RISE Test Facilities for the Measurement of Ultra-Low Flow Rates and Volumes with a Focus on Medical Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement Facility for Flow Rates from 0.25 μL h−1 to 1 mL h−1
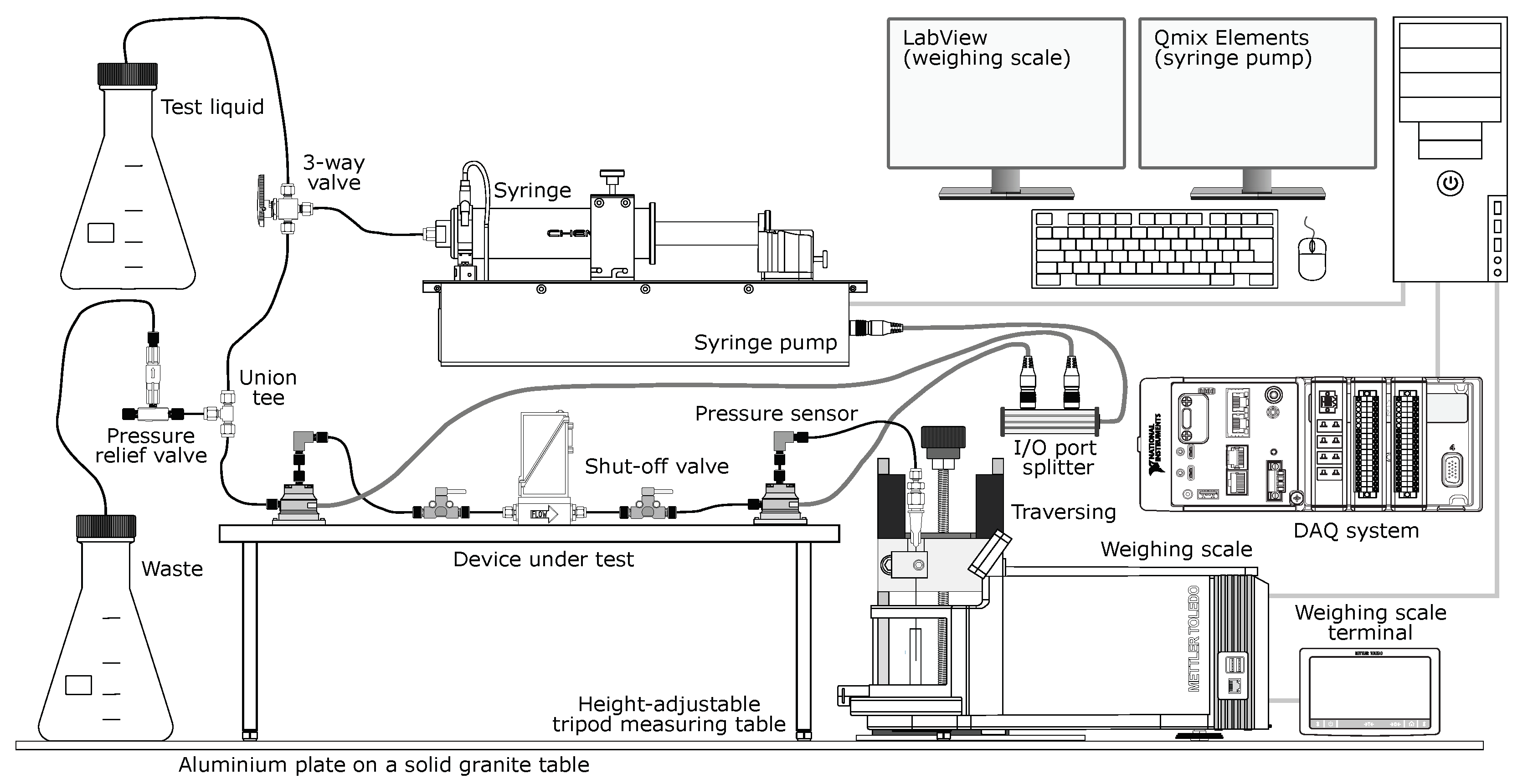
2.1.1. Nanoflow Test Facility
2.1.2. Operating Principle
2.1.3. Data Acquisition (DAQ)
2.2. Measurement Facility for Flow Rates from 1 mL h−1 to 100 mL h−1
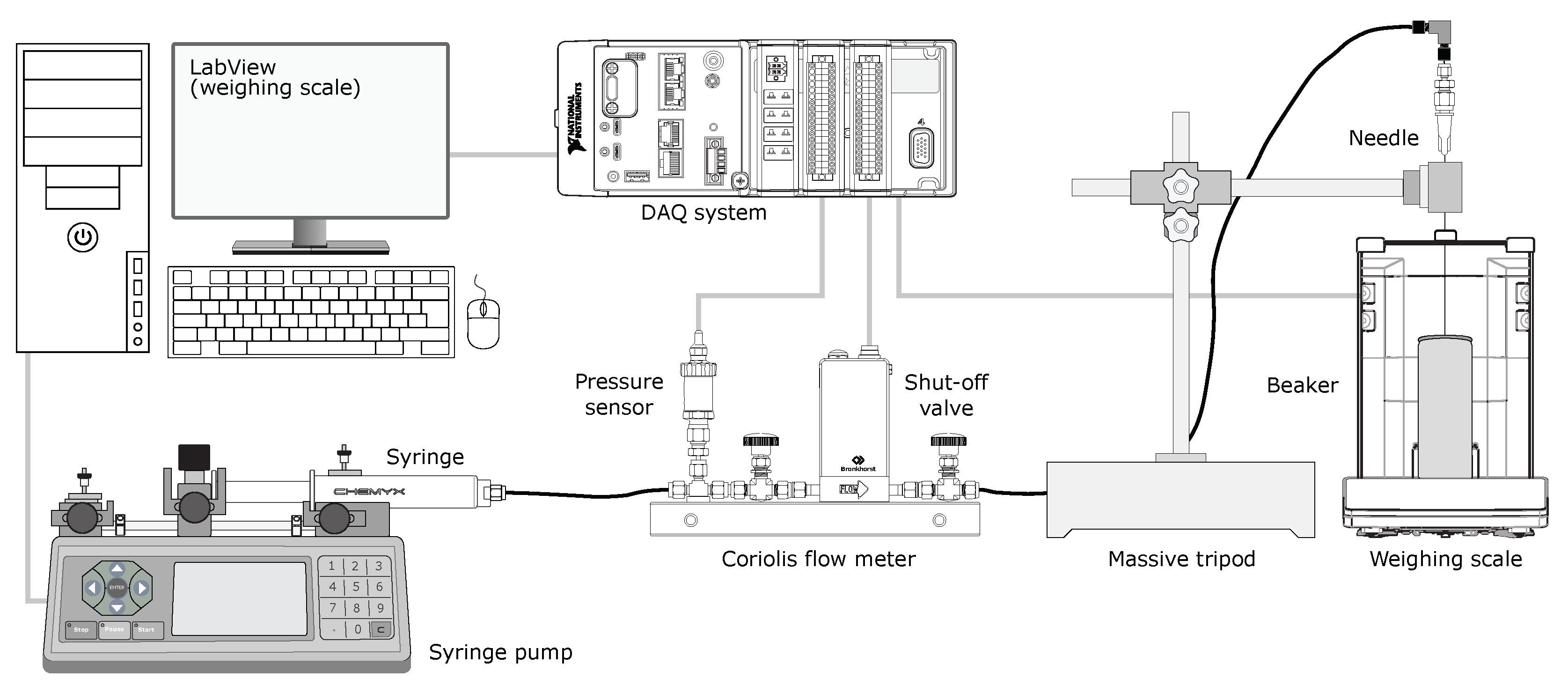
2.2.1. Microflow Test Facility
2.2.2. Operating Principle
2.2.3. Data Acquisition (DAQ)
2.2.4. Design Considerations
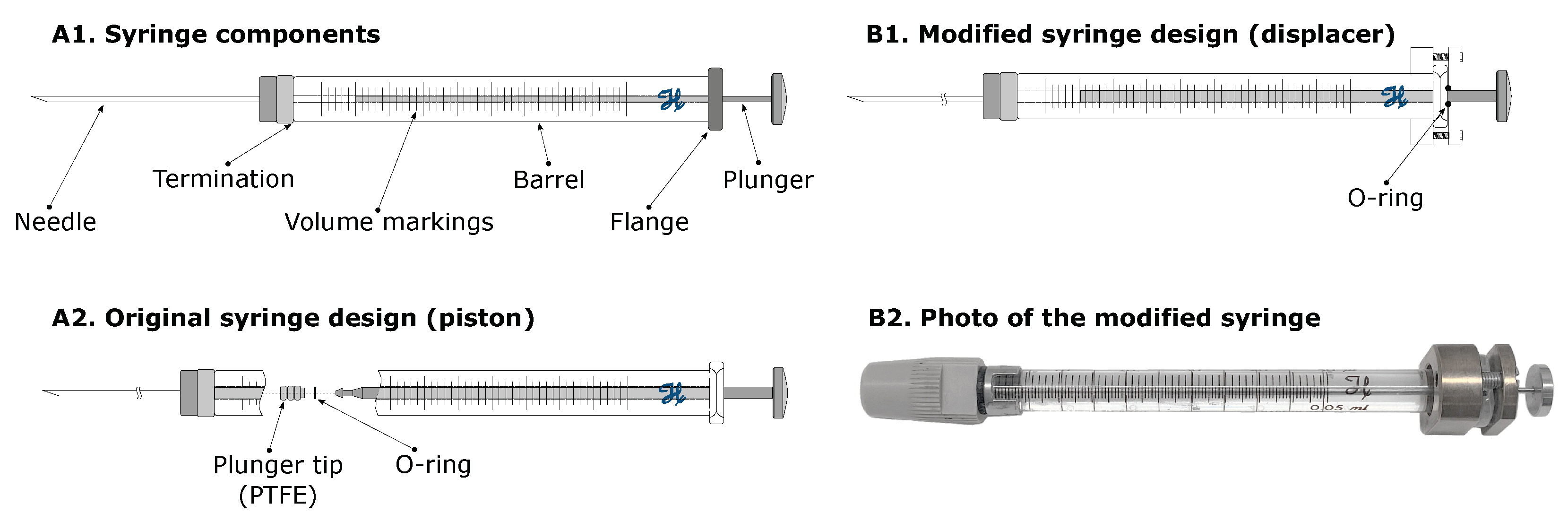
Flow Generation (Syringes)
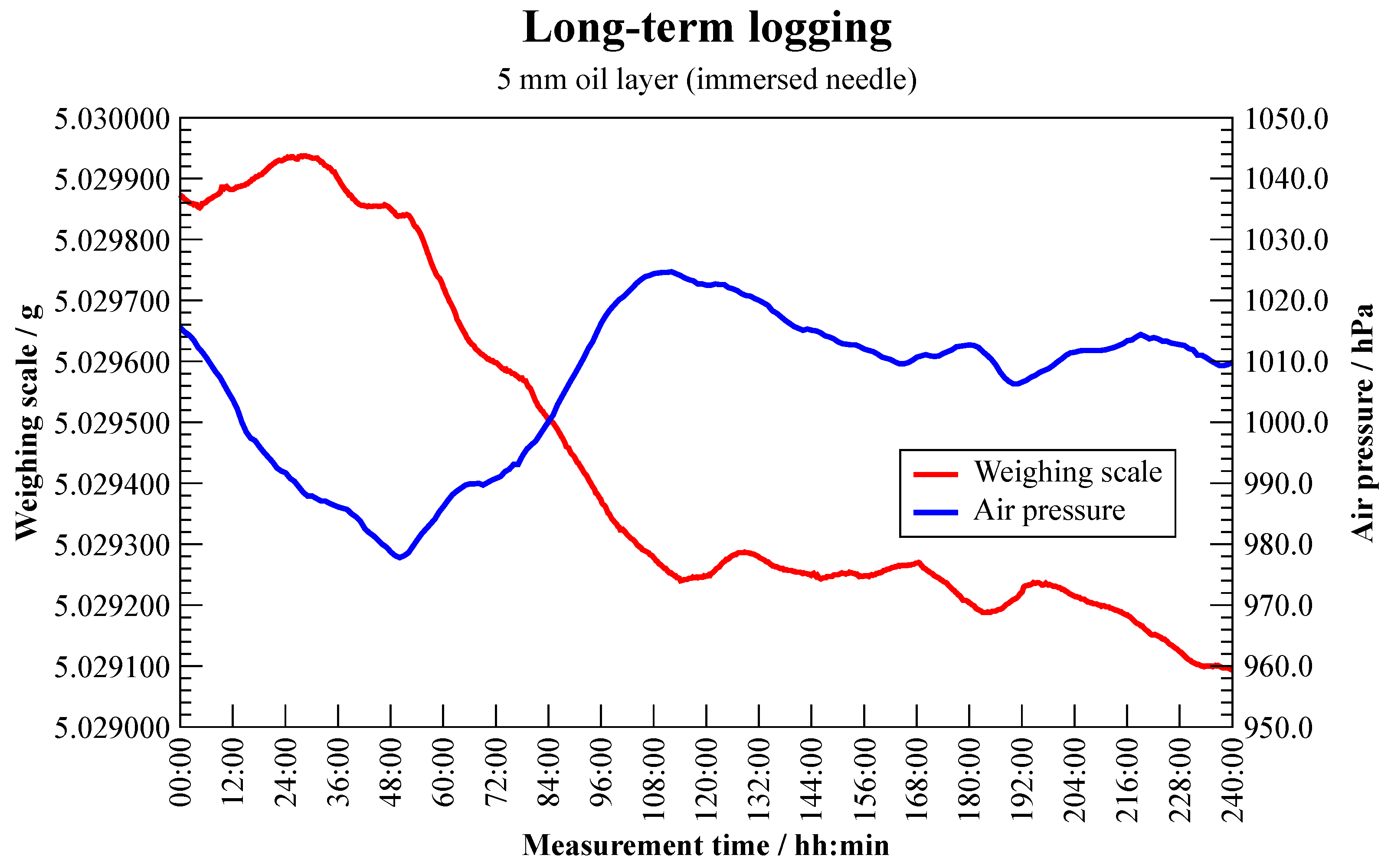
Water Collection (Evaporation)
2.2.5. Measurement Uncertainty
3. Measurement Results
3.1. Tests of Medical Devices
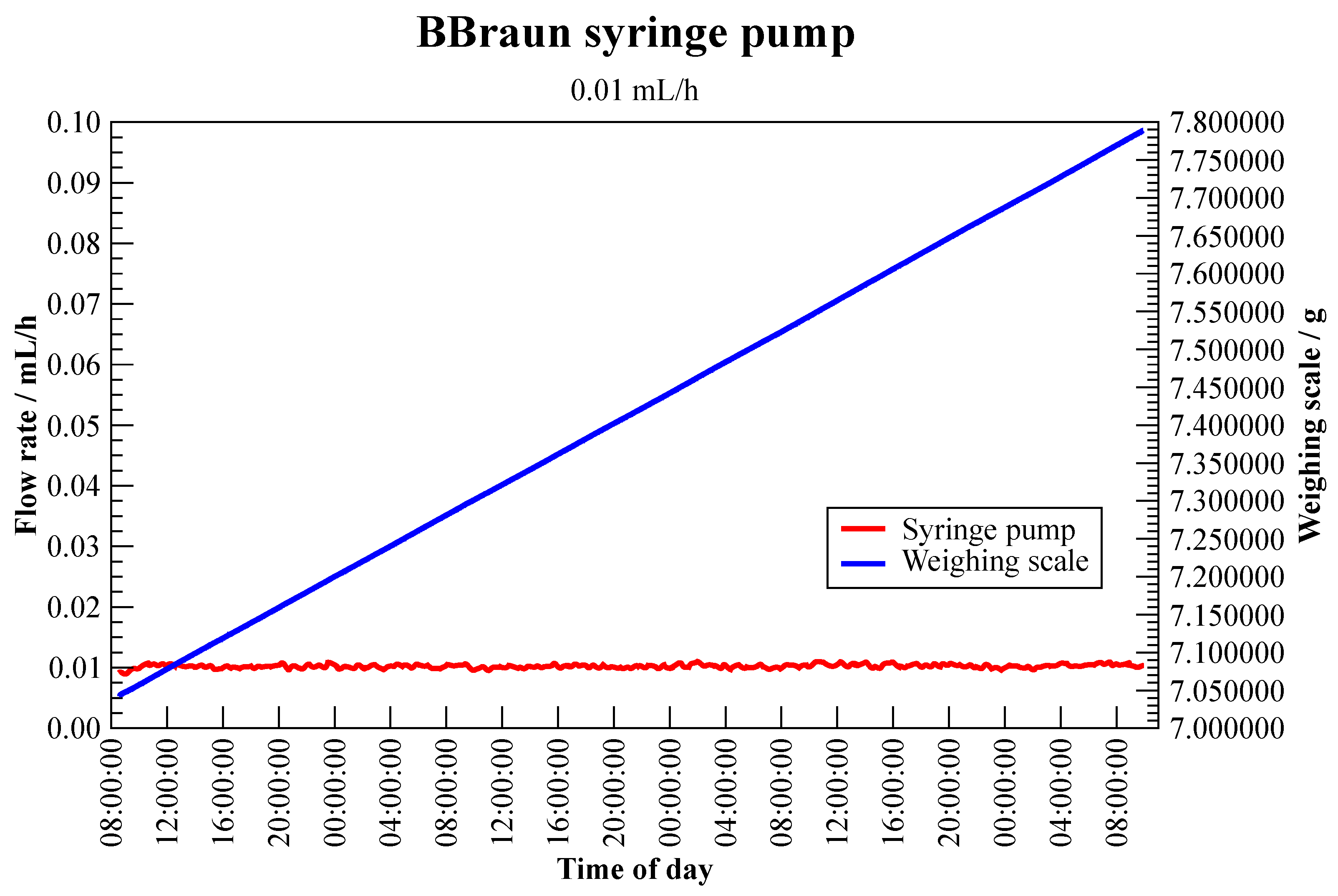
3.1.1. Syringe Pump—BBraun Perfusor Space
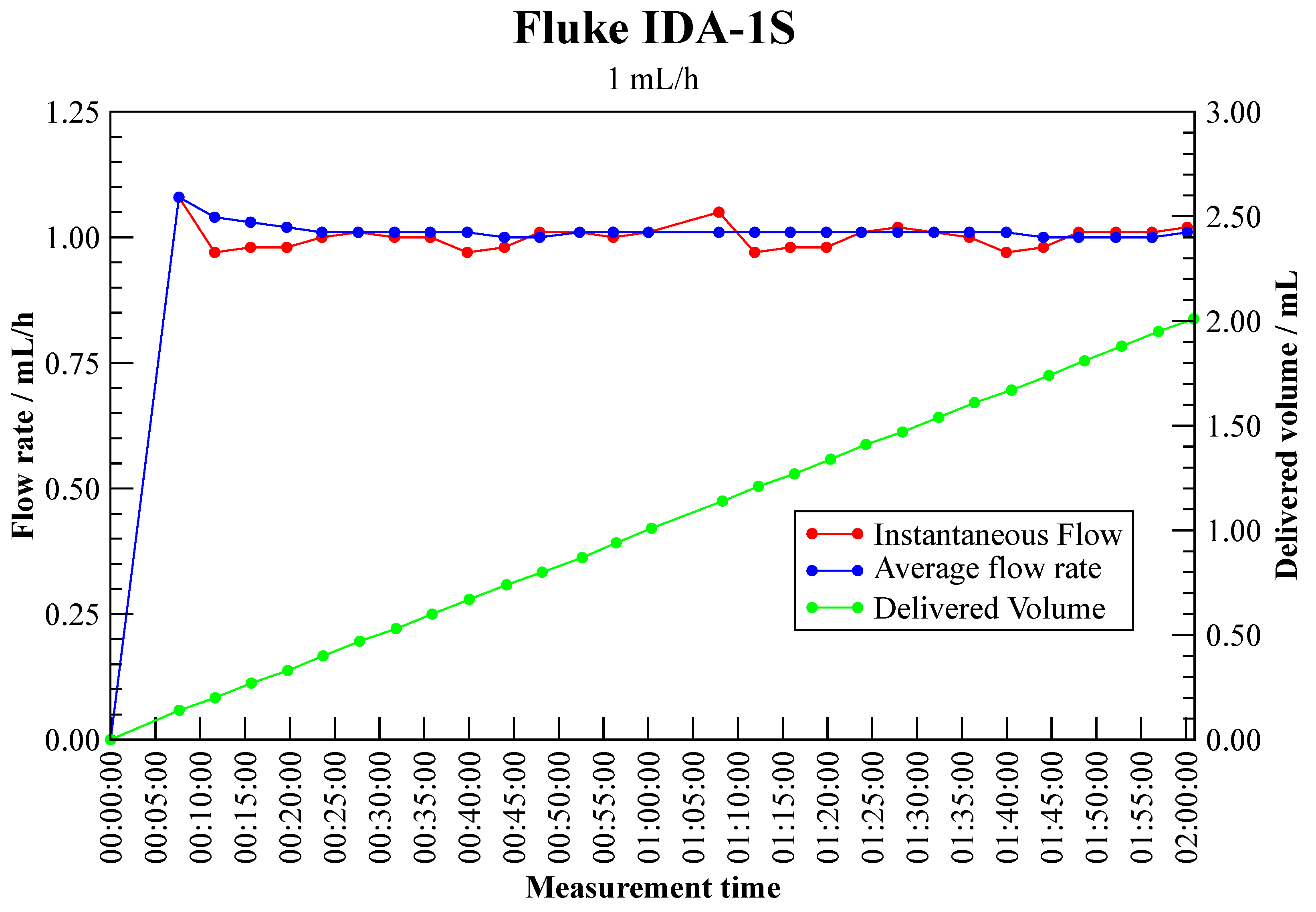
3.1.2. Infusion Device Analyser—Fluke IDA-1S
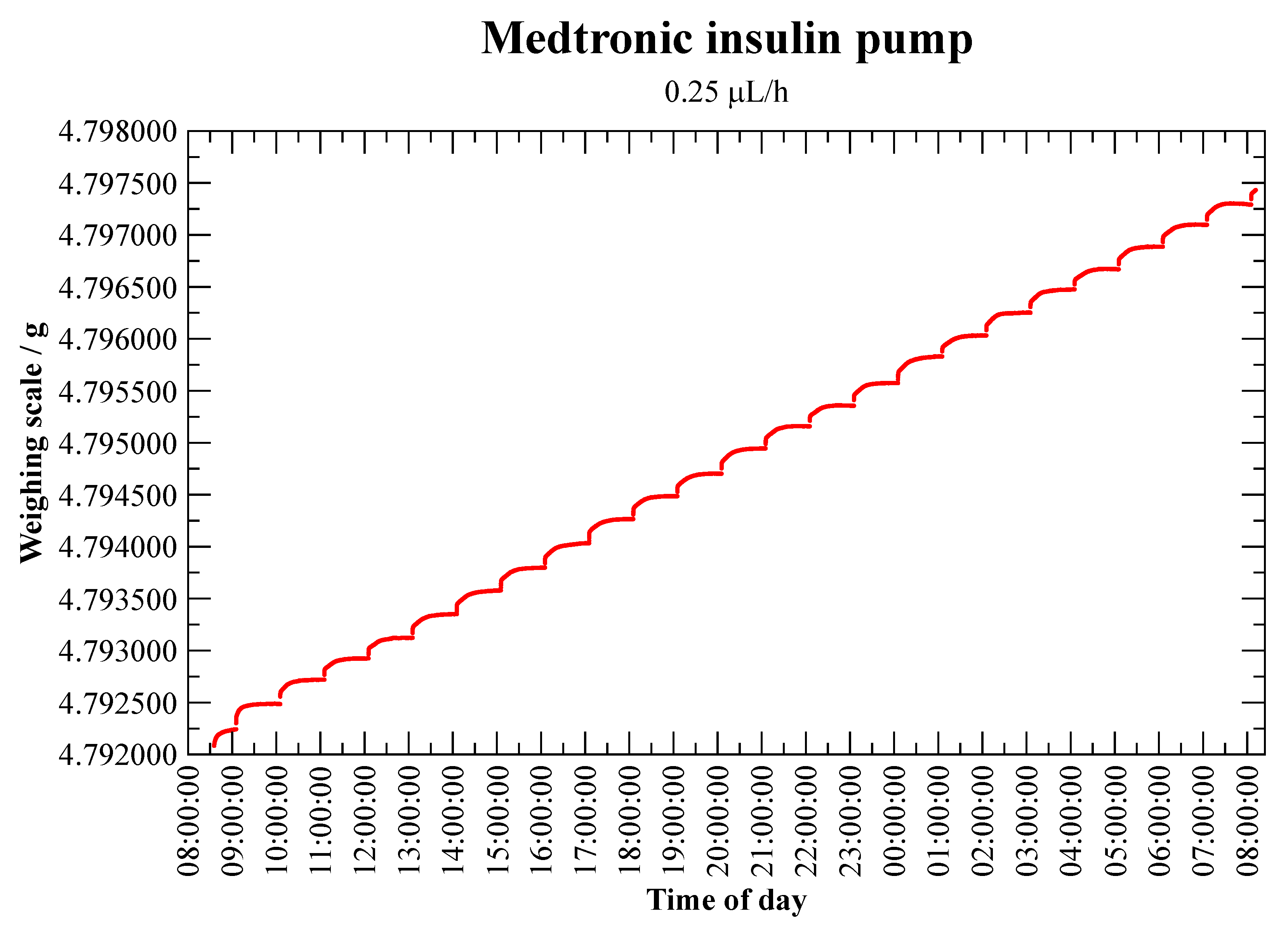
3.1.3. Insulin Pump—Medtronic MiniMed Veo Paradigm 554
3.2. Further Research
3.2.1. Flow Meter (Static Calibration)—Bronkhorst L01
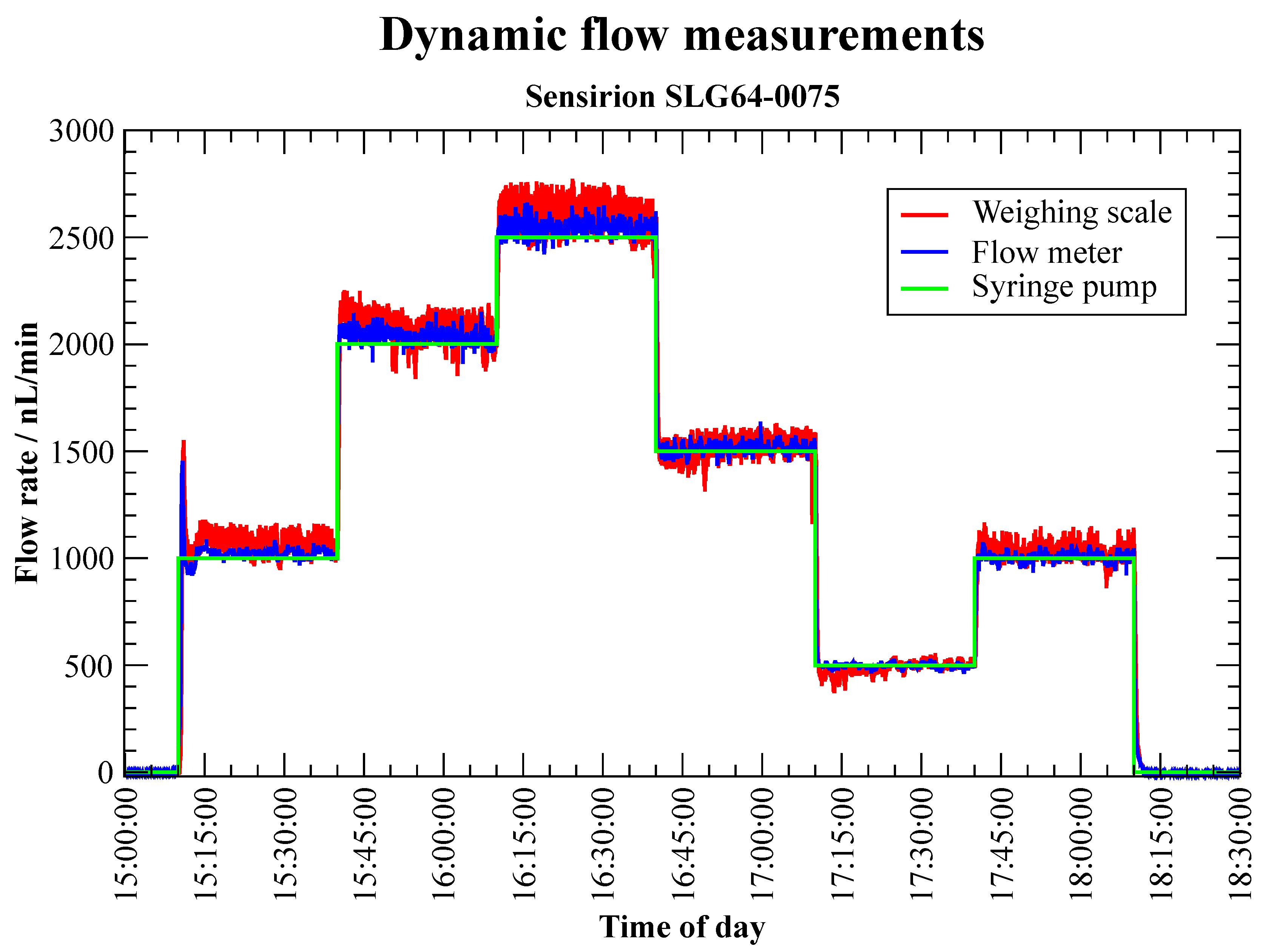
3.2.2. Flow Meter (Dynamic Calibration)—Sensirion SLG64-0075
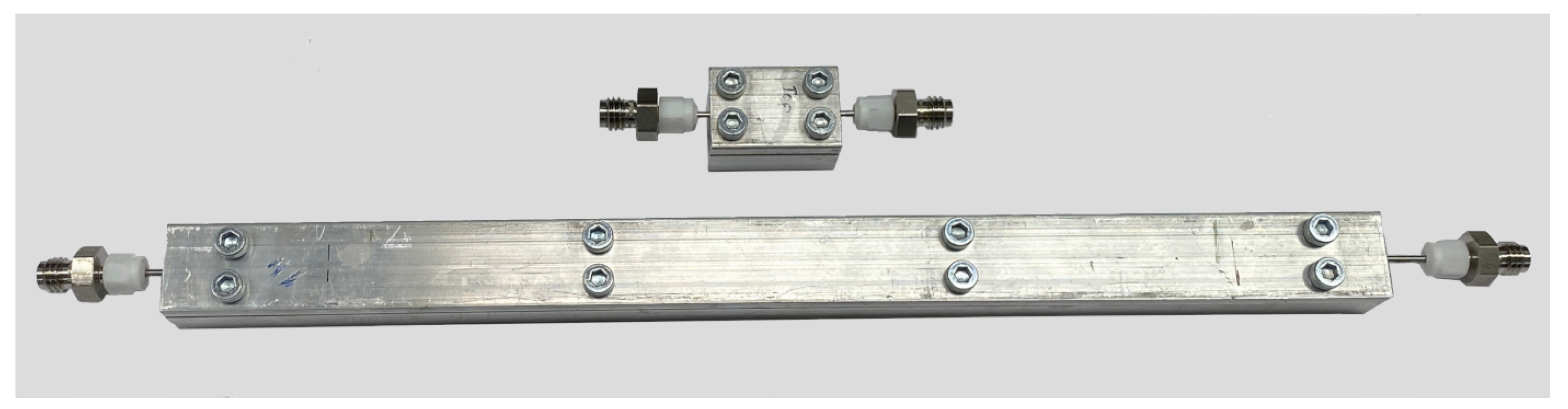
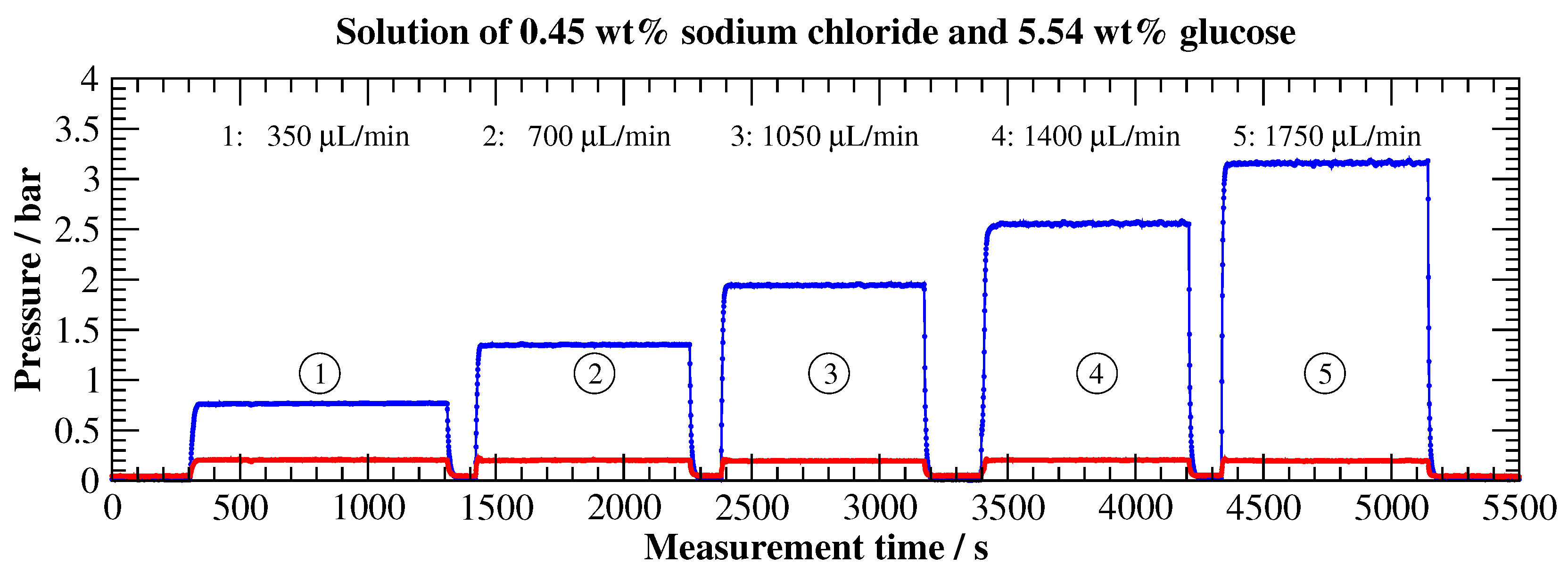
3.2.3. Viscosity Measurement (Pipe Viscometer)
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations and Nomenclature
Abbreviations
| AAMI | Association for the Advancement of Medical Instrumentation |
| CAS | Chemical Abstract Service |
| CFM | Coriolis Flow Meter |
| CSV | Comma-Separated Values |
| DAQ | Data Acquisition |
| DDE | Dynamic Data Exchange |
| DUT | Device Under Test |
| ECRI | Emergency Care Research Institute |
| EMPIR | European Metrology Programme for Innovation and Research |
| EN | European Standard |
| HLPC | High Performance Liquid Chromatography |
| HPW | Highly Purified Water |
| ID | Inner Diameter |
| IEC | International Electrotechnical Commission |
| IDA | Infusion Device Analyser |
| IPQ | Portuguese Institute for Quality |
| ISO | International Organisation for Standardisation |
| JRP | Joint Research Project |
| METAS | The Federal Institute of Metrology |
| MT-SICS | Mettler Toledo Standard Interface Command Set |
| NMI | National Metrology Institute |
| NQIS/EIM | Hellenic Institute of Metrology/National Quality Infrastructure System |
| OD | Outer Diameter |
| RISE | Research Institutes of Sweden |
| TMFM | Thermal Mass Flow Meter |
| TIR | Technical Information Report |
Nomenclature
| D | (Pipe, tube) diameter | [m] |
| Pressure drop (pressure loss) | [Pa] | |
| L | (Pipe, tube) length | [m] |
| Upstream (inlet) pressure | [Pa] | |
| Downstream (outlet) pressure | [Pa] | |
| Mass fraction (percentage by weight) | [–] | |
| Dynamic viscosity | [Pa s] | |
| Kinematic viscosity | [m s] | |
| Density | [kg m] |
References
- Lee, P.T.; Thompson, F.; Thimbleby, H. Analysis of infusion pump error logs and the ir significance for health care. Br. J. Nurs. 2012, 21, S12–S20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemann, L.; Fleming, G.A.; Petrie, J.R.; Holl, R.W.; Bergenstal, R.M.; Peters, A.L. Insulin Pump Risks and Benefits: A Clinical Appraisal of Pump Safety Standards, Adverse Event Reporting, and Research Needs: A Joint Statement of the European Association for the Study of Diabetes and the American Diabetes Association Diabetes Technology Working Group. Diabetes Care 2015, 38, 716–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Lovich, M.A.; Peterfreund, R.A. Quantitative analysis of continuous intravenous infusions in pediatric anesthesia: Safety implications of dead volume, flow rates, and fluid delivery. Pediatr. Anesth. 2011, 21, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Husch, M.; Sullivan, C.; Rooney, D.; Barnard, C.; Fotis, M.; Clarke, J.; Noskin, G. Insights from the sharp end of intravenous medication errors: Implications for infusion pump technology. Qual. Saf. Health Care 2005, 14, 80–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.A.; Jones, R. Risk of Medication Errors With Infusion Pumps: A Study of 1004 Events From 132 Hospitals Across Pennsylvania. Patient Saf. 2019, 1, 60–69. [Google Scholar] [CrossRef]
- ECRI Institute. Top 10 Health Technology Hazards for 2017. Excerpted from: Health Devices. 2016. Available online: https://www.ecri.org/Resources/Whitepapers_and_reports/Haz17.pdf (accessed on 1 August 2022).
- Batista, E.; Furtado, A.; Pereira, J.; Ferreira, M.; Bissig, H.; Graham, E.; Niemann, A.; Timmerman, A.; Alves e Sousa, J.; Ogheard, F.; et al. New EMPIR project—Metrology for Drug Delivery. Flow Meas. Instrum. 2020, 72, 101716. [Google Scholar] [CrossRef]
- Clarkson, D. Accuracy estimations of testing of infusion devices using weighing balances. Med. Eng. Phys. 2002, 24, 229–235. [Google Scholar] [CrossRef]
- Jahn, L.G.; Capurro, J.J.; Levy, B.L. Comparative Dose Accuracy of Durable and Patch Insulin Infusion Pumps. J. Diabetes Sci. Technol. 2013, 7, 1011–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamecke, U.; Waldenmaier, D.; Haug, C.; Ziegler, R.; Freckmann, G. Establishing Methods to Determine Clinically Relevant Bolus and Basal Rate Delivery Accuracy of Insulin Pumps. J. Diabetes Sci. Technol. 2019, 13, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IEC/ISO 60601-2-24:2012; Medical Electrical Equipment—Part 2-24: Particular Requirements for the Basic Safety and Essential Performance of Infusion Pumps and Controllers. International Electrotechnical Commission: Geneva, Switzerland, 2012.
- Bissig, H.; Tschannen, M.; de Huu, M. Water collection techniques at very low flow rates including strong capillary effects. Flow Meas. Instrum. 2020, 73, 101744. [Google Scholar] [CrossRef]
- Batista, E.; Godinho, I.; Martins, R.F.; Mendes, R.; Robarts, J. Development of an experimental setup for microflow measurement using interferometry. Flow Meas. Instrum. 2020, 75, 101789. [Google Scholar] [CrossRef]
- Sousa, J.A.; Batista, E.; Pellegrino, O.; Ribeiro, A.S.; Martins, L.L. Method selection to evaluate measurement uncertainty in microflow applications. J. Phys. Conf. Ser. 2019, 1379, 12033. [Google Scholar] [CrossRef]
- Sousa, J.; Batista, E.; Demeyer, S.; Fischer, N.; Pellegrino, O.; Ribeiro, A.; Martins, L. Uncertainty calculation methodologies in microflow measurements: Comparison of GUM, GUM-S1 and Bayesian approach. Measurement 2021, 181, 109589. [Google Scholar] [CrossRef]
- AAMI TIR101:2021; Fluid Delivery Performance Testing for Infusion Pumps. American National Standards Institute (ANSI): Washington, DC, USA,, 2021.
- Bissig, H.; Tschannen, M.; de Huu, M. Micro-flow facility for traceability in steady and pulsating flow. Flow Meas. Instrum. 2015, 44, 34–42. [Google Scholar] [CrossRef]
- ISO 3696:1987; Water for Analytical Laboratory Use—Specification and Test Methods. International Organization for Standardization: Geneva, Switzerland, 1987.
- Hemmati-Sarapardeh, A.; Hatami, S.; Taghvaei, H.; Naseri, A.; Band, S.S.; Chau, K.W. Designing a committee of machines for modeling viscosity of water-based nanofluids. Eng. Appl. Comput. Fluid Mech. 2021, 15, 1967–1987. [Google Scholar] [CrossRef]
- Bissig, H.; Petter, H.T.; Lucas, P.; Batista, E.; Filipe, E.; Almeida, N.; Ribeiro, L.F.; Gala, J.; Martins, R.; Savanier, B.; et al. Primary standards for measuring flow rates from 100 nl/min to 1 mL/min—Gravimetric principle. Biomed. Eng. Biomed. Tech. 2015, 60, 301–316. [Google Scholar] [CrossRef] [PubMed]
- EURAMET. Calibration Guide No. 18—Guidelines on the Calibration of Non-Automatic Weighing Instruments; EURAMET: Braunschweig, Germany, 2015. [Google Scholar]
- Batista, E.; Almeida, N.; Godinho, I.; Filipe, E. Uncertainty calculation in gravimetric microflow measurements. In Advanced Mathematical and Computational Tools in Metrology and Testing X; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2015; pp. 98–104. [Google Scholar] [CrossRef]
- MeDDII. Report on Calibration Methods for Measuring the Response or Delay Time of Drug Delivery Devices Using Newtonian Liquids for Flow Rates from 5 nL/min to 100 nL/min. EMPIR 18HLT08 MeDDII Deliverable—D1. 2020. Available online: https://drugmetrology.com/wp-content/uploads/2020/10/MeDDII-D1_V1-1.pdf (accessed on 28 June 2022).
- MeDDII. Report on the Results from the Inter-Comparison of the Primary Standards and Improved Measurement Facilities for Flow Rates from 5 nL/min to 100 nL/min (with Uncertainty Targets of 1% (k = 2) for Steady Flow and 2% (k = 2) for Fast Changing Flow). EMPIR 18HLT08 MeDDII Deliverable—D2. 2020. Available online: https://drugmetrology.com/wp-content/uploads/2022/02/MeDD_II_D2-Final.pdf (accessed on 28 June 2022).
- ISO 7886-2:2020; Sterile Hypodermic Syringes for Single Use—Part 2: Syringes for use with Power-Driven Syringe Pumps. International Organization for Standardization: Geneva, Switzerland, 2020.
- Lal, R.; Leelarathna, L. Insulin Pumps. Diabetes Technol. Ther. 2020, 22, 17–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zisser, H. Insulin Pump (Dose-to-Dose) Accuracy: What Does It Mean and When Is It Important? J. Diabetes Sci. Technol. 2014, 8, 1142–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissig, H.; Tschannen, M.; de Huu, M. Traceability of pulsed flow rates consisting of constant delivered volumes at given time interval. Flow Meas. Instrum. 2020, 73, 101729. [Google Scholar] [CrossRef]
- Colin, S. Introduction to microflows. In Microfluidics; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 1–23. [Google Scholar] [CrossRef]



| Chemyx Nexus 3000 | Cetoni neMESYS 290N | |
|---|---|---|
| Drive mechanism | Stepper motor | Stepper motor |
| Step resolution | ||
| (Advance per step) | ||
| Flow rate range | nL min to 500 | nL min (10 μL syringe) to |
| (manufacturer information) | 150 (50 syringe) | |
| with gear box 29:1 | ||
| Syringe size | 140 ; 300 (with self-made | 50 (largest manufacturer |
| holder) | holder) | |
| No. of syringes | 2 | 1 (multi-systems available) |
| Readout / handling | Configuration of the initial | Configuration of the initial |
| values (pump and syringe) via | values (pump and syringe) | |
| keypad or software. | exclusively via software. Output | |
| Information during operation | of the (current) readings via | |
| only via display. | software and in a later log file. |
| Measurement | |
|---|---|
| Flow Rate | Uncertainty |
| U | |
| 5 | |
| 20 | |
| 100 | |
| greater than or | |
| equal to 500 |
| Start Time | Measuring Period | Volume Flow | Volume | Accumulated |
|---|---|---|---|---|
| s | min | nL min | L | Volume L |
| 0 | 30 | 1000 | 30 | 30 |
| 30 | 30 | 2000 | 60 | 90 |
| 60 | 30 | 2500 | 75 | 165 |
| 90 | 30 | 1500 | 45 | 210 |
| 120 | 30 | 500 | 15 | 225 |
| 150 | 30 | 1000 | 30 | 255 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Büker, O.; Stolt, K. RISE Test Facilities for the Measurement of Ultra-Low Flow Rates and Volumes with a Focus on Medical Applications. Appl. Sci. 2022, 12, 8332. https://doi.org/10.3390/app12168332
Büker O, Stolt K. RISE Test Facilities for the Measurement of Ultra-Low Flow Rates and Volumes with a Focus on Medical Applications. Applied Sciences. 2022; 12(16):8332. https://doi.org/10.3390/app12168332
Chicago/Turabian StyleBüker, Oliver, and Krister Stolt. 2022. "RISE Test Facilities for the Measurement of Ultra-Low Flow Rates and Volumes with a Focus on Medical Applications" Applied Sciences 12, no. 16: 8332. https://doi.org/10.3390/app12168332






