Spin Probes as Scavengers of Free Radicals in Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
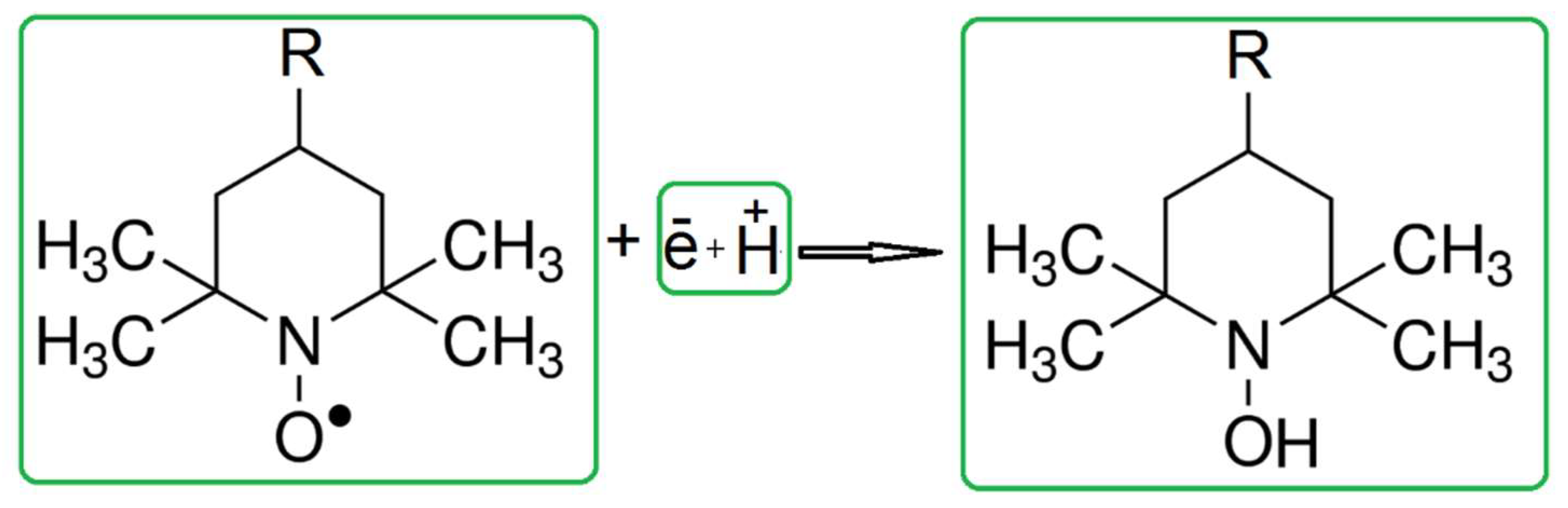
3.1. Experiments with Yeast Cells
3.2. The Effect of the Ratio of Spin Probe Concentration in Solution to Cell Amount on the ESR Spectrum
3.3. Processes of Changing the Concentration of TEMPO and TEMPOL in Cells
3.4. The Influence of Oxygen in the Atmosphere and Vitamin C on Spin Probes in Cell Culture
3.5. Experiments with Cancer Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bil, P.; Ciesielska, S.; Jaksik, R.; Rzeszowska-Wolny, J. Circuits Regulating Superoxide and Nitric Oxide Production and Neutralisation in Dierent Cell Types: Expression of Participating Genes and Changes Induced by Ionizing Radiation. Antioxidants 2000, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, E.; Ivanova, D.; Zhelev, Z.; Bakalova, R.; Gulubova, M.; Aoki, I. Mitochondrial dysfunction and redox imbalance as a diagnostic marker of free radical diseases. Anticancer Res. 2017, 37, 5373–5381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, J.P.; Coutinho, O.P. Free radicals in the regulation of damage and cell death—Basic mechanisms and prevention. Drug Discov. Ther. 2010, 4, 144–167. [Google Scholar] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Das, R.; Banerjee, E.R. Role of free radicals in human inflammatory diseases. AIMS Biophys. 2017, 4, 596–614. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Fernandes, C.; Melo, K.V.; Ferreira, S.S.; Lessa, J.A.; Franco, R.W.A.; Schenk, G.; Pereira, M.D.; Horn, A., Jr. Iron, copper, and manganese complexes with in vitro superoxide dismutase and/or catalase activities that keep Saccharomyces cerevisiae cells alive under severe oxidative stress. Free Radic. Biol. Med. 2015, 80, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and their Implication in Various Diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Peoples, J.N.; Saraf, A.; Ghazal, N.; Pham, T.T.; Kwong, J.Q. Mitochondrial dysfunction and oxidative stress in heart disease. Exp. Mol. Med. 2019, 51, 162. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, M.; Xu, J.; Zhang, H.; Tian, M. Using a TEMPO-based fluorescent probe for monitoring oxidative stress in living cells. Analyst 2011, 136, 4316–4320. [Google Scholar] [CrossRef]
- Weil, J.A.; Bolton, J.R. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications, 2nd ed.; Wiley Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kempe, S.; Metz, H.; Mäder, K. Application of Electron Paramagnetic Resonance (EPR) spectroscopy and imaging in drug delivery research—Chances and challenges. Eur. J. Pharm. Biopharm. 2010, 74, 55–66. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Kruczyński, Z.; Dobosz, B.; Zając, A.; Mackiewicz, A.; Leporowska, E.; Folwaczna, S. EPR Study of Iron Ion Complexes in Human Blood. Appl. Magn. Reson. 2011, 40, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalewska, A.; Krzyminiewski, R.; Dobosz, B.; Mrozińska, J.; Kruczyński, Z. The effect of copper ions on interaction of UV radiation with methacrylic matrix—EPR study. Mater. Chem. Phys. 2013, 43, 440–445. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Kubiak, T. The influence of radiotherapy on ceruloplasmin and transferrin in whole blood of breast cancer patients. Radiat. Environ. Biophys. 2017, 56, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Natsumoto, K.; Krishna, M.C.; Mitchell, J.B. Novel pharmacokinetic measurement using electron paramagnetic resonance spectroscopy and simulation of in vivo decay of various nitroxyl spin probes in mouse blood. J. Pharmacol. Exp. Ther. 2004, 310, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.I.; Kuppusamy, P.; English, S.; Yoo, J.; Irie, A.; Subramanian, S.; Mitchell, J.B.; Krishna, M.C. Feasibility and assessment of non-invasive in vivo redox status using electron paramagnetic resonance imaging. Acta Radiol. 2002, 43, 433–440. [Google Scholar] [CrossRef]
- Villamena, F.A. EPR Spin Trapping. In Reactive Species Detection in Biology: From Fluorescence to Electron Paramagnetic Resonance Spectroscopy, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 163–202. [Google Scholar]
- Headley, C.A.; Hoffman, C.N.; Freisen, J.M.; Han, Y.; Macklin, J.M.; Zweier, J.L.; Rockenbauer, A.; Kuret, J.; Villamena, F.A. Membrane-specific spin trap, 5–dodecylcarbamoyl–5–N–dodecylacetamide–1–pyroline–N–oxide (diC12PO): Theoretical, bioorthogonal fluorescence imaging and EPR studies. Org. Biomol. Chem. 2019, 17, 7694–7705. [Google Scholar] [CrossRef]
- Lewandowski, M.; Gwozdzinski, K. Nitroxides as antioxidants and anticancer drugs. Int. J. Mol. Sci. 2017, 18, 2490. [Google Scholar] [CrossRef] [Green Version]
- Nagasaki, Y. Design and application of redox polymers for nanomedicine. Polym. J. 2018, 50, 821–836. [Google Scholar] [CrossRef]
- Feliciano, C.P.; Nagasaki, Y. Antioxidant Nanomedicine Protects against Ionizing Radiation-Induced Life-Shortening in C57BL/6J Mice. ACS Biomater. Sci. Eng. 2019, 5, 5631–5636. [Google Scholar] [CrossRef]
- Wilcox, C.S. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol. Ther. 2010, 126, 119–145. [Google Scholar] [CrossRef] [Green Version]
- Neil, S.; Huh, J.; Baronas, V.; Li, X.; McFarland, H.F.; Cherukuri, M.; Mitchell, J.B.; Quandt, J.A. Oral administration of the nitroxide radical TEMPOL exhibits immunomodulatory and therapeutic properties in multiple sclerosis models. Brain Behav. Immun. 2017, 62, 332–343. [Google Scholar] [CrossRef]
- Smirnova, T.I.; Smirnov, A.I. Peptide-membrane Interactions by Spin-labeling EPR. Methods Enzymol. 2015, 564, 219–258. [Google Scholar] [PubMed] [Green Version]
- Pavićević, A.; Luo, J.; Popović-Bijelić, A.; Mojović, M. Maleimido-proxyl as an EPR spin label for the evaluation of conformational changes of albumin. Eur. Biophys. J. 2017, 46, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J. ESR as a monitoring method of the interactions between TEMPO-functionalized magnetic nanoparticles and yeast cells. Sci. Rep. 2019, 9, 18733. [Google Scholar] [CrossRef] [PubMed]
- Krzyminiewski, R.; Dobosz, B.; Krist, B.; Schroeder, G.; Kurczewska, J.; Bluyssen, H.A.R. ESR Method in Monitoring of Nanoparticle Endocytosis in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4388. [Google Scholar] [CrossRef]
- Suy, S.; Mitchell, J.B.; Ehleiter, D.; Haimovitz-Friedman, A.; Kasid, U. Nitroxides Tempol and Tempo Induce Divergent Signal Transduction Pathways in MDA-MB 231 Breast Cancer Cells. J. Biol. Chem. 1998, 273, 17871–17878. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Seo, J.E.; Bryce, S.M.; Tan, J.A.; Wu, Q.; Dial, S.L.; Moore, M.M.; Mei, N. Comparative Genotoxicity of TEMPO and 3 of Its Derivatives in Mouse Lymphoma Cells. Toxicol. Sci. 2018, 163, 214–225. [Google Scholar] [CrossRef] [Green Version]
- Rocha, V.C.J.; de Aragão França, L.S.; de Araújo, C.F.; Ng, A.M.; de Andrade, C.M.; Cronemberger Andrade, A.; de Souza Santos, E.; da Cruz Borges-Silva, M.; Garcia Macambira, S.; Noronha-Dutra, A.A.; et al. Protective effects of mito-TEMPO against doxorubicin cardiotoxicity in mice. Cancer Chemother. Pharmacol. 2016, 77, 659–662. [Google Scholar] [CrossRef]
- Ni, R.; Cao, T.; Xiong, S.; Ma, J.; Fan, G.C.; Lacefield, J.C.; Lu, Y.; Le Tissier, S.; Peng, T. Therapeutic inhibition of mitochondrial reactive oxygen species with mito-TEMPO reduces diabetic cardiomyopathy. Free Radic. Biol. Med. 2016, 90, 12–23. [Google Scholar] [CrossRef] [Green Version]
- Akakuru, O.U.; Iqbal, M.Z.; Liu, C.; Xing, J.; Wei, Z.; Jiang, Z.; Fang, Q.; Yuan, B.; Nosike, E.I.; Xia, J.; et al. Self-assembled, biocompatible and biodegradable TEMPO-conjugated nanoparticles enable folate-targeted tumor magnetic resonance imaging. Appl. Mater. Today 2020, 18, 100524. [Google Scholar] [CrossRef]
- Marshall, J.D.; Li, J.Z.; Zhang, Y.; Gong, Y.; St-Pierre, F.; Lin, M.Z.; Schnitzer, M.J. Cell-Type-Specific Optical Recording of Membrane Voltage Dynamics in Freely Moving Mice. Cell 2016, 167, 1650–1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krzyminiewski, R. Computer enhancement of complex spectroscopic spectra resolution. Mol. Phys. Rep. 1994, 6, 174–179. [Google Scholar]
- Jezierska, K.; Macała, A.; Krzyminiewski, R.; Woźniak, P.; Łikowiak, M.; Sękowska-Namiotko, A.; Podraza, W. High Signal Resolution Pulse Oximetry as a Prognostic Indicator of Radiotherapy Toxicity: A Pilot Study. Pulse 2021, 9, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bednarowicz, M.; Dobosz, B.; Krzyminiewski, R.; Hałupka-Bryl, M.; Deptuła, T.; Nagasaki, Y. ESR studies of redox-active PMNT-PEG-PMNT polymer. Mater. Chem. Phys. 2015, 161, 250–255. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Koralewski, M.; Hałupka-Bryl, M. Computer enhancement of ESR spectra of magnetite nanoparticles. J. Magn. Magn. Mater. 2016, 407, 114–121. [Google Scholar] [CrossRef]
- Dobosz, B.R.; Krzyminiewski, J.; Kurczewska, G.S. The influence of surface modification, coating agents and pH value of aqueous solutions on physical properties of magnetite nanoparticles investigated by ESR method. J. Magn. Magn. Mater. 2017, 429, 203–210. [Google Scholar] [CrossRef]
- Atherton, M.; Crossland, W.A. A Single-crystal ENDOR Study of y-Irradiated Pyridoxine Hydrochloride. J. Chem. Soc. Faraday Trans. 1987, 83, 37–42. [Google Scholar] [CrossRef]
- Hedberg, A.; Ehrenberg, A. Resolution enhancement of ESR spectra from irradiated single crystals of glycine. J. Chem. Phys. 1968, 48, 4822–4828. [Google Scholar] [CrossRef] [PubMed]
- Koper, A.; Krzyminiewski, R. Analysis of resonance excitations by linear transformation technique theory. Acta Magn. 1985, II, 3–23. [Google Scholar]
- Madisetti, V.K.; Williams, D.B. The Digital Signal Processing Handbook; CRC/IEEE Press: New York, NY, USA, 1999. [Google Scholar]
- Krzyminiewski, R.; Kowalczyk, R.M.; Bielewicz-Mordalska, A.; Pająk, Z.; Czarnecki, P. Computer enhancement of CW-EPR experimental spectra resolution as a new method in investigation of molecular dynamics in pyridinium tetrafluoroborate. J. Mol. Struct. 1998, 471, 234–249. [Google Scholar] [CrossRef]
- Gan, J.; Ben-Nissan, G.; Arkind, G.; Tarnavsky, M.; Trudeau, D.; Garcia, L.N.; Tawfik, D.S.; Sharon, M. Native mass spectrometry of recombinant proteins from crude cell lysates. Anal. Chem. 2017, 89, 4398–4404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Cong, X.; Liu, W.; Laganowsky, A. Characterisation of membrane protein-lipid interactions by mass spectrometry ion mobility mass spectrometry. J. Am. Soc. Mass Spectrom. 2017, 28, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Bertolin, G.; Le Marchand, G.; Tramier, M. Real-time monitoring of aurora kinase a activation using conformational FRET biosensors in live cells. J. Vis. Exp. 2020, 30, e61611. [Google Scholar] [CrossRef] [PubMed]
- Benaissa, H.; Ounoughi, K.; Aujard, I.; Fischer, E.; Goïame, R.; Nguyen, J.; Tebo, A.G.; Li, C.; Le Saux, T.; Bertolin, G.; et al. Engineering of a fluorescent chemogenetic reporter with tunable color for advanced live-cell imaging. Nat. Commun. 2021, 12, 6989. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, J.H.; Griffin, J.L. An introduction to biological nuclear magnetic resonance spectroscopy. Biol. Rev. Camb. Philos. Soc. 2011, 86, 493–510. [Google Scholar] [CrossRef]
- Grivet, J.P. NMR and microorganisms. Curr. Issues Mol. Biol. 2001, 3, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Bonucci, A.; Ouari, O.; Guigliarelli, B.; Belle, V.; Mileo, E. In-Cell EPR: Progress towards structural studies inside cells. ChemBioChem 2020, 21, 451–460. [Google Scholar] [CrossRef]
- Khramtsov, V.V.; Bobko, A.A.; Tseytlin, M.; Driesschaert, B. Exchange Phenomena in the Electron Paramagnetic Resonance Spectra of the Nitroxyl and Trityl Radicals: Multifunctional Spectroscopy and Imaging of Local Chemical Microenvironment. Anal. Chem. 2017, 89, 4758–4771. [Google Scholar] [CrossRef]
- Smirnov, A.; Smirnova, T.; Morse, P.D. Very high frequency electron paramagnetic resonance of 2,2,6,6-Tetramethyl-1-Piperidinyloxy in 1,2-Dipalmitoyl-sn-Glycero-3-phosphatidylcholine liposomes: Partitioning and molecular dynamics. Biophys. J. 1995, 68, 2350–2360. [Google Scholar] [CrossRef] [Green Version]
- Jeschke, G. Spin Probes and Spin Traps. In Physical Chemistry IV. Part 2: Electron Paramagnetic Resonance; ETH: Zurich, Switzerland, 2016; pp. 75–84. [Google Scholar]
- Krzyminiewski, R.; Kubiak, T.; Dobosz, B.; Schroeder, G.; Kurczewska, J. EPR spectroscopy and imaging of TEMPO-labeled magnetite nanoparticles. Curr. Appl. Phys. 2014, 14, 798–804. [Google Scholar] [CrossRef]
- Dragutan, I.; Mehlhorn, R.J. Modulation of oxidative damage by nitroxide free radicals. Free Radic. Res. 2007, 41, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Prestrelski, S.J.; Tedeschi, N.; Arakawa, T.; Carpenter, J.F. Dehydration-induced conformational transitions in proteins and their inhibition by stabilisers. Biophys. J. 1993, 65, 661–671. [Google Scholar] [CrossRef] [Green Version]















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobosz, B.; Krzyminiewski, R.; Kucińska, M.; Murias, M.; Schroeder, G.; Kurczewska, J. Spin Probes as Scavengers of Free Radicals in Cells. Appl. Sci. 2022, 12, 7999. https://doi.org/10.3390/app12167999
Dobosz B, Krzyminiewski R, Kucińska M, Murias M, Schroeder G, Kurczewska J. Spin Probes as Scavengers of Free Radicals in Cells. Applied Sciences. 2022; 12(16):7999. https://doi.org/10.3390/app12167999
Chicago/Turabian StyleDobosz, Bernadeta, Ryszard Krzyminiewski, Małgorzata Kucińska, Marek Murias, Grzegorz Schroeder, and Joanna Kurczewska. 2022. "Spin Probes as Scavengers of Free Radicals in Cells" Applied Sciences 12, no. 16: 7999. https://doi.org/10.3390/app12167999
APA StyleDobosz, B., Krzyminiewski, R., Kucińska, M., Murias, M., Schroeder, G., & Kurczewska, J. (2022). Spin Probes as Scavengers of Free Radicals in Cells. Applied Sciences, 12(16), 7999. https://doi.org/10.3390/app12167999







