Enzymatically Hydrolysed Common Buckwheat (Fagopyrum esculentum M.) as a Fermentable Source of Oligosaccharides and Sugars
Abstract
:1. Introduction
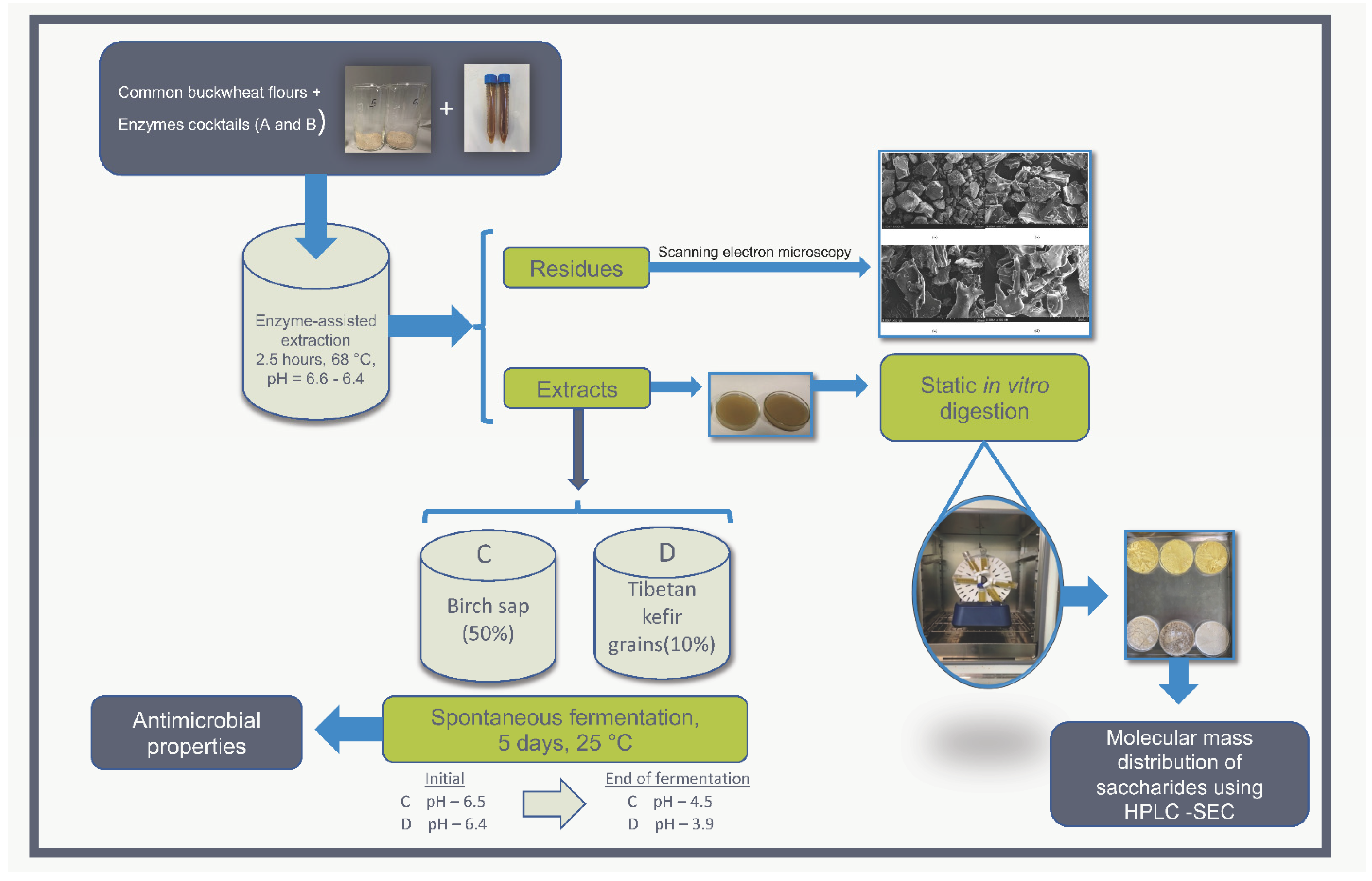
2. Materials and Methods
2.1. Plant Material (Dependent Sub-Sample)
2.2. Enzyme Products (Independent Sub-Sample)
2.3. Enzyme-Assisted Extraction and Spontaneous Fermentation Using Tibetan Kefir Grains and Birch Sap
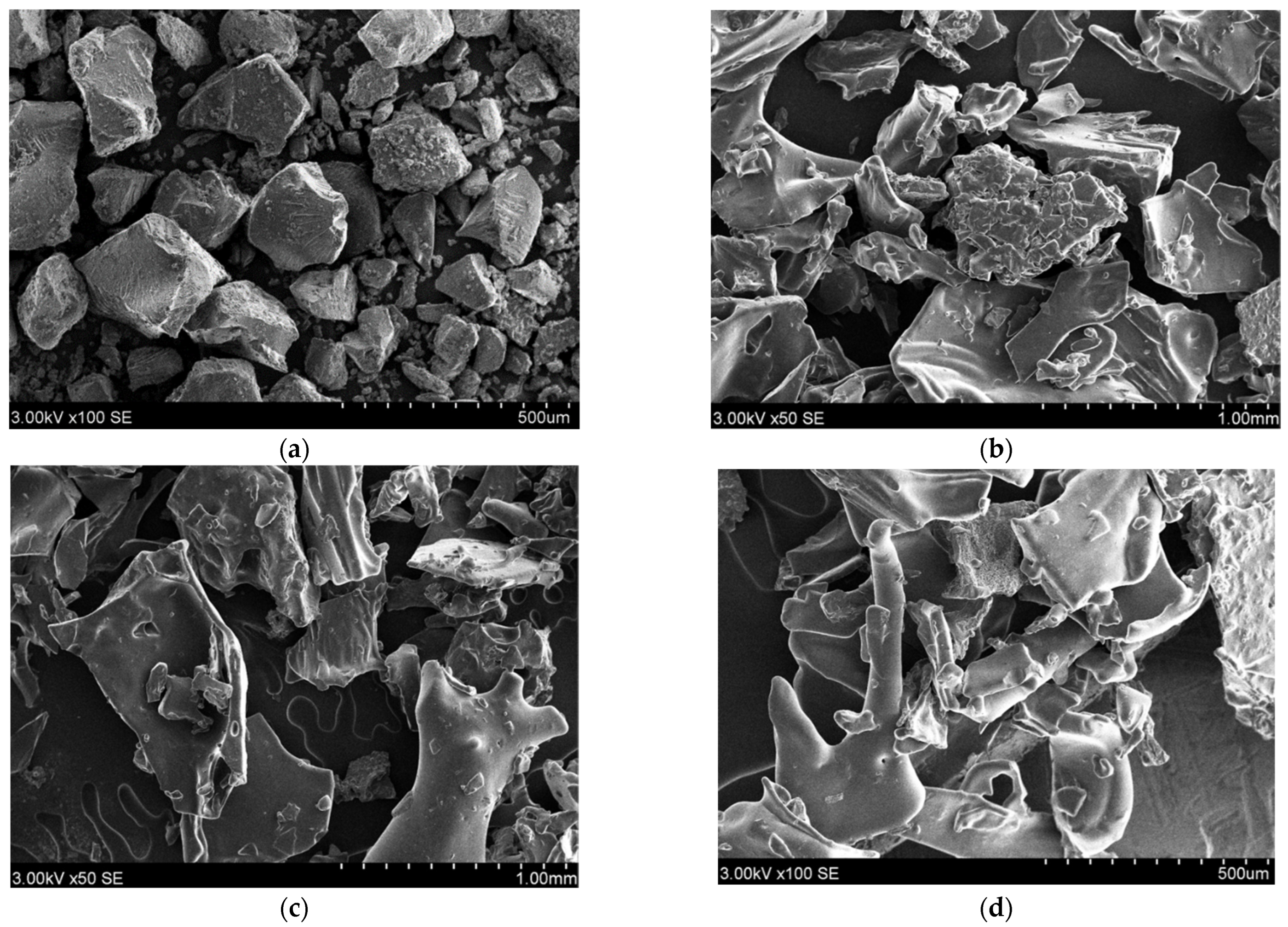
2.4. Scanning Electron Microscopy (SEM) Analysis
2.5. Static In Vitro Digestion
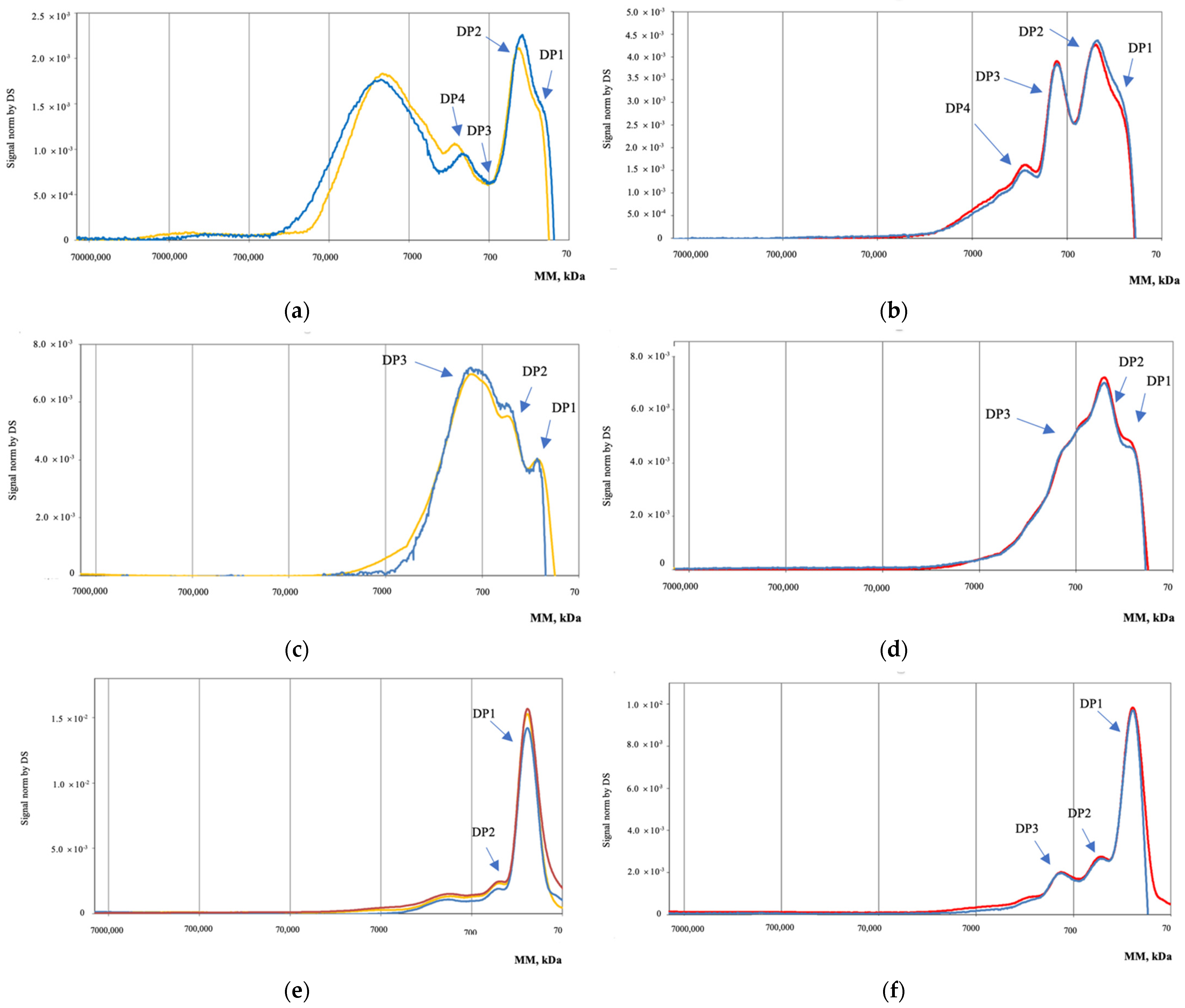
2.6. High-Pressure Liquid Chromatography Size Exclusion for Molecular Mass Distribution (HPLC-SEC)
2.7. Antibacterial Activity Assay
3. Results and Discussion
3.1. Scanning Electron Microscopy (SEM)
3.2. High-Pressure Liquid Chromatography Size Exclusion for Molecular Mass Distribution (HPLC-SEC)
3.3. Antimicrobial Properties against Gram—Positive and Gram—Negative Pathogenic Bacteria
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.Y.; Yue, L.X.; Wang, S.G.; Wang, T.X. Quercitrin restrains the growth and invasion of lung adenocarcinoma cells by regulating gap junction protein beta 2. Bioengineered 2022, 13, 6126–6135. [Google Scholar] [CrossRef] [PubMed]
- Ikari, S.; Yang, Q.; Lu, S.; Liu, Y.; Hao, F.; Tong, G.; Lu, S.; Noda, T. Quercetin in Tartary Buckwheat Induces Autophagy against Protein Aggregations. Antioxidants 2021, 10, 1217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, W.; Guo, X.; Song, G.; Pang, S.; Fang, W.; Peng, Z. Effects of Oats, Tartary Buckwheat, and Foxtail Millet Supplementation on Lipid Metabolism, Oxido-Inflammatory Responses, Gut Microbiota, and Colonic SCFA Composition in High-Fat Diet Fed Rats. Nutrients 2022, 14, 2760. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, Y.; Zhao, Q.; Wang, Y.; Li, C.; Zou, L.; Hu, Y. Effects of tartary buckwheat protein on gut microbiome and plasma metabolite in rats with high-fat diet. Foods 2021, 10, 2457. [Google Scholar] [CrossRef] [PubMed]
- Dabija, A.; Ciocan, M.E.; Chetrariu, A.; Codină, G.G. Buckwheat and Amaranth as Raw Materials for Brewing, a Review. Plants 2022, 11, 756. [Google Scholar] [CrossRef]
- Phiarais, B.P.N.; Mauch, A.; Schehl, B.D.; Zarnkow, M.; Gastl, M.; Herrmann, M.; Zannini, E.; Arendt, E.K. Processing of a top fermented beer brewed from 100% buckwheat malt with sensory and analytical characterisation. J. Inst. Brew. 2010, 116, 265–274. [Google Scholar] [CrossRef]
- Deželak, M.; Zarnkow, M.; Becker, T.; Košir, I.J. Processing of bottom-fermented gluten-free beer-like beverages based on buckwheat and quinoa malt with chemical and sensory characterization. J. Inst. Brew. 2014, 120, 360–370. [Google Scholar] [CrossRef]
- Mondal, S.; Balasubramanian, A.; Biswas, P.; Agrawal, S.; Ghosh, S.; Dey, S. Characterization of pearl millet oligosaccharides and evaluation of their prebiotic potential. Bioact. Carbohydr. Diet. Fibre 2022, 28, 100324. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, M.; Wang, K.; Zhang, Z.; Shah, N.P.; Wei, H. Functional oligosaccharide fermentation in the gut: Improving intestinal health and its determinant factors-A review. Carbohydr. Polym. 2021, 284, 119043. [Google Scholar] [CrossRef]
- Rathod, N.B.; Phadke, G.G.; Tabanelli, G.; Mane, A.; Ranveer, R.C.; Pagarkar, A.; Ozogul, F. Recent advances in bio-preservatives impacts of lactic acid bacteria and their metabolites on aquatic food products. Food Biosci. 2021, 44, 101440. [Google Scholar] [CrossRef]
- Verma, D.K.; Thakur, M.; Singh, S.; Tripathy, S.; Gupta, A.K.; Baranwal, D.; Patel, A.R.; Shah, N.; Utama, G.L.; Niamah, A.K. Bacteriocins as antimicrobial and preservative agents in food: Biosynthesis, separation and application. Food Biosci. 2022, 46, 101594. [Google Scholar] [CrossRef]
- Lappa, I.K.; Terpou, A.; Bosnea, L.A.; Papadaki, A. Lactic acid bacteria and biogenic amines in food: Biological importance and human health. In Lactic Acid Bacteria in Food Biotechnology; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–194. [Google Scholar]
- Alonso-Riaño, P.; Melgosa, R.; Trigueros, E.; Illera, A.; Beltrán, S.; Sanz, M.T. Valorization of brewer’s spent grain by consecutive supercritical carbon dioxide extraction and enzymatic hydrolysis. Food Chem. 2022, 133493. [Google Scholar] [CrossRef] [PubMed]
- Sajib, M.; Falck, P.; Sardari, R.R.; Mathew, S.; Grey, C.; Karlsson, E.N.; Adlercreutz, P. Valorization of Brewer’s spent grain to prebiotic oligosaccharide: Production, xylanase catalyzed hydrolysis, in-vitro evaluation with probiotic strains and in a batch human fecal fermentation model. J. Biotechnol. 2018, 268, 61–70. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Alejandro, R.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2021, 378, 131918. [Google Scholar] [CrossRef] [PubMed]
- Streimikyte, P.; Viskelis, P.; Viskelis, J. Enzymes-Assisted Extraction of Plants for Sustainable and Functional Applications. Int. J. Mol. Sci. 2022, 23, 2359. [Google Scholar] [CrossRef]
- Krakowska-Sieprawska, A.; Kiełbasa, A.; Rafińska, K.; Ligor, M.; Buszewski, B. Modern Methods of Pre-Treatment of Plant material for the extraction of bioactive compounds. Molecules 2022, 27, 730. [Google Scholar] [CrossRef]
- Bishop, P.; Pitts, E.R.; Budner, D.; Thompson-Witrick, K.A. Kombucha: Biochemical and microbiological impacts on the chemical and flavor profile. Food Chem. Adv. 2022, 1, 100025. [Google Scholar] [CrossRef]
- Pang, X.; Han, B.; Huang, X.; Zhang, X.; Hou, L.; Cao, M.; Gao, L.; Hu, G.; Chen, J. Effect of the environment microbiota on the flavour of light-flavour Baijiu during spontaneous fermentation. Sci. Rep. 2018, 8, 3396. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Ye, T.; Yang, X.; Xue, Y.; Shen, Y.; Zhang, Q.; Zheng, X. Effect of lactic acid bacteria and yeasts on the structure and fermentation properties of Tibetan kefir grains. Int. Dairy J. 2021, 114, 104943. [Google Scholar] [CrossRef]
- Mingaila, J.; Čiuldienė, D.; Viškelis, P.; Bartkevičius, E.; Vilimas, V.; Armolaitis, K. The quantity and biochemical composition of sap collected from silver birch (Betula pendula Roth) trees growing in different soils. Forests 2020, 11, 365. [Google Scholar] [CrossRef]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2022, 43, 189–198. [Google Scholar] [CrossRef]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: Evidence for the effects of organic acids. Anaerobe 2012, 18, 530–538. [Google Scholar] [CrossRef] [PubMed]
- O’Bryan, C.; Crandall, P.; Ricke, S.; Ndahetuye, J. Lactic acid bacteria (LAB) as antimicrobials in food products: Types and mechanisms of action. Handb. Nat. Antimicrob. Food Saf. Qual. 2015, 6, 117–129. [Google Scholar]
- Štreimikytė, P.; Urbonavičienė, D.; Balčiūnaitienė, A.; Viškelis, P.; Viškelis, J. Optimization of the Multienzyme-Assisted Extraction Procedure of Bioactive Compounds Extracts from Common Buckwheat (Fagopyrum esculentum M.) and Evaluation of Obtained Extracts. Plants 2021, 10, 2567. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Štreimikytė, P.; Keršienė, M.; Eisinaitė, V.; Jasutienė, I.; Lesauskaitė, V.; Damulevičienė, G.; Knašienė, J.; Leskauskaitė, D. Formulating protein-based beverages for the dysphagia diets of the elderly: Viscosity, protein quality, in vitro digestion, and consumers acceptability. J. Sci. Food Agric. 2020, 100, 3895–3901. [Google Scholar] [CrossRef] [PubMed]
- Puzerytė, V.; Viškelis, P.; Balčiūnaitienė, A.; Štreimikytė, P.; Viškelis, J.; Urbonavičienė, D. Aralia cordata Thunb. as a Source of Bioactive Compounds: Phytochemical Composition and Antioxidant Activity. Plants 2022, 11, 1704. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Fathalla, Z. Investigation into the emerging role of the basic amino acid L-lysine in enhancing solubility and permeability of BCS class II and BCS class IV drugs. Pharm. Res. 2018, 35, 160. [Google Scholar] [CrossRef]
- Brien, R.O.; Hayes, M.; Sheldrake, G.; Tiwari, B.; Walsh, P. Macroalgal Proteins: A Review. Foods 2022, 11, 571. [Google Scholar] [CrossRef]
- Kim, S.; Lim, S. Enhanced antioxidant activity of rice bran extract by carbohydrase treatment. J. Cereal Sci. 2016, 68, 116–121. [Google Scholar] [CrossRef]
- Ngoh, Y.; Gan, C. Enzyme-assisted extraction and identification of antioxidative and α-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Naseri, A.; Marinho, G.S.; Holdt, S.L.; Bartela, J.M.; Jacobsen, C. Enzyme-assisted extraction and characterization of protein from red seaweed Palmaria palmata. Algal Res. 2020, 47, 101849. [Google Scholar] [CrossRef]
- Gómez-García, R.; Martínez-Ávila, G.C.; Aguilar, C.N. Enzyme-assisted extraction of antioxidative phenolics from grape (Vitis vinifera L.) residues. 3 Biotech 2012, 2, 297–300. [Google Scholar] [CrossRef]
- Suraiya, S.; Lee, J.M.; Cho, H.J.; Jang, W.J.; Kim, D.; Kim, Y.; Kong, I. Monascus spp. fermented brown seaweeds extracts enhance bio-functional activities. Food Biosci. 2018, 21, 90–99. [Google Scholar] [CrossRef]
- Izydorczyk, M.S.; McMillan, T.; Bazin, S.; Kletke, J.; Dushnicky, L.; Dexter, J. Canadian buckwheat: A unique, useful and under-utilized crop. Can. J. Plant Sci. 2014, 94, 509–524. [Google Scholar] [CrossRef]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohydr. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Dongmo, S.N.; Sacher, B.; Kollmannsberger, H.; Becker, T. Key volatile aroma compounds of lactic acid fermented malt based beverages–impact of lactic acid bacteria strains. Food Chem. 2017, 229, 565–573. [Google Scholar] [CrossRef]
- Yang, S.; Lin, C.; Aljuffali, I.A.; Fang, J. Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
| Sample Names | Incubation Period | Staphylococcus aureus ATCC 25923 | Staphylococcus epidermidis ATCC 12228 | Enterococcus faecalis ATCC 29212 | Escherichia coli ATCC 25922 | Klebsiella pneumoniae ATCC 13883 | Pseudomonas aeruginosa ATCC 27853 | Proteus vulgaris ATCC 8427 | Bacillus cereus ATCC 11778 | Listeria monocytogenes ATCC 19115 | Candida albicans ATCC 10231 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
| Inhibition Zone, mm | |||||||||||
| FB+BS | 5 days | 4.5 ± 0.3 a | 3.7 ± 0.2 a | 1.5 ± 0.1 a | 0.3 ± 0.1 a | 0.4 ± 0.1 a | 0.4 ± 0.1 a | 0.3 ± 0.2 a | 2.5 ± 0.5 a | 2.6 ± 0.5 a | 0 |
| 12 days | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.8 ± 0.3 a | 0 | |
| FB+TKG | 5 days | 0 | 0 | 2.7 ± 0.3 b | 0.5 ± 0.3 a | 0.7 ± 0.2 b | 0.8 ± 0.2 b | 0.6 ± 0.2 b | 1.1 ± 0.3 b | 1.3 ± 0.2 b | 0 |
| 12 days | 0 | 0 | 2.6 ± 0.3 b | 0.3 ± 0.2 a | 0.6 ± 0.2 b | 0 | 0 | 0 | 0 | 0 | |
| TKG | 5 days | 0 | 2.5 ± 0.1 b | 0 | 0 | 0 | 0 | 0 | 0 | 2.3 ± 0.1 a | 0 |
| 12 days | 0 | 2.4 ± 0.1 b | 0 | 0 | 0.8 ± 0.2 b | 0 | 0 | 0 | 2.6 ± 0.4 a | 0 | |
| BS | 5 days | 3.5 ± 0.2 b | 2.7 ± 0.2 b | 0 | 0 | 0 | 0 | 0 | 0.7 ± 0.3 c | 0 | 0 |
| 12 days | 3.6 ± 0.2 b | 2.8 ± 0.2 b | 0 | 0 | 0 | 0 | 0 | 0.6 ± 0.2 c | 0.5 ± 0.6 c | 0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Streimikyte, P.; Balciunaitiene, A.; Liapman, T.D.; Streimikyte-Mockeliune, Z.; Puzeryte, V.; Borkertas, S.; Viskelis, P.; Viskelis, J. Enzymatically Hydrolysed Common Buckwheat (Fagopyrum esculentum M.) as a Fermentable Source of Oligosaccharides and Sugars. Appl. Sci. 2022, 12, 8210. https://doi.org/10.3390/app12168210
Streimikyte P, Balciunaitiene A, Liapman TD, Streimikyte-Mockeliune Z, Puzeryte V, Borkertas S, Viskelis P, Viskelis J. Enzymatically Hydrolysed Common Buckwheat (Fagopyrum esculentum M.) as a Fermentable Source of Oligosaccharides and Sugars. Applied Sciences. 2022; 12(16):8210. https://doi.org/10.3390/app12168210
Chicago/Turabian StyleStreimikyte, Paulina, Aiste Balciunaitiene, Theodore Daniel Liapman, Zaneta Streimikyte-Mockeliune, Viktorija Puzeryte, Simas Borkertas, Pranas Viskelis, and Jonas Viskelis. 2022. "Enzymatically Hydrolysed Common Buckwheat (Fagopyrum esculentum M.) as a Fermentable Source of Oligosaccharides and Sugars" Applied Sciences 12, no. 16: 8210. https://doi.org/10.3390/app12168210






