Simultaneous Treatment of Swine and Furfural Wastewater Integrated with Lipid Production of Chlorella pyrenoidosa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microalgal Strain
2.2. Wastewater Collection and Pretreatment
2.3. Wastewater Treatment by Microalgae Cultivation
2.4. Analytical Methods
2.4.1. Determination of Microalgal Concentration and Growth Rate
2.4.2. Determination of Water Quality Indexes
2.4.3. Determination of Lipid Content
2.4.4. Statistical Analysis
3. Results and Discussion
3.1. Composition Analysis of Swine and Furfural Wastewater
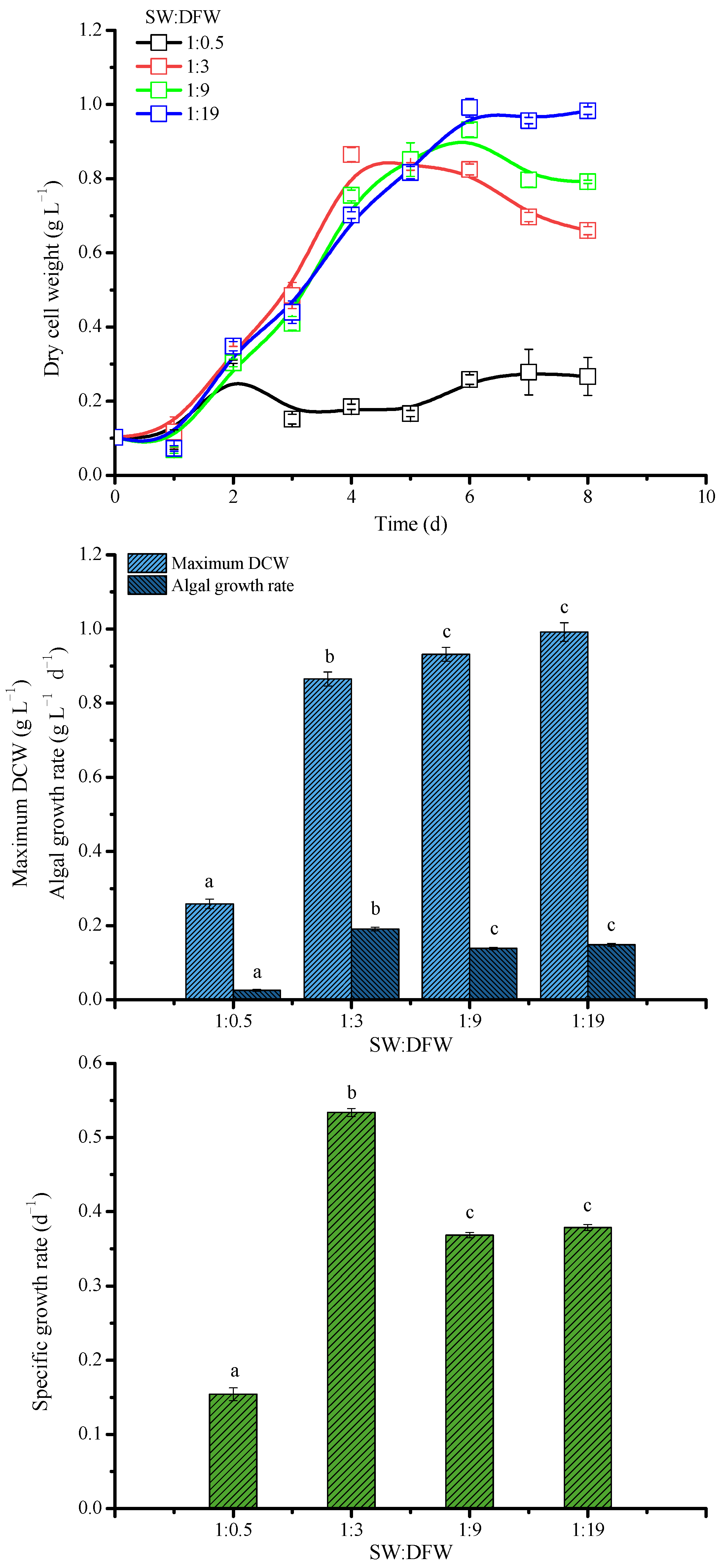
3.2. Growth Performance of Microalgae Cultured in the Mixed Wastewater
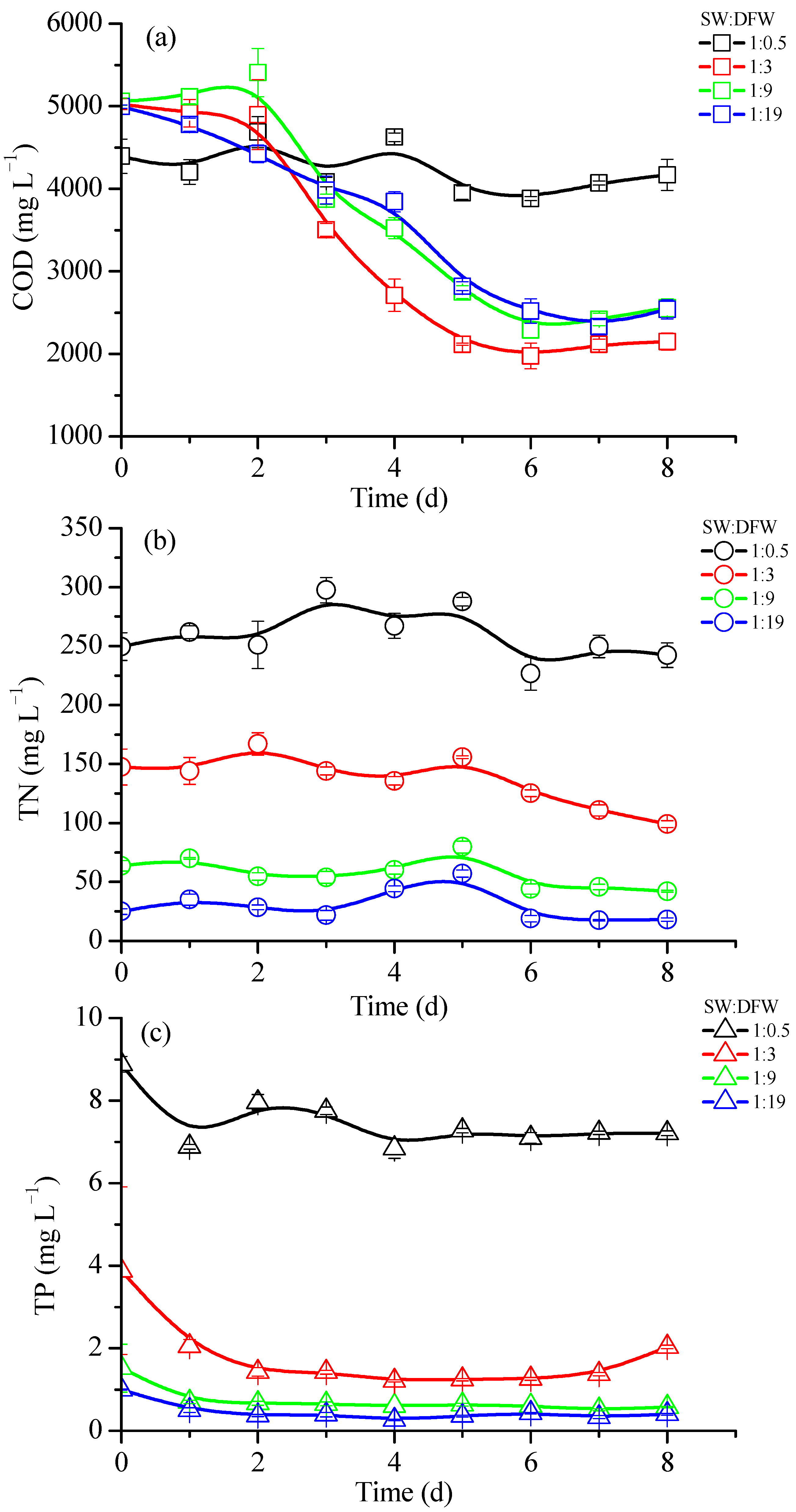
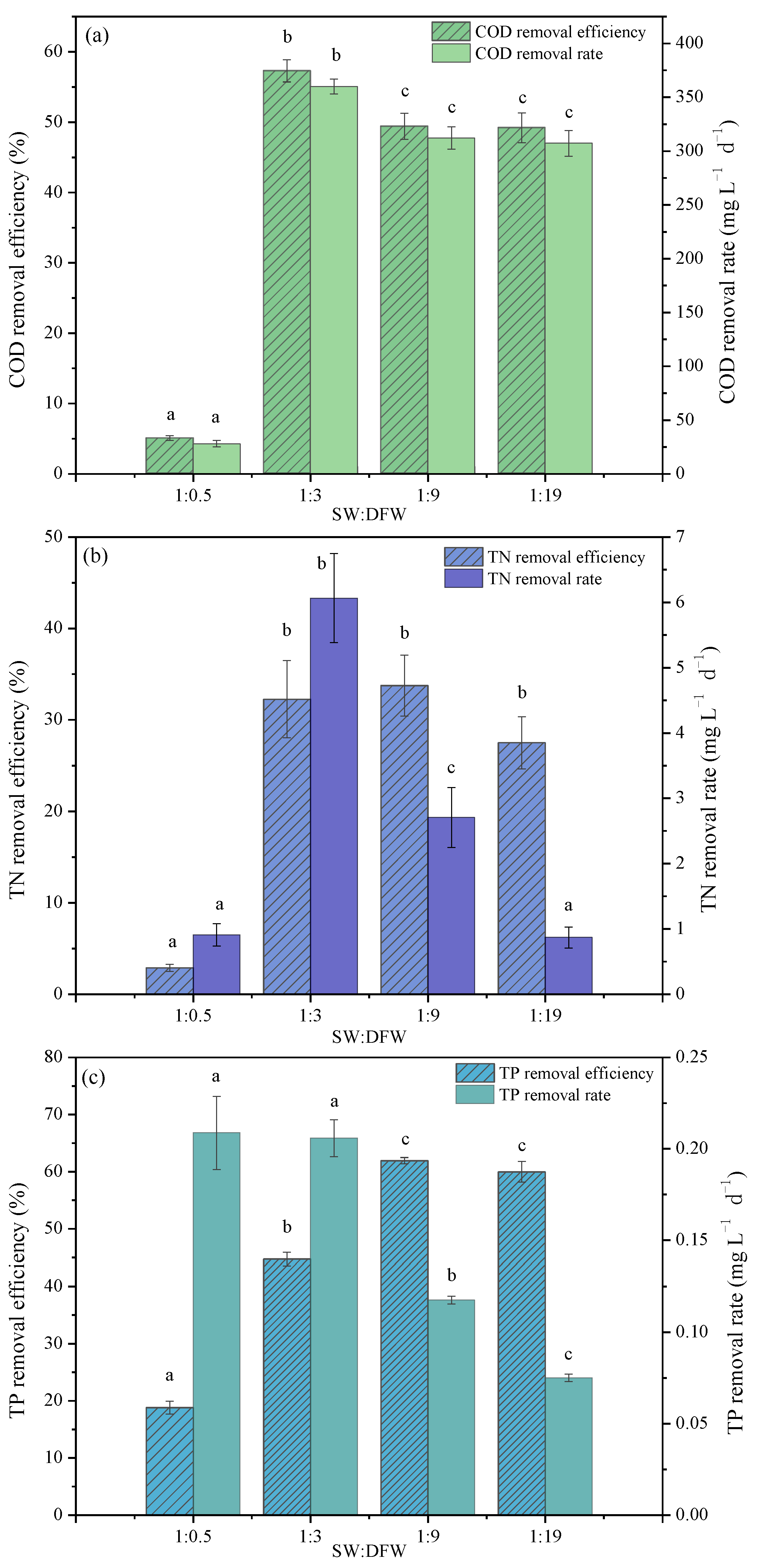
3.3. Influence of Mix Ratio of Wastewaters on Nutrient Removal
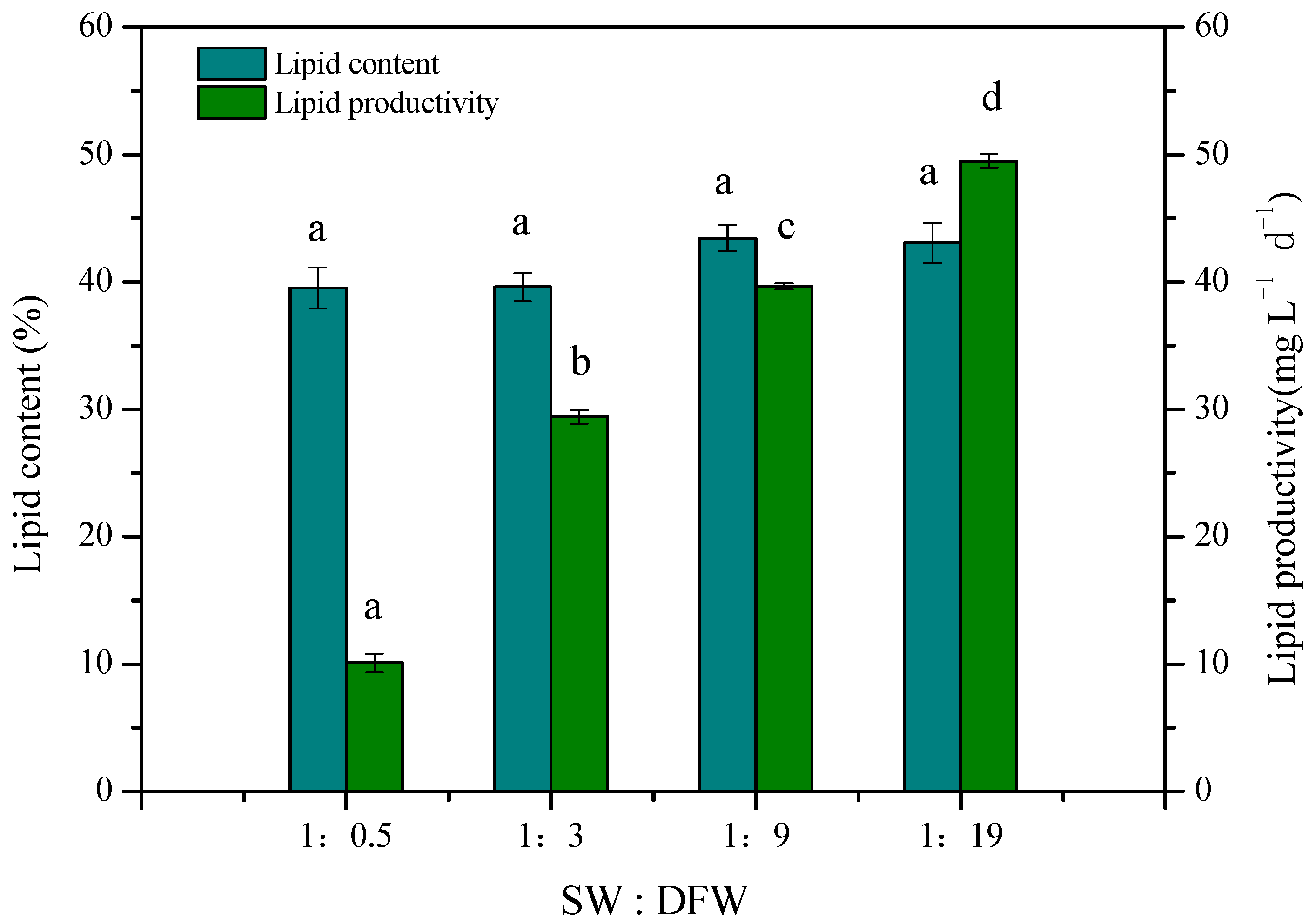
3.4. Lipid Production of Microalgae Cultured in the Mixed Wastewater
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morillas-España, A.; Lafarga, T.; Sánchez-Zurano, A.; Acién-Fernández, F.G.; González-López, C. Microalgae based wastewater treatment coupled to the production of high value agricultural products: Current needs and challenges. Chemosphere 2021, 291, 132968. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Mishra, A.; Pant, D.; Malaviya, P. Recent advances in microalgae-based remediation of industrial and non-industrial wastewaters with simultaneous recovery of value-added products. Bioresour. Technol. 2021, 344, 126129. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process Eng. 2020, 40, 101747. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Chen, C.-Y.; Chang, J.-S. Resource recovery from wastewaters using microalgae-based approaches: A circular bioeconomy perspective. Bioresour. Technol. 2020, 302, 122817. [Google Scholar] [CrossRef]
- Nagarajan, D.; Kusmayadi, A.; Yen, H.-W.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Current advances in biological swine wastewater treatment using microalgae-based processes. Bioresour. Technol. 2019, 289, 121718. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, C.; Zhang, Y.; Ho, S.H. Optimizing real swine wastewater treatment with maximum carbohydrate production by a newly siolated indigenous microalga Parachlorella kessleri QWY28. Bioresour. Technol. 2019, 289, 121702. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Ngo, H.; Guo, W.; Chang, S.; Nguyen, D.; Kumar, S. Microalgae biomass from swine wastewater and its conversion to bioenergy. Bioresour. Technol. 2018, 275, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Cheng, J.J.; Cobb, K.; Zhou, C.; Zhou, N.; Addy, M.; Chen, P.; Yan, X.; Ruan, R. Tribonema sp. and Chlorella zofingiensis co-culture to treat swine wastewater diluted with fishery wastewater to facilitate harvest. Bioresour. Technol. 2019, 297, 122516. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Hong, Y.; Zhao, G.-P.; Zhang, H.-K.; Zhai, Q.-Y.; Wang, Q. Microalgae-based swine wastewater treatment: Strain screening, conditions optimization, physiological activity and biomass potential. Sci. Total Environ. 2021, 807, 151008. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Choi, Y.Y.; Sim, S.J. Emerging prospects of mixotrophic microalgae: Way forward to sustainable bioprocess for environmental remediation and cost-effective biofuels. Bioresour. Technol. 2020, 300, 122741. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xiong, H.; Hui, Z.; Zeng, X. Mixotrophic cultivation of Chlorella pyrenoidosa with diluted primary piggery wastewater to produce lipids. Bioresour. Technol. 2012, 104, 215–220. [Google Scholar] [CrossRef]
- Tan, X.-B.; Yang, L.-B.; Zhang, W.-W.; Zhao, X.-C. Lipids production and nutrients recycling by microalgae mixotrophic culture in anaerobic digestate of sludge using wasted organics as carbon source. Bioresour. Technol. 2019, 297, 122379. [Google Scholar] [CrossRef] [PubMed]
- Mubashar, M.; Ahmad, Z.; Li, C.; Zhang, H.; Xu, C.; Wang, G.; Qiu, D.; Song, L.; Zhang, X. Carbon-negative and high-rate nutrient removal using mixotrophic microalgae. Bioresour. Technol. 2021, 340, 125731. [Google Scholar] [CrossRef]
- Liu, J.; Yin, J.; Ge, Y.; Han, H.; Liu, M.; Gao, F. Improved lipid productivity of Scenedesmus obliquus with high nutrient removal efficiency by mixotrophic cultivation in actual municipal wastewater. Chemosphere 2021, 285, 131475. [Google Scholar] [CrossRef]
- Zheng, H.; Wu, X.; Zou, G.; Zhou, T.; Liu, Y.; Ruan, R. Cultivation of Chlorella vulgaris in manure-free piggery wastewater with high-strength ammonium for nutrients removal and biomass production: Effect of ammonium concentration, carbon/nitrogen ratio and pH. Bioresour. Technol. 2018, 273, 203–211. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Z.; Noor, R.S.; Cheng, Q.; Chu, X.; Qu, B.; Zhen, F.; Sun, Y. Furfural wastewater pretreatment of corn stalk for whole slurry anaerobic co-digestion to improve methane production. Sci. Total Environ. 2019, 674, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Bong, K.; Mubashir, M.; Cheng, C.; Lam, M.; Lim, J.; Ho, Y.; Lee, K.; Munawaroh, H.; Show, P. Microalgae Cultivation in Palm Oil Mill Effluent (POME) Treatment and Biofuel Production. Sustainability 2021, 13, 3247. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Yap, Y.J.; Zaid, H.F.M.; Lam, M.K.; Lim, J.W.; Ho, Y.C.; Tao, Y. Enhancing microalga Chlorella sorokiniana CY-1 biomass and lipid production in palm oil mill effluent (POME) using novel-designed photobioreactor. Bioengineered 2020, 11, 61–69. [Google Scholar] [CrossRef]
- Hussain, F.; Shah, S.Z.; Ahmad, H.; Abubshait, S.A.; Abubshait, H.A.; Laref, A.; Manikandan, A.; Kusuma, H.S.; Iqbal, M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021, 137, 110603. [Google Scholar] [CrossRef]
- Purba, L.D.A.; Thman, F.S.O.; Yuzir, A.; Mohamad, S.E.; Iwamoto, K.; Abdullah, N.; Shimizu, K.; Hermana, J. Enhanced cultivation and lipid production of isolated microalgae strains using municipal wastewater. Environ. Technol. Innov. 2022, 27, 102444. [Google Scholar] [CrossRef]
- Nguyen, T.D.P.; Nguyen, D.H.; Lim, J.W.; Chang, C.-K.; Leong, H.Y.; Tran, T.N.T.; Vu, T.B.H.; Show, P.L. Investigation of the Relationship between Bacteria Growth and Lipid Production Cultivating of Microalgae Chlorella Vulgaris in Seafood Wastewater. Energies 2019, 12, 2282. [Google Scholar] [CrossRef]
- Rosli, S.S.; Wong, C.Y.; Yunus, N.M.; Lam, M.K.; Show, P.L.; Cheng, C.K.; Wang, D.K.; Da Oh, W.; Lim, J.W. Optimum interaction of light intensity and CO2 concentration in bioremediating N-rich real wastewater via assimilation into attached microalgal biomass as the feedstock for biodisel production. Process Saf. Environ. Prot. 2020, 141, 355–365. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Yin, J.-Y.; Han, H.-F.; Ge, Y.-M.; Wang, Z.-Y.; Bao, X.-Y.; Gao, F. Enhancements of lipid productivity and phosphorus utilization efficiency of Chlorella pyrenoidosa by iron and acetate supplements in actual municipal wastewater. Renew. Energy 2021, 170, 927–935. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, Z.; Wei, D.; Chen, W.; Xie, J. Mixotrophic Chlorella pyrenoidosa as cell factory for ultrahigh-efficient removal of ammonium from catalyzer wastewater with valuable algal biomass coproduction through short-time acclimation. Bioresour. Technol. 2021, 333, 125151. [Google Scholar] [CrossRef]
- Cheng, P.; Chu, R.; Zhang, X.; Song, L.; Chen, D.; Zhou, C.; Yan, X.; Cheng, J.J.; Ruan, R. Screening of the dominant Chlorella pyrenoidosa for biofilm attached culture and feed production while treating swine wastewater. Bioresour. Technol. 2020, 318, 124054. [Google Scholar] [CrossRef]
- Tan, X.-B.; Wang, L.; Wan, X.-P.; Zhou, X.-N.; Yang, L.-B.; Zhang, W.-W.; Zhao, X.-C. Growth of Chlorella pyrenoidosa on different septic tank effluents from rural areas for lipids production and pollutants removal. Bioresour. Technol. 2021, 339, 125502. [Google Scholar] [CrossRef]
- Gan, K.; Mou, X.; Xu, Y.; Wang, H. Application of ozonated piggery wastewater for cultivation of oil-rich Chlorella pyrenoidosa. Bioresoure Technol. 2014, 171, 285–290. [Google Scholar] [CrossRef]
- Purcell, L.C.; King, C.A.J.A.J. Total Nitrogen Determination in Plant Material by Persulfate Digestion. Agron. J. 1996, 88, 111–113. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, P.; Addy, M.; Zhang, R.; Deng, X.; Ma, Y.; Cheng, Y.; Hussain, F.; Chen, C.; Liu, Y.; et al. Carbon-dependent alleviation of ammonia toxicity for algae cultivation and associated mechanisms exploration. Bioresour. Technol. 2018, 249, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Yang, H.-L.; Li, C.; Peng, Y.-Y.; Lu, M.-M.; Jin, W.-H.; Bao, J.-J.; Guo, Y.-M. Effect of organic carbon to nitrogen ratio in wastewater on growth, nutrient uptake and lipid accumulation of a mixotrophic microalgae Chlorella sp. Bioresour. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Li, D.; Chu, H.; Zhou, X.; Zhang, Y.; Yu, H. Nutrients removal and lipids production by Chlorella pyrenoidosa cultivation using anaerobic digested starch wastewater and alcohol wastewater. Bioresour. Technol. 2015, 181, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cheirsilp, B.; Torpee, S. Enhanced growth and lipid production of microalgae under mixotrophic culture condition: Effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour. Technol. 2012, 110, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, T.; Ma, J.; Zhao, Q.; Wensel, P.; Lian, J.; Chen, S. A kinetic model of heterotrophic and mixotrophic cultivation of the potential biofuel organism microalgae Chlorella sorokiniana. Algal Res. 2022, 64, 102701. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, L.; Luo, X.; Zheng, Z. Effects of various LED light wavelengths and intensities on the performance of purifying synthetic domestic sewage by microalgae at different influent C/N ratios. Ecol. Eng. 2013, 51, 24–32. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Y.; Feng, J.; Liu, Q.; Nan, F.; Xie, S. Nutrients removal from undiluted cattle farm wastewater by the two-stage process of microalgae-based wastewater treatment. Bioresour. Technol. 2018, 264, 311–318. [Google Scholar] [CrossRef]
- Xia, L.; Yang, H.; He, Q.; Hu, C. Physiological responses of freshwater oleaginous microalgae Desmodesmus sp. NMX451 under nitrogen deficiency and alkaline pH-induced lipid accumulation. J. Appl. Phycol. 2015, 27, 649–659. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Rai, M.P.; Nigam, S.; Sharma, R. Response of growth and fatty acid compositions of Chlorella pyrenoidosa under mixotrophic cultivation with acetate and glycerol for bioenergy application. Biomass Bioenergy 2013, 58, 251–257. [Google Scholar] [CrossRef]
- Moon, M.; Kim, C.W.; Park, W.-K.; Yoo, G.; Choi, Y.-E.; Yang, J.-W. Mixotrophic growth with acetate or volatile fatty acids maximizes growth and lipid production in Chlamydomonas reinhardtii. Algal Res. 2013, 2, 352–357. [Google Scholar] [CrossRef]
- Avidan, O.; Brandis, A.; Rogachev, I.; Pick, U. Enhanced acetyl-CoA production is associated with increased triglyceride accumulation in the green alga Chlorella desiccata. J. Exp. Bot. 2015, 66, 3725–3735. [Google Scholar] [CrossRef]
- Lin, W.; Li, P.; Liao, Z.; Luo, J. Detoxifification of ammonium to Nannochloropsis oculata and enhancement of lipid production by mixotrophic growth with acetate. Bioresoure Technol. 2017, 227, 404–407. [Google Scholar] [CrossRef]
- León-Vaz, A.; León, R.; Díaz-Santos, E.; Vigara, J.; Raposo, S. Using agro-industrial wastes for mixotrophic growth and lipids production by the green microalga Chlorella sorokiniana. New Biotechnol. 2019, 51, 31–38. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Swine Wastewater (SW) | Furfural Wastewater (FW) | SW:DFW a = 1:0.5 | SW:DFW = 1:3 | SW:DFW = 1:9 | SW:DFW = 1:19 |
|---|---|---|---|---|---|---|
| COD (mg L−1) | 4316.40 ± 396.56 | 50,978.67 ± 544.95 | 4394.00 ± 207.12 | 5026.01 ± 65.91 | 5056.67 ± 42.34 | 4996.33 ± 21.91 |
| TN (mg L−1) | 425.41 ± 17.82 | 33.47 ± 3.52 | 249.55 ± 11.73 | 147.56 ± 4.71 | 63.73 ± 2.34 | 24.92 ± 1.67 |
| TP (mg L−1) | 14.18 ± 1.56 | 1.01 ± 0.04 | 8.88 ± 0.06 | 3.68 ± 0.03 | 1.52 ± 0.01 | 1.00 ± 0.01 |
| pH | 6.52 ± 0.12 | 3.10 ± 0.51 | 7.1 ± 0.20 | 7.1 ± 0.21 | 7.1 ± 0.18 | 7.1 ± 0.20 |
| COD/TN ratio | 10 ± 1 | 1540 ± 157 | 18 ± 1 | 34 ± 3 | 79 ± 5 | 202 ± 20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhang, C.; Zhang, H.; Yao, T.; Du, Y.; Cheng, Z.; Zhang, A.-H.; Zhang, D.; Zhang, Z. Simultaneous Treatment of Swine and Furfural Wastewater Integrated with Lipid Production of Chlorella pyrenoidosa. Appl. Sci. 2022, 12, 8144. https://doi.org/10.3390/app12168144
Huang J, Zhang C, Zhang H, Yao T, Du Y, Cheng Z, Zhang A-H, Zhang D, Zhang Z. Simultaneous Treatment of Swine and Furfural Wastewater Integrated with Lipid Production of Chlorella pyrenoidosa. Applied Sciences. 2022; 12(16):8144. https://doi.org/10.3390/app12168144
Chicago/Turabian StyleHuang, Jianke, Chao Zhang, Han Zhang, Ting Yao, Yi Du, Zheng Cheng, Ai-Hua Zhang, Daofeng Zhang, and Zhen Zhang. 2022. "Simultaneous Treatment of Swine and Furfural Wastewater Integrated with Lipid Production of Chlorella pyrenoidosa" Applied Sciences 12, no. 16: 8144. https://doi.org/10.3390/app12168144







