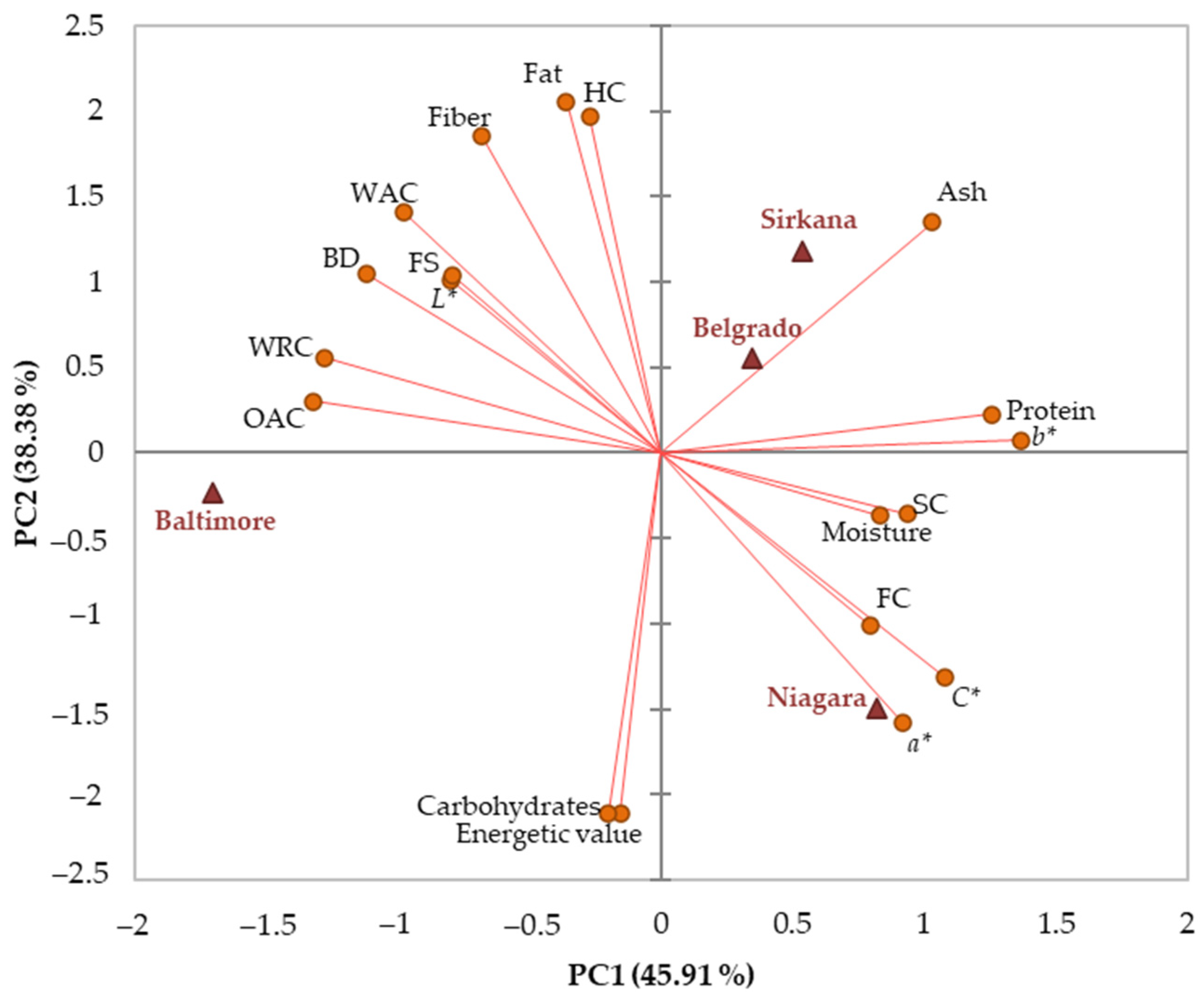
3.5. Relationships between Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Surbhi, S.; Verma, R.C.; Deepak, R.; Jain, H.K.; Yadav, K.K. A Review: Food, Chemical Composition and Utilization of Carrot (Daucus carota L.) Pomace. Int. J. Chem. Stud. 2018, 6, 2921–2926. [Google Scholar]
- Heimendinger, J.; Chapelsky, D. Dietary Phytochemicals in Cancer Prevention and Treatment. In The National 5 a Day for Better Health Program; Springer: Boston, MA, USA, 1996; pp. 199–206. ISBN 978-1-4613-0399-2. [Google Scholar]
- Alam, M.S.; Gupta, K.; Khaira, H.; Javed, M. Quality of dried carrot pomace powder as affected by pretreatments and methods of drying. Agric. Eng. Int. CIGR J. 2013, 15, 236–243. [Google Scholar]
- Di Donato, P.; Finore, I.; Anzelmo, G.; Lama, L.; Nicolaus, B.; Poli, A. Biomass and biopolymer production using vegetable wastes as cheap substrates for extremophiles. Chem. Eng. Trans. 2014, 38, 163–168. [Google Scholar] [CrossRef]
- Tańska, M.; Zadernowski, R.; Konopka, I. The quality of wheat bread supplemented with dried carrot pomace. Polish J. Nat. Sci. 2007, 22, 126–135. [Google Scholar] [CrossRef]
- Nawirska, A.; Kwaśniewska, M. Dietary fibre fractions from fruit and vegetable processing waste. Food Chem. 2005, 91, 221–225. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Bao, B.; Chang, K.C. Carrot Pulp Chemical Composition, Color, and Water-holding Capacity as Affected by Blanching. J. Food Sci. 1994, 59, 1159–1161. [Google Scholar] [CrossRef]
- Singh, B.; Panesar, P.S.; Nanda, V. Utilization of Carrot Pomace for the Preparation of a Value Added Product. World J. Dairy Food Sci. 2006, 1, 22–27. [Google Scholar]
- Kırbaş, Z.; Kumcuoglu, S.; Tavman, S. Effects of apple, orange and carrot pomace powders on gluten-free batter rheology and cake properties. J. Food Sci. Technol. 2019, 56, 914–926. [Google Scholar] [CrossRef]
- Lotfi Shirazi, S.; Koocheki, A.; Milani, E.; Mohebbi, M. Production of high fiber ready-to-eat expanded snack from barley flour and carrot pomace using extrusion cooking technology. J. Food Sci. Technol. 2020, 57, 2169–2181. [Google Scholar] [CrossRef]
- Coțovanu, I.; Batariuc, A.; Mironeasa, S. Characterization of quinoa seeds milling fractions and their effect on the rheological properties of wheat flour dough. Appl. Sci. 2020, 10, 7225. [Google Scholar] [CrossRef]
- Bordei, D.; Bahrim, G.; Pâslaru, V.; Gasparotti, C.; Elisei, A.; Banu, I.; Ionescu, L.; Codină, G. Quality Control in the Bakery Industry-Analysis Methods. Galați Acad. 2007, 1, 203–212. [Google Scholar]
- Oladiran, D.A.; Emmambux, N.M. Nutritional and Functional Properties of Extruded Cassava-Soy Composite with Grape Pomace. Starch 2018, 70, 1700298. [Google Scholar] [CrossRef]
- Elkhalifa, A.E.O.; Bernhardt, R. Combination Effect of Germination and Fermentation on Functional Properties of Sorghum Flour. Curr. J. Appl. Sci. Technol. 2018, 30, 1–12. [Google Scholar] [CrossRef]
- Raghavendra, S.N.; Rastogi, N.K.; Raghavarao, K.S.M.S.; Tharanathan, R.N. Dietary fiber from coconut residue: Effects of different treatments and particle size on the hydration properties. Eur. Food Res. Technol. 2004, 218, 563–567. [Google Scholar] [CrossRef]
- Okaka, J.C.; Potter, N.N. Functional and Storage Properties of Cowpea Powder-Wheat Flour Blends in Breadmaking. J. Food Sci. 1977, 42, 828–833. [Google Scholar] [CrossRef]
- Sucheta; Misra, N.N.; Yadav, S.K. Extraction of pectin from black carrot pomace using intermittent microwave, ultrasound and conventional heating: Kinetics, characterization and process economics. Food Hydrocoll. 2020, 102, 105592. [Google Scholar] [CrossRef]
- Sucheta; Chaturvedi, K.; Yadav, S.K. Ultrasonication assisted salt-spices impregnation in black carrots to attain anthocyanins stability, quality retention and antimicrobial efficacy on hot-air convective drying. Ultrason. Sonochem. 2019, 58, 104661. [Google Scholar] [CrossRef]
- Joda, B.A.; Abed Al-Kadhim, Z.M.; Ahmed, H.J.; Al-Khalaf, A.K.H. A Convenient Green Method to Synthesize β-Carotene from Edible Carrot and Nanoparticle Formation. Karbala Int. J. Mod. Sci. 2022, 8, 20–27. [Google Scholar] [CrossRef]
- Jayesree, N.; Hang, P.K.; Priyangaa, A.; Krishnamurthy, N.P.; Ramanan, R.N.; Turki, M.S.A.; Charis, M.G.; Ooi, C.W. Valorisation of carrot peel waste by water-induced hydrocolloidal complexation for extraction of carote and pectin. Chemosphere 2021, 272, 129919. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Zdunek, A. Use of FT-IR Spectra and PCA to the Bulk Characterization of Cell Wall Residues of Fruits and Vegetables along a Fraction Process. Food Biophys. 2013, 8, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Grewal, R.B. Nutritional evaluation and utilization of carrot pomace powder for preparation of high fiber biscuits. J. Food Sci. Technol. 2007, 44, 56–58. [Google Scholar]
- Amin, S.; Jung, S.; Kang, I.; Duval, A. Valorization of Baby Carrot Processing Waste. J. Culin. Sci. Technol. 2021, 1–17. [Google Scholar] [CrossRef]
- Sharoba, A.; Farrag, M.; Abd El-Salam, A. Utilization of Some Fruits and Vegetables Wastes As a Source of Dietary Fibers in Cake Making. J. Food Dairy Sci. 2013, 4, 433–453. [Google Scholar] [CrossRef]
- Damodaran, S.; Parkin, K.L.; Fennema, O.R. Fennema’s Food Chemistry; CRC Press: Boca Raton, FL, USA, 2007; ISBN 1420020528. [Google Scholar]
- Jaime, L.; Mollá, E.; Fernández, A.; Martín-Cabrejas, M.A.; López-Andréu, F.J.; Esteban, R.M. Structural carbohydrate differences and potential source of dietary fiber of onion (Allium cepa L.) tissues. J. Agric. Food Chem. 2002, 50, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Wani, T.A.; Wani, S.M.; Masoodi, F.A.; Gani, A. Incorporation of carrot pomace powder in wheat flour: Effect on flour, dough and cookie characteristics. J. Food Sci. Technol. 2016, 53, 3715–3724. [Google Scholar] [CrossRef] [PubMed]
- Negi, T.; Vaidya, D. Functional Properties of Apple Pomace Powder. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 589–595. [Google Scholar] [CrossRef]
- Grover, S.S.; Chauhan, G.S.; Masoodi, F.A. Effect of particle size on surface properties of apple pomace. Int. J. Food Prop. 2003, 6, 1–7. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Lech, K.; Łysiak, G.P.; Figiel, A. Effect of different drying techniques on physical properties, total polyphenols and antioxidant capacity of blackcurrant pomace powders. LWT-Food Sci. Technol. 2017, 78, 114–121. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Bawoł, A.; Czapski, J.; Kidoń, M. Studies on Drying of Purple Carrot Roots. Dry. Technol. 2009, 27, 1325–1331. [Google Scholar] [CrossRef]
- Ando, Y.; Maeda, Y.; Mizutani, K.; Wakatsuki, N.; Hagiwara, S.; Nabetani, H. Impact of blanching and freeze-thaw pretreatment on drying rate of carrot roots in relation to changes in cell membrane function and cell wall structure. LWT-Food Sci. Technol. 2016, 71, 40–46. [Google Scholar] [CrossRef]
- Kaur, P.; Ghoshal, G.; Jain, A. Bio-utilization of fruits and vegetables waste to produce β-carotene in solid-state fermentation: Characterization and antioxidant activity. Process Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- Thummajitsakul, S.; Samaikam, S.; Tacha, S.; Silprasit, K. Study on FTIR spectroscopy, total phenolic content, antioxidant activity and anti-amylase activity of extracts and different tea forms of Garcinia schomburgkiana leaves. LWT 2020, 134, 110005. [Google Scholar] [CrossRef]

| Variety | Protein (%) | Fat (%) | Ash (%) | Fiber (%) | Moisture (%) | Carbohydrates (%) | Energetic Value (kcal/100 g) |
|---|---|---|---|---|---|---|---|
| Baltimore | 6.87 ± 0.06 c | 1.00 ± 0.02 b | 5.29 ± 0.04 c | 28.69 ± 0.58 c | 4.04 ± 0.02 b | 54.13 ± 0.52 b | 339.40 ± 40.18 b |
| Belgrado | 8.01 ± 0.06 b | 1.01 ± 0.03 b | 5.89 ± 0.02 a | 33.34 ± 0.25 a | 3.78 ± 0.01 b | 48.00 ± 0.24 c | 333.96 ± 47.87 b |
| Niagara | 8.84 ± 0.12 a | 0.70 ± 0.02 c | 5.56 ± 0.03 b | 20.09 ± 0.08 d | 5.88 ± 0.46 a | 58.95 ± 0.65 a | 343.32 ± 34.64 a |
| Sirkana | 9.14 ± 0.06 a | 1.13 ± 0.03 a | 5.89 ± 0.01 a | 31.40 ± 0.70 b | 5.91 ± 0.15 a | 46.55 ± 0.64 c | 332.00 ± 35.81 b |
| Variety | HC (%) | WAC (%) | OAC (%) | WRC (g/g) | FC (%) | FS (%) | SC (mL/g) | BD (g/cm³) |
|---|---|---|---|---|---|---|---|---|
| Baltimore | 67.94 ± 2.67 a | 16.99 ± 0.24 a | 37.31 ± 0.32 a | 7.64 ± 0.03 a | 5.00 ± 0.00 b | 96.00 ± 0.00 a | 25.95 ± 0.01 b | 0.56 ± 0.00 a |
| Belgrado | 66.81 ± 2.24 a | 15.96 ± 0.44 ab | 34.72 ± 0.85 c | 5.33 ± 0.05 c | 7.00 ± 0.00 a | 94.00 ± 0.00 b | 25.96 ± 0.01 b | 0.46 ± 0.00 b |
| Niagara | 57.96 ± 0.78 b | 11.59 ± 0.01 c | 34.33 ± 0.25 c | 4.76 ± 0.01 d | 7.00 ± 0.00 a | 94.00 ± 0.00 b | 27.22 ± 0.00 a | 0.45 ± 0.00 b |
| Sirkana | 68.26 ± 4.51 a | 15.15 ± 0.37 b | 35.29 ± 1.68 b | 6.10 ± 0.00 b | 5.00 ± 0.00 b | 96.00 ± 0.00 a | 27.20 ± 0.01 a | 0.56 ± 0.00 a |
| Variety | L* (Adim.) | a* (Adim.) | b* (Adim.) | C* (Adim.) |
|---|---|---|---|---|
| Baltimore | 73.30 ± 0.15 a | 8.61 ± 1.68 b | 18.85 ± 0.16 b | 20.77 ± 0.62 c |
| Belgrado | 71.30 ± 0.09 b | 10.21 ± 0.15 b | 19.47 ± 0.05 a | 21.98 ± 0.10 b |
| Niagara | 66.02 ± 0.08 d | 13.05 ± 0.02 a | 19.61 ± 0.06 a | 23.55 ± 0.04 a |
| Sirkana | 69.44 ± 0.01 c | 9.25 ± 0.06 b | 19.56 ± 0.04 a | 21.63 ± 0.05 a |
| Measured Wavenumber (cm−1) | Wavenumber from the Literature (cm−1) | Assignment | Origin |
|---|---|---|---|
| 3324 | 3000–3600 | O–H and N–H stretch C–H | Water, alcohols, phenols, carbohydrates, peroxides polysaccharides, lipids, and carbohydrates |
| 2898 | 2891 | –CH stretching vibrations | Galacturonic acid |
| 1717, 1736 | 1700–1799 | C=O | Lipids, beta carotene |
| 1649 | 1600–1706 | Amide I of proteins, C–O, C–N, CNN | Proteins |
| 1606 | 1600–1630 | COO- antisymmetric stretching | Polygalacturonic acid, carboxylate (pectin ester group) |
| 1557 | 1460–1590 | Amide II of proteins, N–H, C–N | Proteins |
| 1421 | 1421–1428 | CH3 symmetric bending | Cellulose |
| 1367 | 1370 | CH2 bending | Xyloglucan, Cellulose |
| 1329 | 1320–1330 | Ring vibration | Pectin |
| 1247 | 1243 | C–O stretching | Pectin |
| 1147 | 1147 | O–C–O asymmetric stretching | Xyloglucan (glycosidic link) |
| 1104 | 1103–1115 | C–O stretching, C–C stretching | Cellulose (C2-O2) |
| 1032 | 1030 | C–O stretching, C–C stretching | Cellulose (C6-H2-O6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, M.I.; Ungureanu-Iuga, M.; Mironeasa, S. Carrot Pomace Characterization for Application in Cereal-Based Products. Appl. Sci. 2022, 12, 7989. https://doi.org/10.3390/app12167989
Luca MI, Ungureanu-Iuga M, Mironeasa S. Carrot Pomace Characterization for Application in Cereal-Based Products. Applied Sciences. 2022; 12(16):7989. https://doi.org/10.3390/app12167989
Chicago/Turabian StyleLuca, Marian Ilie, Mădălina Ungureanu-Iuga, and Silvia Mironeasa. 2022. "Carrot Pomace Characterization for Application in Cereal-Based Products" Applied Sciences 12, no. 16: 7989. https://doi.org/10.3390/app12167989






