Preparation of Al2O3 Multichannel Cylindrical-Tube-Type Microfiltration Membrane with Surface Modification
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
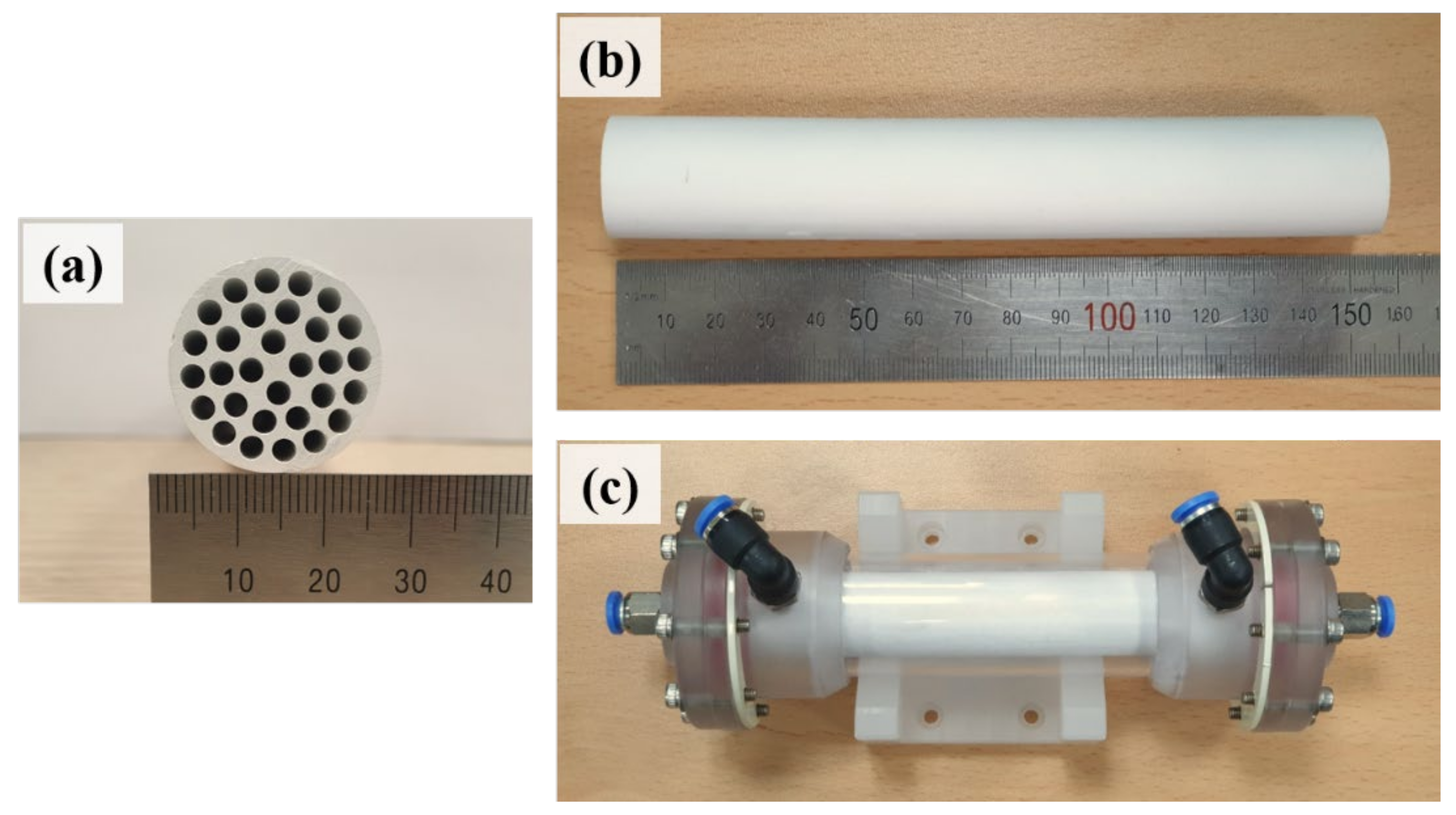
3.1. Multichannel Tubular Support
3.2. Alumina Microfiltration Membrane
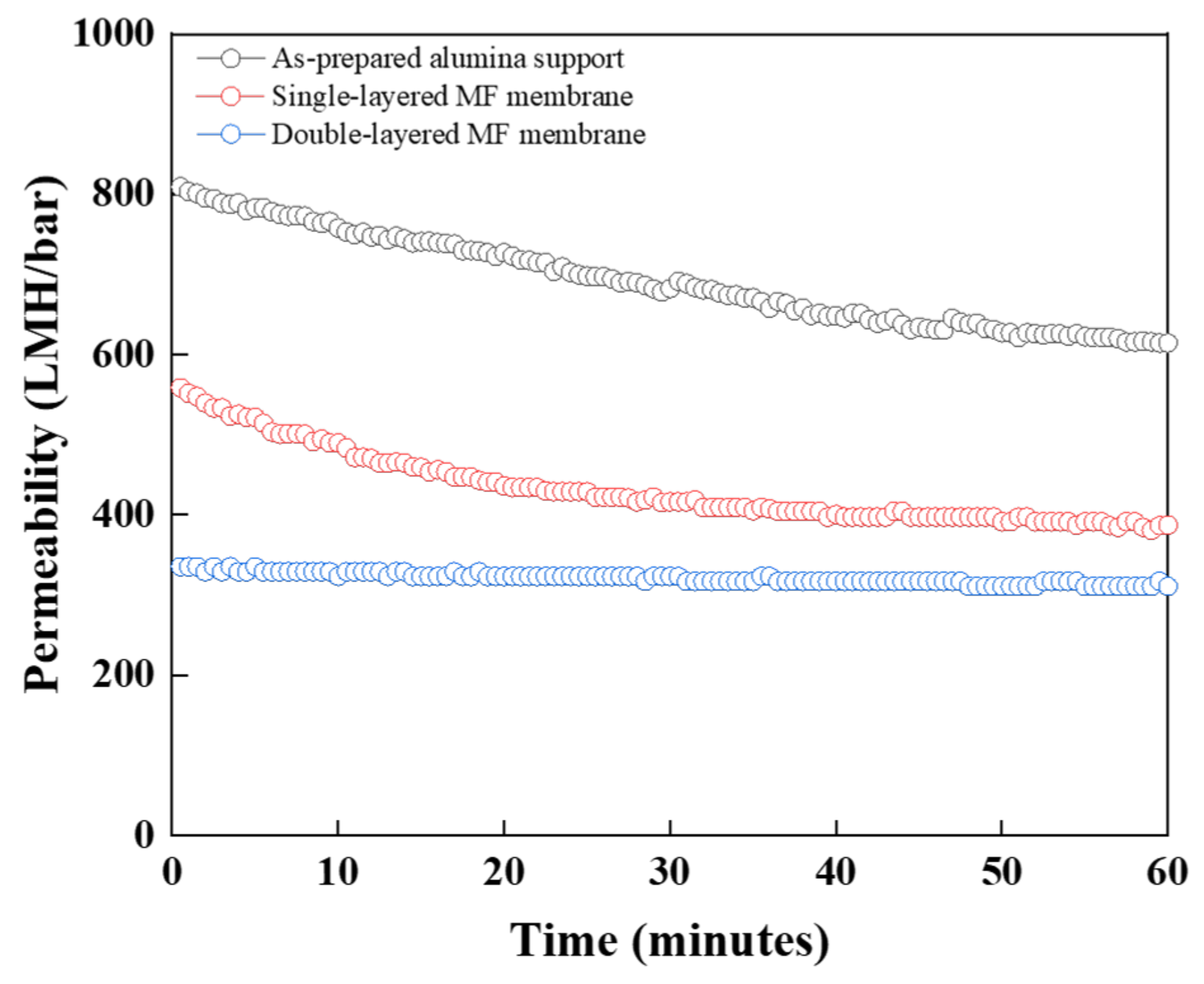
3.3. Pure Water Permeability
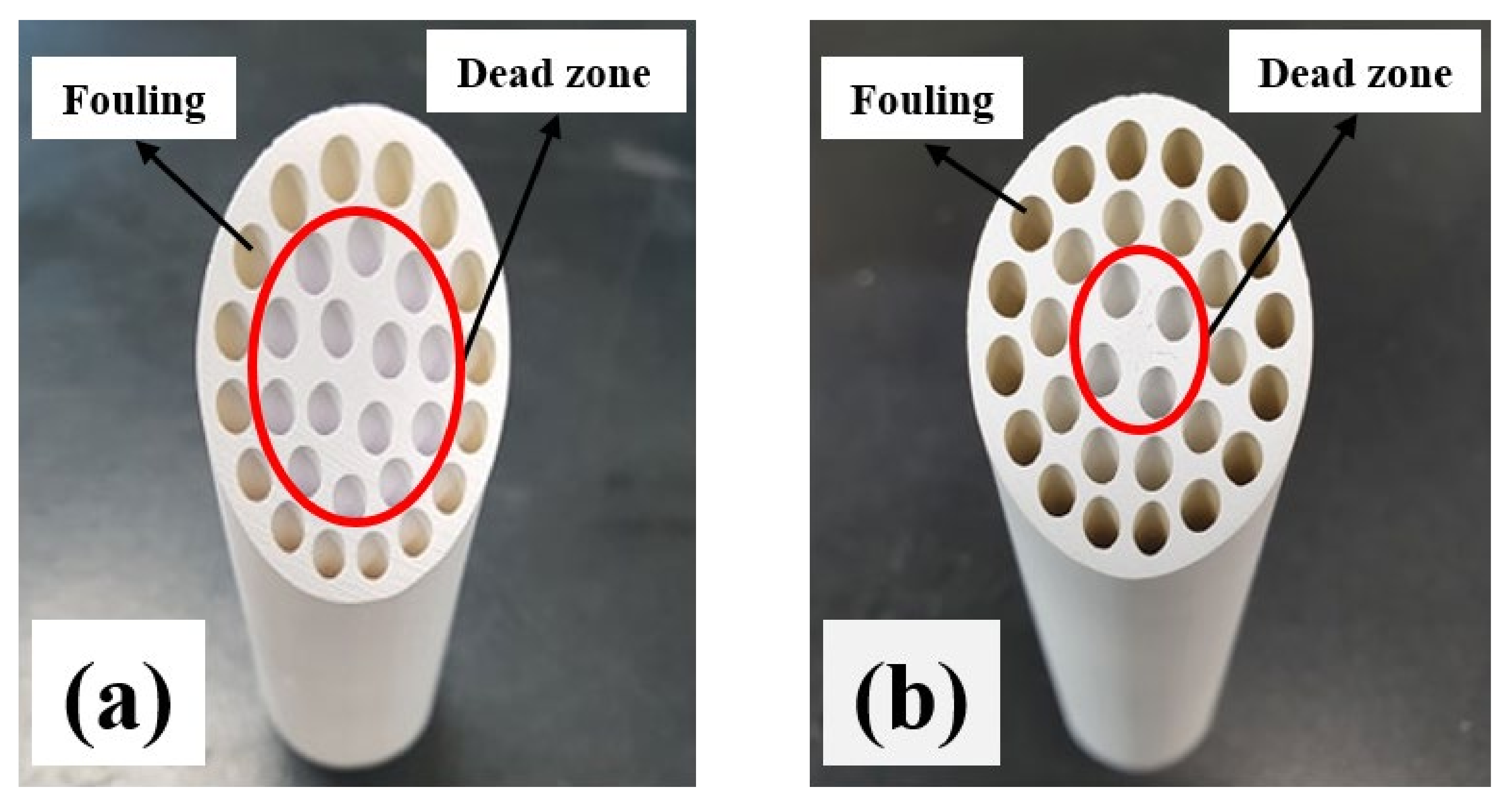
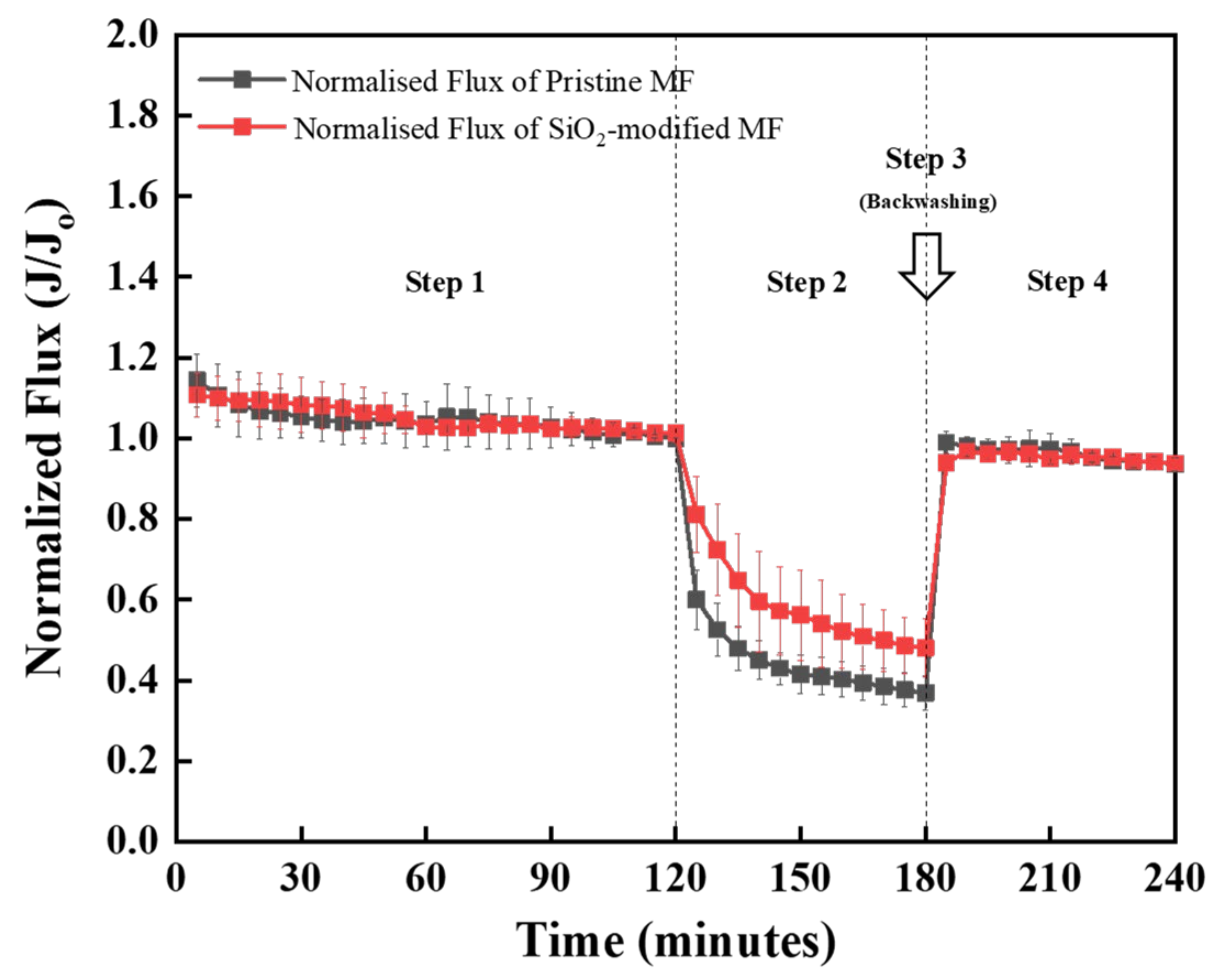
3.4. Surface Modification for Antifouling Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fane, A.G.; Wang, R.; Jia, Y. Membrane Technology: Past, Present and Future. In Membrane and Desalination Technologies; Wang, L.K., Chen, J.P., Hung, Y.-T., Shammas, N.K., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 1–45. [Google Scholar] [CrossRef]
- Studart, A.R.; Gonzenbach, U.T.; Tervoort, E.; Gauckler, L.J. Processing Routes to Macroporous Ceramics: A Review. J. Am. Ceram. Soc. 2006, 89, 1771–1789. [Google Scholar] [CrossRef]
- Cheong, H.; Cho, W.-S.; Ha, J.-S.; Kim, C.-S.; Choi, D.-K.; Cheong, D.-S. Structural evolution of alumina membrane prepared on an alumina support using a sol–gel method. J. Alloys Compd. 1999, 290, 304–309. [Google Scholar] [CrossRef]
- Gonzenbach, U.T.; Studart, A.R.; Steinlin, D.; Tervoort, E.; Gauckler, L.J. Processing of Particle-Stabilized Wet Foams Into Porous Ceramics. J. Am. Ceram. Soc. 2007, 90, 3407–3414. [Google Scholar] [CrossRef]
- Raether, F.; Iuga, M. Effect of particle shape and arrangement on thermoelastic properties of porous ceramics. J. Eur. Ceram. Soc. 2006, 26, 2653–2667. [Google Scholar] [CrossRef]
- Ha, J.-H.; Bukhari, S.Z.A.; Lee, J.; Song, I.-H. The preparation and characterizations of an alumina support layer as a free-standing membrane for microfiltration. Ceram. Int. 2015, 41, 13372–13380. [Google Scholar] [CrossRef]
- Nair, B.N. Synthesis, characterisation and gas permeation studies on microporous silica and alumina-silica membranes for separation of propane and propylene. J. Membr. Sci. 1996, 116, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Rao, A.P.; Desai, N.V.; Rangarajan, R. Inorganic membranes: New materials for separation technology. J. Sci. Ind. Res. 1997, 56, 518–522. [Google Scholar]
- Yang, Z.; Cheng, J.; Yang, C.; Liang, B. CFD-based optimization and design of multi-channel inorganic membrane tubes. Chin. J. Chem. Eng. 2016, 24, 1375–1385. [Google Scholar] [CrossRef]
- Ha, J.-H.; Bukhari, S.Z.A.; Lee, J.; Song, I.-H.; Park, C. Preparation processes and characterizations of alumina-coated alumina support layers and alumina-coated natural material-based support layers for microfiltration. Ceram. Int. 2016, 42, 13796–13804. [Google Scholar] [CrossRef]
- Ha, J.-H.; Bukhari, S.Z.A.; Lee, J.; Song, I.-H. The membrane properties of alumina-coated alumina support layers and alumina-coated diatomite–kaolin composite support layers. Adv. Appl. Ceram. 2017, 117, 1–8. [Google Scholar] [CrossRef]
- Meng, S.; Zhang, M.; Yao, M.; Qiu, Z.; Hong, Y.; Lan, W.; Xia, H.; Jin, X. Membrane Fouling and Performance of Flat Ceramic Membranes in the Application of Drinking Water Purification. Water 2019, 11, 2606. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Lin, B.; Zhou, J.-E.; Zhang, X.; Ling, Y.; Liu, X.; Meng, G.; Hampshire, S. Corrosion resistance characterization of porous alumina membrane supports. Mater. Charact. 2011, 62, 409–418. [Google Scholar] [CrossRef]
- Dilaver, M.; Hocaoglu, S.M.; Soydemir, G.; Dursun, M.; Keskinler, B.; Koyuncu, I.; Ağtaş, M. Hot wastewater recovery by using ceramic membrane ultrafiltration and its reusability in textile industry. J. Clean. Prod. 2018, 171, 220–233. [Google Scholar] [CrossRef]
- Damas, S.B.; Alcaina-Miranda, M.I.; Bes-Piá, A.; Iborra-Clar, M.I.; Iborra-Clar, A.; Mendoza-Roca, J.-A. Ceramic membrane behavior in textile wastewater ultrafiltration. Desalination 2010, 250, 623–628. [Google Scholar] [CrossRef]
- Nandi, B.; Das, B.; Uppaluri, R.; Purkait, M. Microfiltration of mosambi juice using low cost ceramic membrane. J. Food Eng. 2009, 95, 597–605. [Google Scholar] [CrossRef]
- Chang, Y.; Ling, Z.; Liu, Y.; Hu, X.; Li, Y. A simple method for fabrication of highly ordered porous α-alumina ceramic membranes. J. Mater. Chem. 2012, 22, 7445–7448. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kerker, S.; Schmitz, O.; Schmidt, A.A.; Czermak, P. Evaluation of the fouling potential of ceramic membrane configurations designed for the treatment of oilfield produced water. Sep. Sci. Technol. 2017, 53, 349–363. [Google Scholar] [CrossRef]
- Ha, J.-H.; Lee, S.; Bukhari, S.Z.A.; Lee, J.; Song, I.-H.; Lee, S.J.; Choi, J. Effects of preparation conditions on the membrane properties of alumina-coated silicon carbide supports. J. Ceram. Soc. Jpn. 2018, 126, 860–869. [Google Scholar] [CrossRef]
- Wang, F.; Lee, J.; Ha, J.-H.; Song, I.-H. Surface modification of alumina membranes via a sol-gel process for antifouling properties. Mater. Lett. 2017, 191, 200–202. [Google Scholar] [CrossRef]
- Lee, J.; Ha, J.-H.; Song, I.-H.; Park, J.-W. Effect of SiO2 coating on alumina microfiltration membranes on flux performance in membrane fouling process. J. Ceram. Soc. Jpn. 2019, 127, 35–43. [Google Scholar] [CrossRef]
- Esfahani, M.R.; Stretz, H.A.; Wells, M.J. Comparing humic acid and protein fouling on polysulfone ultrafiltration membranes: Adsorption and reversibility. J. Water Process Eng. 2015, 6, 83–92. [Google Scholar] [CrossRef]
- Yeh, T.-S.; Sacks, M.D. Effect of Particle Size Distribution on the Sintering of Alumina. J. Am. Ceram. Soc. 1988, 71, C-484–C-487. [Google Scholar] [CrossRef]
- Ma, J.; Lim, L. Effect of particle size distribution on sintering of agglomerate-free submicron alumina powder compacts. J. Eur. Ceram. Soc. 2002, 22, 2197–2208. [Google Scholar] [CrossRef]
- Shqau, K.; Mottern, M.L.; Yu, D.; Verweij, H. Preparation of defect-free porous alumina membrane supports by colloidal filtration. In Proceedings of the 6th Pacific Rim Conference on Multimedia, Jeju Island, Korea, 11–13 November 2005; pp. 20–21. [Google Scholar]
- Shqau, K.; Mottern, M.L.; Yu, D.; Verweij, H. Preparation and Properties of Porous alpha-Al2O3 Membrane Supports. J. Am. Ceram. Soc. 2006, 89, 1790–1794. [Google Scholar] [CrossRef]
- Qi, H.; Niu, S.; Jiang, X.; Xu, N. Enhanced performance of a macroporous ceramic support for nanofiltration by using α-Al2O3 with narrow size distribution. Ceram. Int. 2013, 39, 2463–2471. [Google Scholar] [CrossRef]
- Cooper, A.; Floreani, R.; Ma, H.; Bryers, J.D.; Zhang, M. Chitosan-based nanofibrous membranes for antibacterial filter applications. Carbohydr. Polym. 2012, 92, 254–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukhari, S.Z.A.; Ha, J.-H.; Lee, J.; Song, I.-H. Viscosity Study to Optimize a Slurry of Alumina Mixed with Hollow Microspheres. J. Korean Ceram. Soc. 2015, 52, 403–409. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, R.; Bergström, L. Electroacoustic and rheological properties of aqueous Ce-ZrO2 (Ce-TZP) suspensions. J. Eur. Ceram. Soc. 1997, 17, 537–548. [Google Scholar] [CrossRef]
- Briscoe, B.J.; Khan, A.U.; Luckham, P. Optimising the dispersion on an alumina suspension using commercial polyvalent electrolyte dispersants. J. Eur. Ceram. Soc. 1998, 18, 2141–2147. [Google Scholar] [CrossRef]
- Wang, L.; Song, L. Flux decline in crossflow microfiltration and ultrafiltration: Experimental verification of fouling dynamics. J. Membr. Sci. 1999, 160, 41–50. [Google Scholar] [CrossRef]
- Doleěk, P. Mathematical modelling of permeate flow in multichannel ceramic membrane. J. Membr. Sci. 1995, 100, 111–119. [Google Scholar] [CrossRef]
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Mariñas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Mohamamdi, T.; Moghadam, M.K. Chemical cleaning of reverse osmosis membranes. Desalination 2001, 134, 77–82. [Google Scholar] [CrossRef]
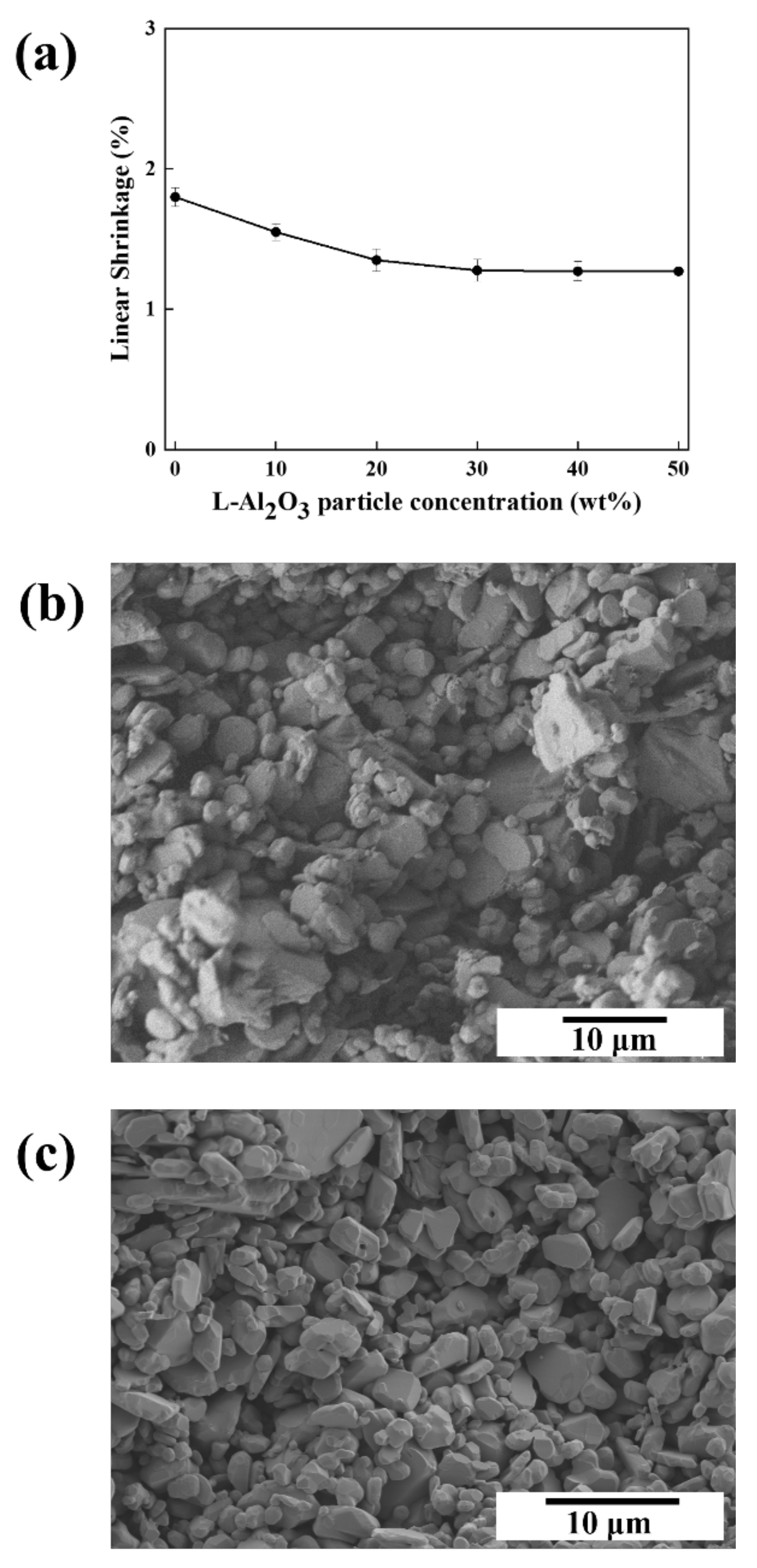
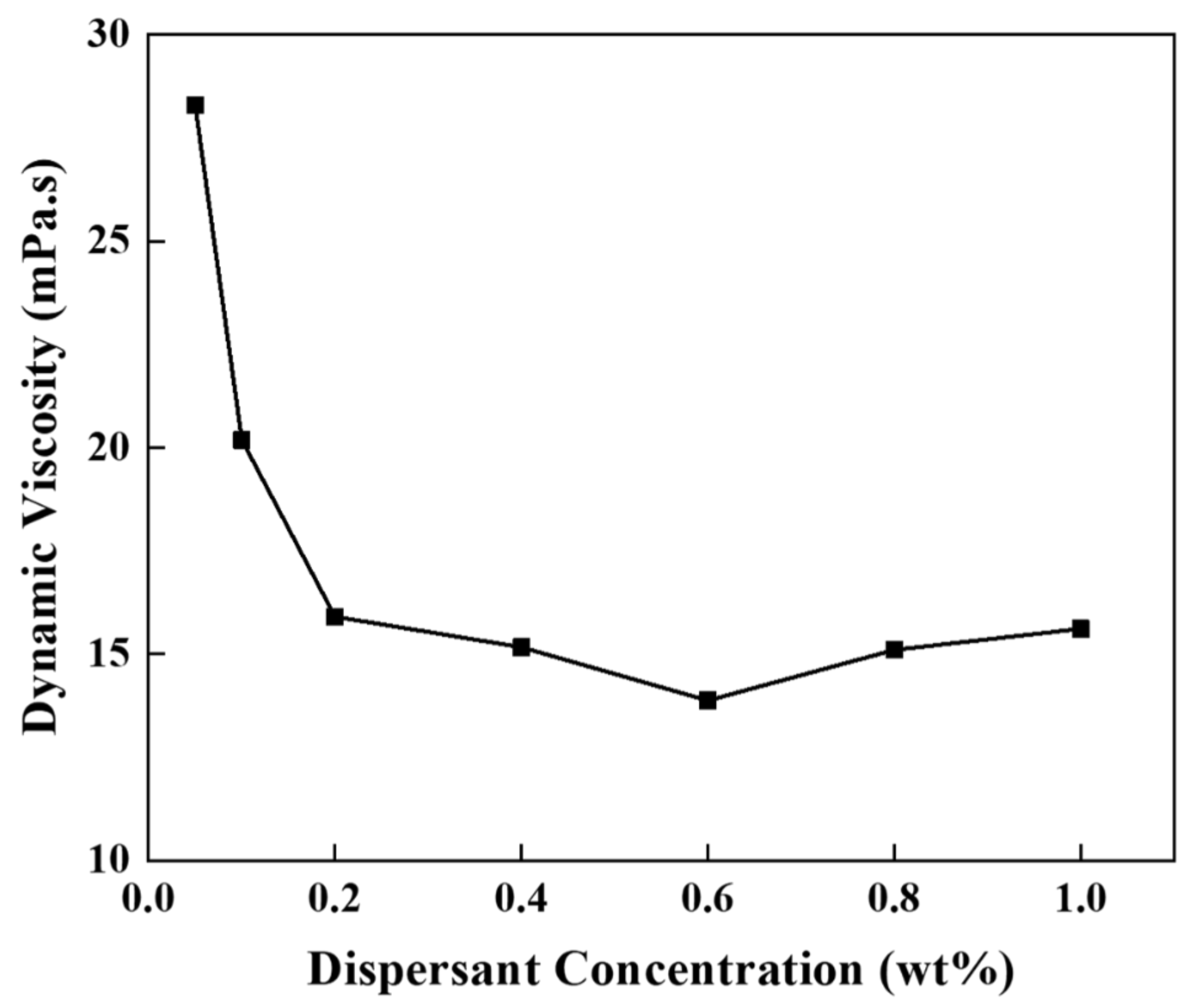
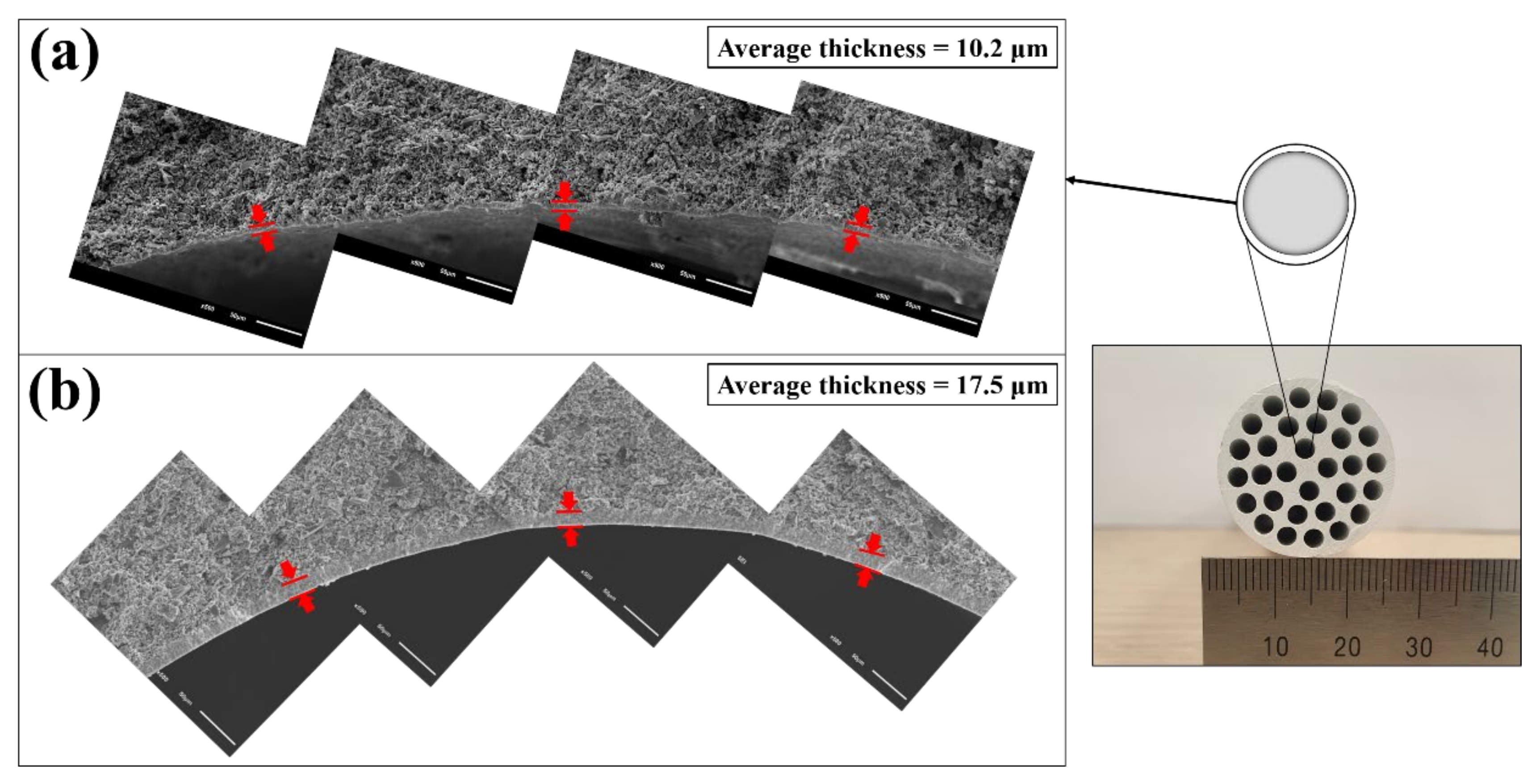
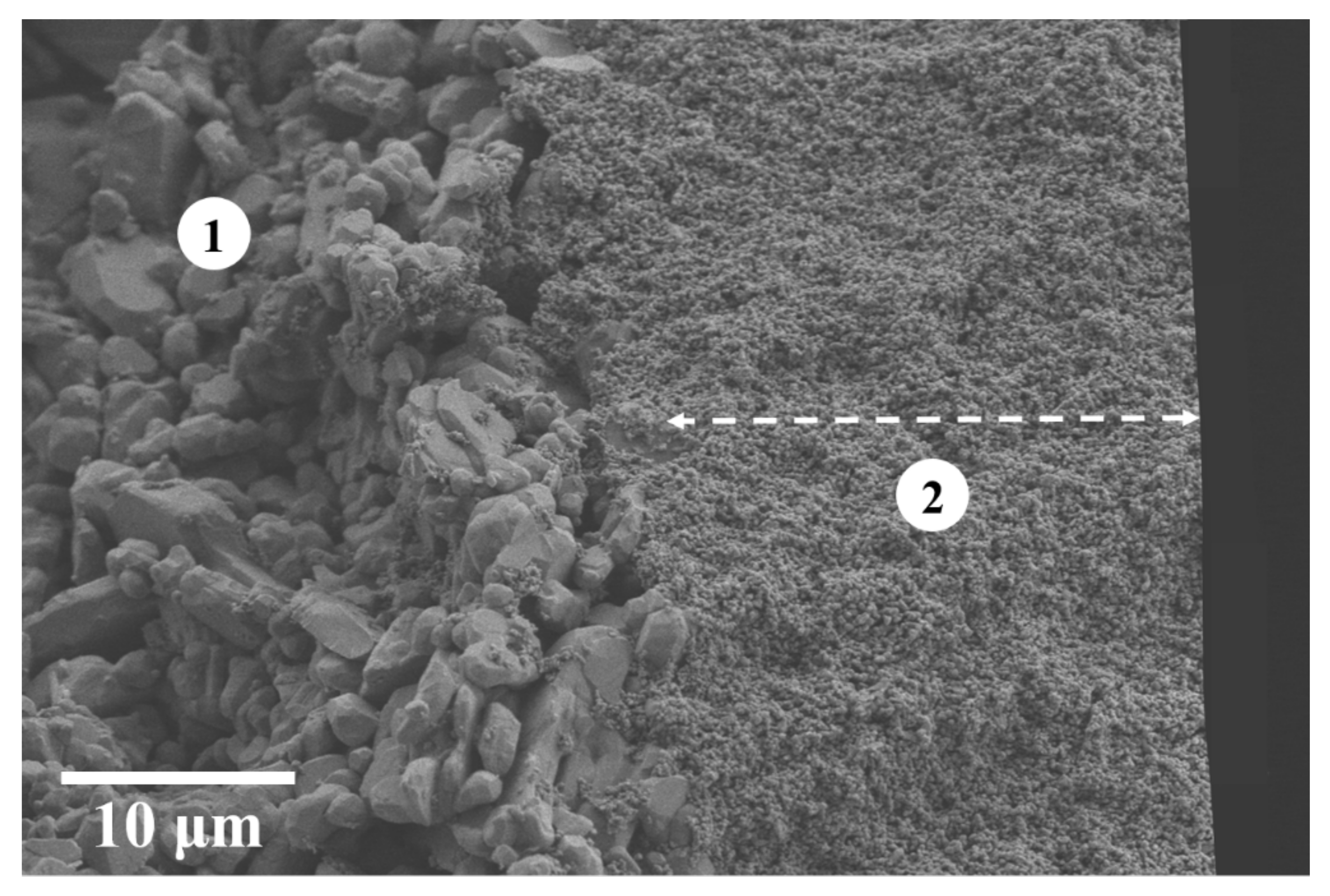

| Slurry Composition | |||
|---|---|---|---|
| Powder | Solvents | Binder | |
| AKP-30 | H2O | IPA | PVA |
| 8 wt% | 57 wt% | 33 wt% | 2 wt% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naseer, D.; Ha, J.-H.; Lee, J.; Song, I.-H. Preparation of Al2O3 Multichannel Cylindrical-Tube-Type Microfiltration Membrane with Surface Modification. Appl. Sci. 2022, 12, 7993. https://doi.org/10.3390/app12167993
Naseer D, Ha J-H, Lee J, Song I-H. Preparation of Al2O3 Multichannel Cylindrical-Tube-Type Microfiltration Membrane with Surface Modification. Applied Sciences. 2022; 12(16):7993. https://doi.org/10.3390/app12167993
Chicago/Turabian StyleNaseer, Danyal, Jang-Hoon Ha, Jongman Lee, and In-Hyuck Song. 2022. "Preparation of Al2O3 Multichannel Cylindrical-Tube-Type Microfiltration Membrane with Surface Modification" Applied Sciences 12, no. 16: 7993. https://doi.org/10.3390/app12167993







