Exploring the Genetic Basis of Dens Evaginatus Using Whole-Exome Sequencing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Whole-Exome Sequencing (WES) Analysis
3. Results
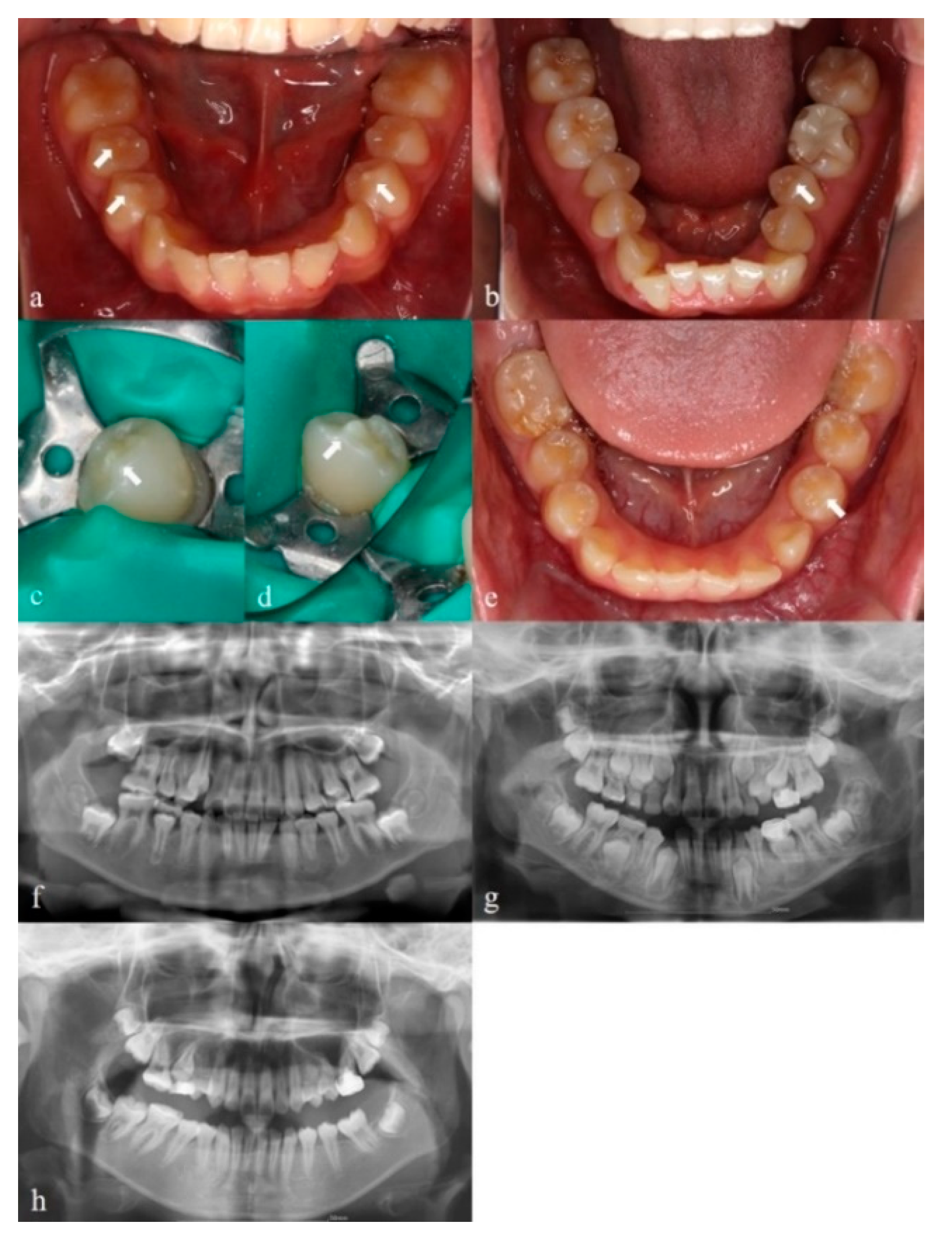
3.1. Clinical Findings
3.2. Genetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woelfel, J.B. Dental Anatomy: Its Relevance to Dentistry, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1990. [Google Scholar]
- Manuja, N.; Chaudhary, S.; Nagpal, R.; Rallan, M. Bilateral dens evaginatus (talon cusp) in permanent maxillary lateral incisors: A rare developmental dental anomaly with great clinical significance. BMJ Case Rep. 2013, 2013, bcr2013009184. [Google Scholar] [CrossRef]
- Hattab, F.N.; Yassin, O.M.; al-Nimri, K.S. Talon Cusp—Clinical Significance and Management: Case Reports. Quintessence Int. 1995, 26, 115–120. [Google Scholar] [PubMed]
- Levitan, M.E.; Himel, V.T. Dens evaginatus: Literature review, pathophysiology, and comprehensive treatment regimen. J. Endod. 2006, 32, 1–9. [Google Scholar] [CrossRef]
- Hattab, F.N.; Yassin, O.M.; al-Nimri, K.S. Talon cusp in permanent dentition associated with other dental anomalies: Review of literature and reports of seven cases. ASDC J. Dent. Child. 1996, 63, 368–376. [Google Scholar] [PubMed]
- Yip, W.K. The prevalence of dens evaginatus. Oral Surg. Oral Med. Oral Pathol. 1974, 38, 80–87. [Google Scholar] [CrossRef]
- Ash, M. Wheeler’s Dental Anatomy, Physiology and Occlusion, 8th ed.; W. B. Saunders: Philadelphia, PA, USA, 2003; pp. 241–242. [Google Scholar]
- Echeverri, E.A.; Wang, M.M.; Chavaria, C.; Taylor, D.L. Multiple dens evaginatus: Diagnosis, management, and complications: Case report. Pediatr. Dent. 1994, 16, 314–317. [Google Scholar]
- Priddy, W.L.; Carter, H.G.; Auzins, J. Dens evaginatus—An anomaly of clinical significance. J. Endod. 1976, 2, 51–52. [Google Scholar] [CrossRef]
- Hülsmann, M. Dens invaginatus: Aetiology, classification, prevalence, diagnosis, and treatment considerations. Int. Endod. J. 1997, 30, 79–90. [Google Scholar] [CrossRef]
- Chen, J.W.; Huang, G.T.; Bakland, L.K. Dens evaginatus: Current treatment options. J. Am. Dent. Assoc. 2020, 151, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Stecker, S.S.; Peterson, V.S.; Beiraghi, S.; Myers, S.L.; Bowles, W.R. Dens evaginatus: Just another cusp? Northwest Dent. 2015, 94, 31–33. [Google Scholar]
- Kocsis, G.; Marcsik, A.; Kokai, E.; Kocsis, K. Supernumerary occlusal cusps on permanent human teeth. Acta Biol. Szeged. 2002, 46, 71–82. [Google Scholar]
- Merrill, R.G. Occlusal anomalous tubercles on premolars of Alaskan eskimos and Indians. Oral Surg. Oral Med. Oral Pathol. 1964, 17, 484–496. [Google Scholar] [CrossRef]
- Curzon, M.E.; Curzon, J.A.; Poyton, H.G. Evaginated odontomes in the Keewatin Eskimo. Br. Dent. J. 1970, 129, 324–328. [Google Scholar] [CrossRef]
- Leigh, R. Dental pathology of the Eskimo. Dent. Cosmos. 1925, 67, 884–898. [Google Scholar]
- Geist, J.R. Dens evaginatus. Case report and review of the literature. Oral Surg. Oral Med. Oral Pathol. 1989, 67, 628–631. [Google Scholar] [CrossRef]
- Kimura, R.; Yamaguchi, T.; Takeda, M.; Kondo, O.; Toma, T.; Haneji, K.; Hanihara, T.; Matsukusa, H.; Kawamura, S.; Maki, K.; et al. A common variation in EDAR is a genetic determinant of shovel-shaped incisors. Am. J. Hum. Genet. 2009, 85, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lozić, B.; Ljubković, J.; Pandurić, D.G.; Saltvig, I.; Kutsche, K.; Krželj, V.; Zemunik, T. Oculo-facio-cardio-dental syndrome in three succeeding generations: Genotypic data and phenotypic features. Braz. J. Med. Biol. Res. 2012, 45, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.E.; Dixon, G.H.; Graber, R.B. Dens evaginatus (tuberculated cusps): Genetic and treatment considerations. Oral Surg. Oral Med. Oral Pathol. 1978, 46, 831–836. [Google Scholar] [CrossRef]
- Davis, P.J.; Brook, A.H. The presentation of talon cusp: Diagnosis, clinical features, associations and possible aetiology. Br. Dent. J. 1986, 160, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, C.; Hudson, K.L.; Anderson, K.V. The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 1988, 52, 269–279. [Google Scholar] [CrossRef]
- Fawzy El-Sayed, K.M.; Klingebiel, P.; Dörfer, C.E. Toll-like receptor expression profile of human dental pulp stem/progenitor cells. J. Endod. 2016, 42, 413–417. [Google Scholar] [CrossRef]
- Durand, S.H.; Flacher, V.; Roméas, A.; Carrouel, F.; Colomb, E.; Vincent, C.; Magloire, H.; Couble, M.L.; Bleicher, F.; Staquet, M.J.; et al. Lipoteichoic acid increases TLR and functional chemokine expression while reducing dentin formation in in vitro differentiated human odontoblasts. J. Immunol. 2006, 176, 2880–2887. [Google Scholar] [CrossRef] [PubMed]
- Pääkkönen, V.; Rusanen, P.; Hagström, J.; Tjäderhane, L. Mature human odontoblasts express virus-recognizing toll-like receptors. Int. Endod. J. 2014, 47, 934–941. [Google Scholar] [CrossRef]
- Staquet, M.J.; Carrouel, F.; Keller, J.F.; Baudouin, C.; Msika, P.; Bleicher, F.; Kufer, T.A.; Farges, J.C. Pattern-recognition receptors in pulp defense. Adv. Dent. Res. 2011, 23, 296–301. [Google Scholar] [CrossRef]
- Keller, J.F.; Carrouel, F.; Colomb, E.; Durand, S.H.; Baudouin, C.; Msika, P.; Bleicher, F.; Vincent, C.; Staquet, M.J.; Farges, J.C. Toll-like receptor 2 activation by lipoteichoic acid induces differential production of pro-inflammatory cytokines in human odontoblasts, dental pulp fibroblasts and immature dendritic cells. Immunobiology 2010, 215, 53–59. [Google Scholar] [CrossRef]
- Arana-Chavez, V.E.; Massa, L.F. Odontoblasts: The cells forming and maintaining dentine. Int. J. Biochem. Cell Biol. 2004, 36, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Andrukhov, O. Toll-Like Receptors and Dental Mesenchymal Stromal Cells. Front. Oral Health. 2021, 2, 648901. [Google Scholar] [CrossRef]
- Zhou, B.B.; Elledge, S.J. The DNA damage response: Putting checkpoints in perspective. Nature 2000, 408, 433–439. [Google Scholar] [CrossRef]
- Wu, L.; Luo, K.; Lou, Z.; Chen, J. MDC1 regulates intra-S-phase checkpoint by targeting NBS1 to DNA double-strand breaks. Proc. Natl. Acad. Sci. USA 2008, 105, 11200–11205. [Google Scholar] [CrossRef]
- Chrzanowska, K.H.; Kleijer, W.J.; Krajewska-Walasek, M.; Białecka, M.; Gutkowska, A.; Goryluk-Kozakiewicz, B.; Michałkiewicz, J.; Stachowski, J.; Gregorek, H.; Lysón-Wojciechowska, G.; et al. Eleven Polish patients with microcephaly, immunodeficiency, and chromosomal instability: The Nijmegen breakage syndrome. Am. J. Med. Genet. 1995, 57, 462–471. [Google Scholar] [CrossRef]
- Maraschio, P.; Spadoni, E.; Tanzarella, C.; Antoccia, A.; Di Masi, A.; Maghnie, M.; Varon, R.; Demuth, I.; Tiepolo, L.; Danesino, C. Genetic heterogeneity for a Nijmegen breakage-like syndrome. Clin. Genet. 2003, 63, 283–290. [Google Scholar] [CrossRef]
- Olczak-Kowalczyk, D.; Dembowska-Bagińska, B.; Gregorek, H.; Wakulińska, A.; Pietrucha, B.; Chrzanowska, K.H. Dental Aspect of Nijmegen Breakage Syndrome. Nowa Stomatol. 2009, 3–8. [Google Scholar]
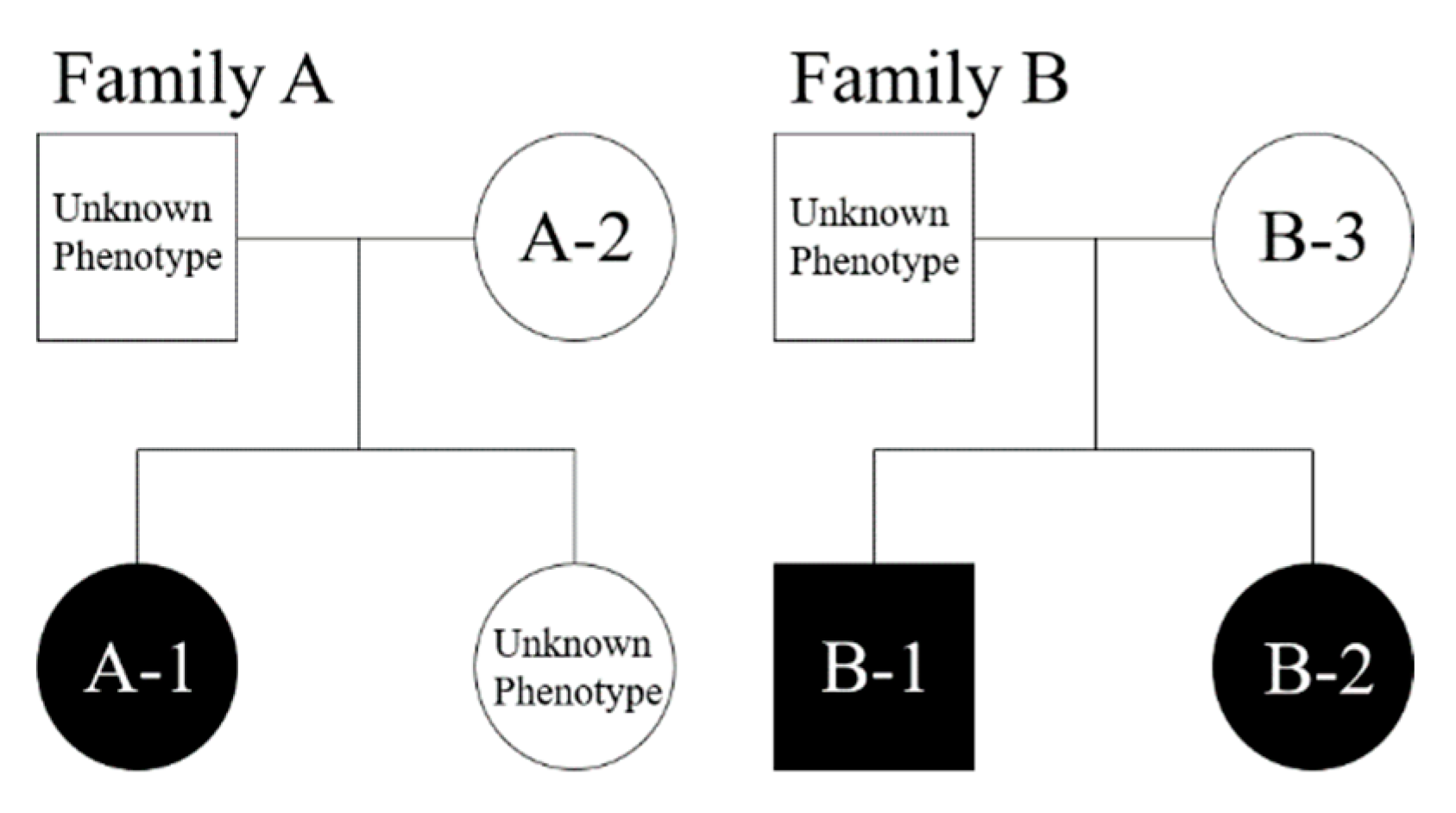
| Subject * | Sex | Dental Arch | Dens Evaginatus | Inclusion Type |
|---|---|---|---|---|
| A-1 | Female | Lower | Left and right first premolar, right second premolar | Bilateral |
| A-2 | Female | Lower | Left second premolar | Monolateral |
| B-1 | Male | Lower | Left and right first premolar | Bilateral |
| B-2 | Female | Lower | Left first premolar | Monolateral |
| B-3 | Female | - | - | - |
| Patient/Gene | TLR3 | MDC1 | ||
|---|---|---|---|---|
| A-1 * | c.2491delA | c.3908C>T | ||
| A-2 * | c.2491delA | c.3908C>T | ||
| B-1 * | c.1443C>G | c.1825G>A | ||
| B-2 * | c.1443C>G | c.1825G>A | ||
| B-3 | ||||
| Gene | GenBank Accession Number | Nucleotide Substitution | Amino Acid Substitution | Variant ID | MAF | CADD (Scaled) | PolyPhen2 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GnomAD | GnomAD EAS | 1000 Genomes | ExAC | |||||||
| TLR3 | NM_003265 | c.1443C>G | p.Ser481Arg | rs751882299 | 0.00003229 | 0 | 0 | 0.000008283 | 12.35 | Benign |
| NM_003265 | c.2491delA | p.Lys831fs | - | 0 | 0 | 0 | 0 | - | - | |
| MDC1 | NM_014641 | c.3908C>T | p.Pro1303Leu | rs763250651 | 0 | 0 | 0 | 0.000008275 | 17.49 | Damaging |
| NM_014641 | c.G1825A | p.Glu609Lys | rs763544794 | 0 | 0 | 0 | 8.98 × 10−6 | 14.83 | Benign | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Hosomichi, K.; Kim, Y.-I.; Tajima, A.; Yamaguchi, T. Exploring the Genetic Basis of Dens Evaginatus Using Whole-Exome Sequencing. Appl. Sci. 2022, 12, 8962. https://doi.org/10.3390/app12188962
Park H, Hosomichi K, Kim Y-I, Tajima A, Yamaguchi T. Exploring the Genetic Basis of Dens Evaginatus Using Whole-Exome Sequencing. Applied Sciences. 2022; 12(18):8962. https://doi.org/10.3390/app12188962
Chicago/Turabian StylePark, Heetae, Kazuyoshi Hosomichi, Yong-Il Kim, Atsushi Tajima, and Tetsutaro Yamaguchi. 2022. "Exploring the Genetic Basis of Dens Evaginatus Using Whole-Exome Sequencing" Applied Sciences 12, no. 18: 8962. https://doi.org/10.3390/app12188962
APA StylePark, H., Hosomichi, K., Kim, Y.-I., Tajima, A., & Yamaguchi, T. (2022). Exploring the Genetic Basis of Dens Evaginatus Using Whole-Exome Sequencing. Applied Sciences, 12(18), 8962. https://doi.org/10.3390/app12188962







