Oral Lesions in Pediatric Subjects: SARS-CoV-2 Infection and COVID-19 Vaccination
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
- P—Population: young subjects (<18 years old) who have tested positive for SARS-CoV-2 or have received at least one dose of the COVID-19 vaccine;
- C—Comparison: adult subjects (>18 years) who tested positive for SARS-CoV-2 or received at least one dose of COVID-19 vaccine;
- O—Outcome(s): oral mucosal lesions associated with SARS-CoV-2 infection or COVID-19 vaccines.
2.2. Search Strategy
- 1.
- Oral lesion OR oral manifestation OR oral signs
- 2.
- COVID-19 OR SARS-CoV-2 OR Coronavirus disease 2019 OR novel coronavirus
- 3.
- Pediatric OR child OR children.
2.3. Study Selection Process
2.4. Data Extraction and Collection
- -
- author, year and journal of publication, full citation of the article, study design, and funding;
- -
- population sample size, gender, mean age, comorbidities, current therapies for comorbidities, COVID-19 severity and treatment, and the time between disease onset and oral lesion occurrence;
- -
- oral lesion macroscopic and microscopic features, diagnostic procedures and treatment.
- -
- author, year and journal of publication, full citation of the article, study design and funding;
- -
- population sample size, gender, mean age, comorbidities, current therapies for comorbidities, COVID-19, COVID-19 vaccine type and the number of doses administered, and the time between vaccine administration and oral lesion occurrence;
- -
- oral lesion macroscopic and microscopic features, diagnostic procedures and treatment.
2.5. Data Synthesis
- -
- to assess the overall prevalence of oral lesions following SARS-CoV-2 infection and COVID-19 vaccination in pediatric subjects;
- -
- to rank primary oral lesions following SARS-CoV-2 infection and COVID-19 vaccination in descending order of occurrence;
- -
- to estimate the frequency and type of primary oral lesions described following SARS-CoV-2 infection in pediatric subjects in relation to age, gender, comorbidities, ongoing treatments, and COVID-19 severity and therapies of the cases;
- -
- to estimate the frequency and type of the primary oral lesions described following COVID-19 vaccination in pediatric subjects in relation to age, gender, comorbidities, ongoing treatments, and history of COVID-19, and to vaccine type and administered dose of the cases.
2.6. Quality Assessment
- -
- Low = low risk of bias for all domains;
- -
- Moderate = low or moderate risk of bias for all domains;
- -
- Serious = serious risk of bias in at least one domain, but not at critical risk of bias in any domain;
- -
- Critical = critical risk of bias in at least one domain.
3. Results
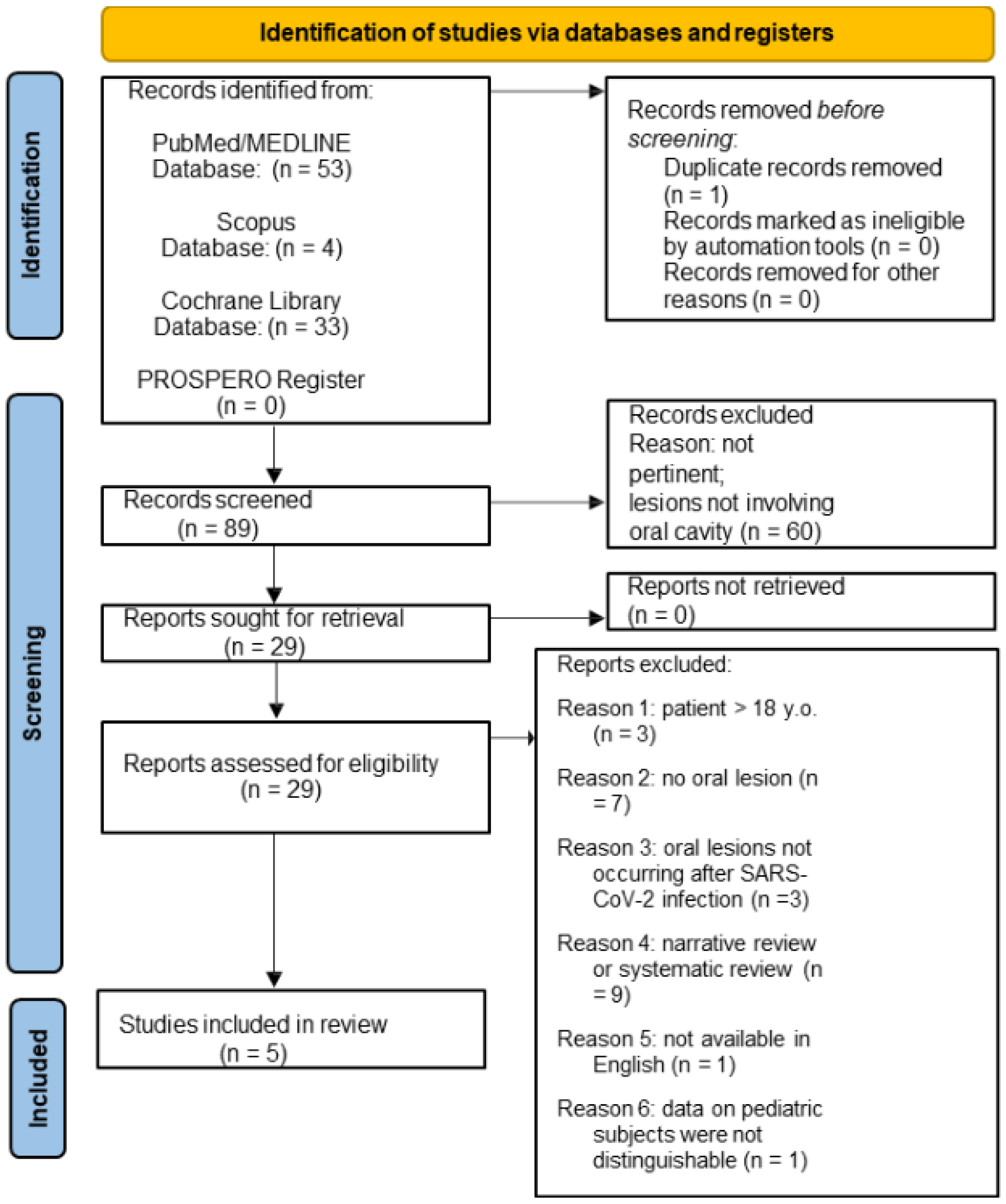
3.1. Study Selection
3.1.1. Oral Lesions in Pediatric SARS-CoV-2 Positive Subjects
3.1.2. Oral Lesions in Pediatric Subjects Following COVID-19 Vaccination
3.2. Study Characteristics and Qualitative Synthesis
3.2.1. Oral Lesions in Pediatric SARS-CoV-2 Positive Subjects
3.2.2. Oral Lesions Diagnosed in Pediatric Subjects following COVID-19 Vaccination
3.3. Quality Assessment
4. Discussion
4.1. Oral Lesions in Pediatric SARS-CoV-2 Positive Subjects
4.2. Oral Lesions Pediatric Subjects Following COVID-19 Vaccination
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, S.; Villagomez Montero, M.T.; Rowe, K.; Kirton, R.; Kunik, F., Jr. Epidemiology, pathogenesis, clinical presentations, diagnosis and treatment of COVID-19: A review of current evidence. Expert Rev. Clin. Pharmacol. 2021, 14, 601–621. [Google Scholar] [CrossRef]
- Caggiano, M.; Amato, M.; Di Spirito, F.; Galdi, M.; Sisalli, L. mRNA COVID-19: Vaccine and Oral Lichen Planus: A case report. Oral Dis. 2022. advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Štefan, M.; Dlouhý, P.; Bezdíčková, L. Vaccinationagainst COVID-19. Klin MikrobiolInfekc Lek 2021, 27, 49–60. [Google Scholar]
- Nikolopoulou, G.B.; Maltezou, H.C. COVID-19 in Children: Where do we Stand? Arch. Med. Res. 2022, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Tracker European Centre for Disease Prevention and Control (europa.eu). Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-vaccine-tracker (accessed on 2 September 2022).
- Children and COVID-19 Vaccination Trends (aap.org). Available online: https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-vaccination-trends/ (accessed on 12 August 2022).
- COVID-19 Vaccines: Key Facts. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-key-facts#vaccination-in-children-section (accessed on 13 August 2022).
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1–e34. [Google Scholar] [CrossRef]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: A key to evidence-based decisions. ACP J. Club 1995, 123, 12–13. [Google Scholar] [CrossRef]
- Data Collection form for Intervention Reviews for RCTs and non-RCTs-Template. Forms, The Cochrane Collaboration. Data Extraction. s.l.: Cochrane Developmental, Psychosocial and Learning Problems, 3 April 2014. Available online: https://view.officeapps.live.com/op/view.aspx?src=https%3A%2F%2Fdplp.cochrane.org%2Fsites%2Fdplp.cochrane.org%2Ffiles%2Fpublic%2Fuploads%2FCDPLPG%2520data%2520collection%2520form%2520for%2520intervention%2520reviews%2520for%2520RCTs%2520and%2520non-RCTs.doc&wdOrigin=BROWSELINK (accessed on 20 July 2022).
- Di Spirito, F.; Iandolo, A.; Amato, A.; Caggiano, M.; Raimondo, A.; Lembo, S.; Martina, S. Prevalence, Features and Degree of Association of Oral Lesions in COVID-19: A Systematic Review of Systematic Reviews. Int. J. Environ. Res. Public Health 2022, 19, 7486. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. Br. Med. J. 2016, 355, i4919. [Google Scholar] [CrossRef]
- Akca, U.K.; Kesici, S.; Ozsurekci, Y.; Aykan, H.H.; Batu, E.D.; Atalay, E.; Demir, S.; Sag, E.; Vuralli, D.; Bayrakci, B.; et al. Kawasaki like disease in children with COVID 19. Rheumatol. Int. 2020, 40, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Cant, A.; Bhujel, N.; Harrison, M. Oral ulceration as presenting feature of paediatric inflammatory multisystem syndrome associated with COVID-19. J. Oral Maxillofac. Surg. 2020, 58, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Jones, V.G.; Mills, M.; Suarez, D.; Hogan, C.A.; Yeh, D.; Segal, J.B.; Nguyen, E.L.; Barsh, G.R.; Maskatia, S.; Mathew, R. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp. Pediatr. 2020, 10, 537–540. [Google Scholar] [CrossRef]
- Labé, P.; Ly, A.; Sin, C.; Nasser, M.; Chapelon-Fromont, E.; Ben Saïd, P.; Mahé, E. Erythema multiforme and Kawasaki disease associated with COVID-19 infection in children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 539–541. [Google Scholar] [CrossRef]
- Al Ameer, H.H.; Alkadhem, S.M.; Busaleh, F.; AlKhwaitm, S.; Llaguno, M.B.B. Multisystem Inflammatory Syndrome in Children Temporally Related to COVID-19: A Case Report from Saudi Arabia. Cureus 2020, 12, e10589. [Google Scholar] [CrossRef]
- Bardellini, E.; Bondioni, M.P.; Amadori, F.; Veneri, F.; Lougaris, V.; Meini, A.; Plebani, A.; Majorana, A. Non-specific oral and cutaneous manifestations of Coronavirus Disease 2019 in children. Med. Oral Patol. Oral Y CirugíaBuca 2021, 26, 549–553. [Google Scholar] [CrossRef]
- Dima, M.; Enatescu, I.; Craina, M.; Petre, I.; Iacob, E.R.; Iacob, D. First neonates with severe acute respiratory syndrome coronavirus 2 infection in Romania: Three case reports. Medicine 2020, 99, e21284. [Google Scholar] [CrossRef]
- Falah, N.; Hashmi, S.; Ahmed, Z.; Jaan, A.; Akhtar, A.; Khalid, F.; Frooque, U.; Shera, M.T.; Ali, S.; Javed, A. Kawasaki Disease-Like Features in 10 Pediatric COVID-19 Cases: A Retrospective Study. Cureus 2020, 12, 11–35. [Google Scholar] [CrossRef]
- Marinescu, A.R.; Lazureanu, V.E.; Musta, V.F.; Nicolescu, N.D.; Mocanu, A.; Cut, T.G.; Muresan, C.O.; Tudoran, C.; Licker, M.; Laza, R. Severe Thrombocytopenic Purpura Associated with COVID-19 in a Pediatric Patient. Infect. Drug Resist. 2022, 15, 3405–3415. [Google Scholar] [CrossRef]
- Bogs, T.; Saleh, N.; Yavuz, S.T.; Fazeli, W.; Ganschow, R.; Schreiner, F. Aseptic Meningitis, Mucocutaneous Lesions and Arthritis after COVID-19 Vaccination in a 15-Year-Old Boy. Vaccines 2022, 10, 325. [Google Scholar] [CrossRef]
- Petruzzi, M.; Galleggiante, S.; Messina, S.; Della Vella, F. Oral erythema multiforme after Pfzer-BioNTech COVID-19 vaccination: A report of four cases. BMC Oral Health 2022, 22, 90. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Põld, A.; Kateeb, E.; Attia, S. Oral Adverse Events Following COVID-19 Vaccination: Analysis of VAERS Reports. Front. Public Health 2022, 10, 952781. [Google Scholar] [CrossRef] [PubMed]
- Furlanetto, D.L.; Crighton, A.; Topping, G.V. Differences in methodologies of measuring the prevalence of oral mucosal lesions in children and adolescents. Int. J. Paediatr. Dent. 2006, 16, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Iranmanesh, B.; Khalili, M.; Amiri, R.; Zartab, H.; Aflatoonian, M. Oral manifestations of COVID-19 disease: A review article. Dermatol. Ther. 2021, 34, e14578. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Williams, D.M. Classification and diagnostic criteria for oral lesions in HIV infection. EC-Clearinghouse on Oral Problems Related to HIV Infection and WHO Collaborating Centre on Oral Manifestations of the Immunodeficiency Virus. J. Oral Pathol. Med. 1993, 22, 289–291. [Google Scholar]
- Orilisi, G.; Mascitti, M.; Togni, L.; Monterubbianesi, R.; Tosco, V.; Vitiello, F.; Santarelli, A.; Putignano, A.; Orsini, G. Oral Manifestations of COVID-19 in Hospitalized Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 12511. [Google Scholar] [CrossRef]
- Di Spirito, F.; Pelella, S.; Argentino, S.; Sisalli, L.; Sbordone, L. Oral manifestations and the role of the oral healthcare workers in COVID-19. Oral Dis. 2022, 28, 1003–1004. [Google Scholar] [CrossRef]
- Martín Carreras-Presas, C.; Amaro Sánchez, J.; López-Sánchez, A.F.; Jané-Salas, E.; Somacarrera Pérez, M.L. Oral vesiculobullous lesions associated with SARS-CoV-2 infection. Oral Dis. 2021, 27 (Suppl. 3), 710–712. [Google Scholar] [CrossRef]
- Patton, L.L.; Phelan, J.A.; Ramos-Gomez, F.J.; Nittayananta, W.; Shiboski, C.H.; Mbuguye, T.L. Prevalence and classification of HIV-associated oral lesions. Oral Dis. 2002, 8, 98–109. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Living Guidance for Clinical Management of COVID-19: Living Guidance. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-2 (accessed on 23 November 2021).
- Di Spirito, F.; Amato, A.; Di Palo, M.P.; Contaldo, M.; D’Ambrosio, F.; Lo Giudice, R.; Amato, M. Oral Lesions Following Anti-SARS-CoV-2 Vaccination: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 10228. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, N. Reported orofacial adverse effects of COVID-19 vaccines: The knowns and the unknowns. J. Oral Pathol. Med. 2021, 50, 424–427. [Google Scholar] [CrossRef]
- Riad, A. Oral side effects of COVID-19 vaccine. Br. Dent. J. 2021, 230, 59. [Google Scholar] [CrossRef]
- Smyth, R.M.; Gargon, E.; Kirkham, J.; Cresswell, L.; Golder, S.; Smyth, R.; Williamson, P. Adverse drug reactions in children--a systematic review. PLoS ONE 2012, 7, e24061. [Google Scholar] [CrossRef] [PubMed]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Calabria, E.; Canfora, F.; Mascolo, M.; Varricchio, S.; Mignogna, M.D.; Adamo, D. Autoimmune mucocutaneous blistering diseases after SARS-CoV-2 vaccination: A Case report of Pemphigus Vulgaris and a literature review. Pathol. Res. Pract. 2022, 232, 153834. [Google Scholar] [CrossRef] [PubMed]
- Borg, L.; Mericieca, L.; Mintoff, D.; Micallef, D.; Pisani, D.; Betts, A.; Scerri, L. Pfizer-BioNTech SARS-CoV-2 mRNA vaccine-associated erythema multiforme. JEADV 2022, 36, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Hertel, M.; Schmidt-Westhausen, A.M.; Wendy, S.; Heiland, M.; Nahles, S.; Preissner, R.; Preissner, S. Onset of Oral Lichenoid Lesions and Oral Lichen Planus Following COVID-19 Vaccination: A Retrospective Analysis of about 300,000 Vaccinated Patients. Vaccines 2022, 10, 480. [Google Scholar] [CrossRef]
- Maeda, K.; Yamashita, D.; Takenobu, T. Ulcers on the bilateral palate mucosa following mRNA-based vaccination for coronavirus disease 2019 (COVID-19): A case report. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 283–286. [Google Scholar] [CrossRef]
- Saibene, A.M.; Alliata, A.; Cozzi, A.T.; Ottavi, A.; Spagnolini, S.; Pipolo, C.; Maccari, A.; Felisati, G. Erythema Multiforme Major following SARS-CoV-2 vaccine. Clin. Case Rep. 2021, 9, e04947 . [Google Scholar] [CrossRef]
- Dash, S.; Sirka, C.S.; Mishra, S.; Viswan, P. COVID-19 vaccine-induced Stevens–Johnson syndrome. Clin. Exp. Dermatol 2021, 46, 1567–1625. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Toia, M.; Stevanello, N.; Maggi, F.; Forlani, G. An episode of oral mucositis after the first administration of the ChAdOx1 COVID-19 vaccine. Oral Dis. 2021. advance online publication. [Google Scholar] [CrossRef]
- Troeltzsch, M.; Gogl, M.; Berndt, R.; Troeltzsch, M. Oral lichen planus following the administration of vector-based COVID-19 vaccine (Ad26.COV2.S). Oral Dis. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hatami, P.; Aryanian, Z.; Niknam Asl, H.; Goodarzi, A. Mucocutaneous adverse effects following COVID-19 vaccination: A case series with a comprehensive review of the literature. Iran. J. Dermatol. 2021, 24, 331–338. [Google Scholar]
- Sharda, P.; Mohta, A.; Ghiya, B.C.; Mehta, R.D. Development of oral lichen planus after COVID-19 vaccination—A rare case report. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 80–157. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, A.; Miladi, R.; Barary, M.; Shirvani, M.; Ebrahimpour, S.; Aryanian, Z.; Mohseni Afshar, Z. COVID-19 vaccine-related new-onset lichen planus. Clin. Case Rep. 2022, 10, e05323. [Google Scholar] [CrossRef]
- Colonna, C.; Restano, L.; Monzani, N.; Zussino, M.; Ponziani, A.; Cambiaghi, S.; Cavalli, R. Rare and common manifestations of COVID-19 in children. EADV Clin. Pr. 2022, 1, 21–30. [Google Scholar] [CrossRef]
- Dondi, A.; Sperti, G.; Gori, D.; Guaraldi, F.; Montalti, M.; Parini, L.; Piraccini, B.M.; Lanari, M.; Neri, I. Epidemiology and clinical evolution of non-multisystem inflammatory syndrome (MIS-C) dermatological lesions in pediatric patients affected by SARS-CoV-2 infection: A systematic review of the literature. Eur. J. Pediatr. 2022, 10, 1–17. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Barile, G.; Brienza, N.; Capodiferro, S.; Vestito, M.C.; Crudele, L.; Procacci, V.; Ingravello, G.; Maiorano, E.; et al. Covid-19 Symptomatic Patients with Oral Lesions: Clinical and Histopathological Study on 123 Cases of the University Hospital Policlinic of Bari with a Purpose of a New Classification. J. Clin. Med. 2021, 10, 757. [Google Scholar] [CrossRef]
- Di Spirito, F.; Contaldo, M.; Amato, A.; Di Palo, M.P.; Pantaleo, G.; Amato, M. COVID-19 Vaccine and Oral Lesions: Putative Pathogenic Mechanisms. Oral Dis. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Di Spirito, F.; Iacono, V.J.; Iandolo, A.; Amato, A.; Sbordone, L. Evidence-based recommendations on periodontal patients during and after the COVID-19 Era: Challenging infectious evidence-based recommendations on periodontal practice and the management of periodontal patients during and after the COVID-19 era: Challenging infectious diseases spread by air-borne transmission. Open Dent. 2021, 15, 325–336. [Google Scholar] [CrossRef]







| Study Eligibility | Inlcusion Criteria | Non-Inclusion Criteria |
|---|---|---|
| Sources | ||
| Databases | Electronic | No restrictions |
| Publication language | English language | Non English language |
| Publication date | No restrictions | No restrictions |
| Publication status | Published/In press/Ahead of print | Submitted |
| Study characteristics | ||
| Type | Clinical | In vitro/Pre-clinical in vivo |
| Design | Prospective | Systematic review |
| Retrospective | Narrative review | |
| Case-control | Conference paper | |
| Case-series and report | Oral communication | |
| Letter to the Editors | Books and chapter | |
| Study sample size | No restrictions | No restrictions |
| Population | ||
| Age | <18 years old | ≥18 years old |
| Gender | No restrictions | No restrictions |
| Characteristics and conditions | No restrictions | Pregnancy; lactation |
| Comorbidities | No restrictions | No restrictions |
| Ongoing treatment | No restrictions | No restrictions |
| History of COVID-19 | No restrictions | No restrictions |
| Intervention | ||
| Type | SARS-CoV-2 infection (confirmed) | None |
| Comparison | Oral lesions following SARS-CoV-2 infection in adult subjects | None |
| Outcomes | Oral lesions | (Likely) Pre-existing oral lesions |
| Other oro-facial manifestations | ||
| Macroscopic features | Erosions and Ulcers (Aphthous-like, Erythema Multiforme-like, Herpetiform-like) | No restrictions |
| Plaques (white and red) | ||
| Vesicles and Bullae | ||
| Maculae and Petechiae | ||
| Others | ||
| Microscopic features | Cyto/Histopathology | None |
| Study Eligibility | Inlcusion Criteria | Non-Inclusion Criteria |
|---|---|---|
| Sources | ||
| Databases | Electronic | No restrictions |
| Publication language | English language | Non English language |
| Publication date | No restrictions | No restrictions |
| Publication status | Published/In press/Ahead of print | Submitted |
| Study characteristics | ||
| Type | Clinical | In vitro/Pre-clinical in vivo |
| Design | Prospective | Systematic review |
| Retrospective | Narrative review | |
| Case-control | Conference paper | |
| Case-series and report | Oral communication | |
| Letter to the Editors | Books and chapter | |
| Study sample size | No restrictions | No restrictions |
| Population | ||
| Age | <18 years old | ≥18 years old |
| Gender | No restrictions | No restrictions |
| Characteristics and conditions | No restrictions | Pregnancy; lactation |
| Comorbidities | No restrictions | No restrictions |
| Ongoing treatment | No restrictions | No restrictions |
| History of COVID-19 | No restrictions | No restrictions |
| Intervention | ||
| Type | WHO Emergency Use Listing approved and EMA authorized COVID-19 vaccine | Other COVID-19 vaccines |
| Dosing | At least one dose | None |
| Comparison | Oral lesions following SARS-CoV-2 vaccine in adult subjects No COVID-19 vaccine | Oral lesions following other COVID-19 vaccines |
| Outcomes | Oral lesions | (Likely) Pre-existing oral lesions |
| Oral lesions in SARS-CoV-2 positive subjects | ||
| Other oro-facial manifestations | ||
| Macroscopic features | Erosions and Ulcers (Aphthous-like, Erythema Multiforme-like, Herpetiform-like) | |
| Plaques (white and red) | None | |
| Vesicles and Bullae | ||
| Maculae and Petechiae | ||
| Others | ||
| Microscopic features | Cyto/Histopathology | None |
| Studies | Population | COVID-19 | Reported Primary Oral Lesions | Oral Lesions Diagnosis, Therapy and Progression |
|---|---|---|---|---|
| Akca, 2020 Rheumatology International [15] Case series No funding | Sample size: n = 4 (n = 2 with oral lesions) Mean age: 8.5 y.o (7–10 y.o.) Gender ratio: 1M/1F Comorbidities: MD Ongoing treatments: MD | Severity 2 hospitalizations, 1 death Treatment Intubation, 2 IVIG, azithromycin, hydroxychloroquine, ritonavir, lopinavir, favipiravir, tocilizumab, anakinra, corticosteroid (20 mg/d), mesenchymal stem cell treatments, VV-ECMO, inotropic therapy Time to oral lesions onset: MD | Erosion and Ulcers n = 1 Number: N/D Distribution: N/D Location: N/D oral mucosa Cyto/Histopathology: MD Maculae and Petechiae n = 1 Erythema n = 1 Number: N/D Distribution: N/D Location: N/D oral mucosa Cyto/Histopathology: MD Others n = 1 N/D changes n = 1 Number: N/D Distribution: N/D Location: N/D oral cavity, lips Cyto/Histopathology: MD | Diagnosis Others: incomplete KD n = 1 KD n = 1 Diagnostic procedure(s) (+) 2 RT-PCR Serological exam: Mild elevation of liver transaminases Lymphopenia Thrombocytopenia2 ↑ D-dimer ↑ Lactate dehydrogenase ↑ CRP (26.4 mg/dL) ↑ ESR (96 mm/h) ↑ Brain-natruretric peptide ↑ Ferritin (1019.6 µg/L) ↑ Triglyceride Chest CT: 2 bilateral diffuse ground-glass density areas ECHO: pulmonary hypertension Therapy: MD Progression: MD |
| Al Ameer, 2020 Cureus [19] Case report No funding | Sample size: n = 1 Mean age: 13 y.o Gender ratio: 1F Comorbidities: G6PD deficiency, MIS-C mimicking Kawasaki disease Ongoing treatments: MD | Severity hospitalization and death Treatment Respiratory support with a high-flow nasal cannula with 2 L/min flow, mechanically ventilation, normal saline bolus 60 mL/kg/h, IV immunoglobulins 2 g/kg single dose, methylprednisolone 2 mg/kg/dose twice d, antibiotic, antiemetic, antiviral favipiravir, tocilizumab IL-6 inhibitor, low molecular weight heparin, milrinone, epinephrine, norepinephrine Time to oral lesions onset: MD | Maculae and Petechiae n = 1 Erythema n = 1 Number: N/D Distribution: N/D Location: lips Cyto/Histopathology: MD Others n = 1 Fissures n = 1 Number: Multiple Distribution: N/D Location: lips Cyto/Histopathology: MD | Diagnosis Others: Erythematous cracked lips n = 1 Diagnostic procedure(s) (+) RT-PCRBT measurement: 39.6 °C for five days HR: 130 bpm BP: 66/32 mmHg Abdominal ultrasound: mesenteric lymphadenitis Chest X-ray: cardiomegaly, acute respiratory distress syndrome Echocardiogram: mild mitral regurgitation, mild pericardial effusion, moderate depression in left ventricle function Serological exam: (↑) high-sensitivity troponin (0.454 ng/mL); LDH (258 units/L); Direct bilirubin (0,26 mg/dL); AST (44 units/L); PT (22 s); INR (1.40); ESR (101 mm/h); Ferritin (800 ng/mL) (↓) Ca+ 1.71 mmol/L; Na+ 125 mmol/L; K+ 3.0 mmol/L; SO4+ 0.63 mmol/L, Albumin 28.7 g/L; PCO2 34(-) CMV, EBV, HSV, blood and urine colture Therapy: MD Progression: MD |
| Bardellini, 2021 Med Oral Patol Oral CirBucal [20] Retrospective study No funding | Sample size: n = 27 (n = 15 with oral lesion) Mean age: 4.2 y.o (3 months-14 y.o.) Gender ratio: 19M/8F Comorbidities: MD Ongoing treatments: MD | Severity: MD Treatment: MD Time to oral lesions onset: MD | Others n = 15 Pseudomembranous candidiasis n = 2 (7.4%) Number: N/D Distribution: N/D Location: N/D Cyto/Histopathology: MD Geographic tongue n = 1 (3.7%) Number: N/D Distribution: N/D Location: tongue Cyto/Histopathology: MD Coated tongue n = 2 (7.4%) Number: N/D Distribution: N/D Location: tongue Cyto/Histopathology: MD Hyperaemic pharynx n = 10 (37%)Number: N/D Distribution: N/D Location: pharynx Cyto/Histopathology: MD | Diagnosis Others n = 15 Oral candidiasis n = 2 Geographic tongue n = 1 Coated tongue n = 2 Hyperaemic pharynx n = 10 Diagnostic procedure(s) 15 BT: > 38 °C 10 BT: 37.5°–38 °C 27 PCO2: > 92% Therapy: MD Progression: MD |
| Cant, 2020 British Journal of Oral and Maxillofacial Surgery [16] Case Report No funding | Sample size: n = 1 Mean age: 9 y.o Gender ratio: 1M Comorbidities: dystonia, epilepsy Ongoing treatments: MD | Severity hospitalization Treatment Topical hydrocortisone 2.5 mg tablets Time to oral lesions onset: MD | Erosion and Ulcers n = 1 Ulcers n = 1 Number: N/D Distribution: N/D Location: N/D Cyto/Histopathology: MD Others n = 1 Swollen n = 1 Number: N/D Distribution: N/D Location: lips Cyto/Histopathology: MD | Diagnosis Ulcers n = 1 Others: swollen n = 1 Diagnostic procedure(s): MD Therapy: MD Progression: MD |
| Dima, 2020 Medicine [21] Case series No funding | Sample size: n = 3 Mean age: 13.3 d (10–15 d) Gender ratio: 2M/1F Comorbidities: 1 left cryptorchidism Ongoing treatments: MD | Severity 3 hospitalizations Treatment 2 vitamin D, 3 topical cream for erythema, 1 eye drops, 1 Ampicillin (100 mg/kgc/d), 1 Gentamicin (4 mg/kgc/d), 1 human immunoglobulins, 1 aminophylline (3 × 0.3 mL/d); 1 parental nutrition Time to oral lesions onset: MD | Others n = 3 Oral candidiasis n = 3 Number: N/D Distribution: N/D Location: N/D Cyto/Histopathology: MD | Diagnosis Others: oral candidiasis n = 3 Diagnostic procedure(s) (+) RT-PCR (n = 3) Serological exam (n = 3): any relevant modification Chest X-ray (n = 3): no pathologic findings (n = 2); medium degree of hilar parenchymal infiltration and a slight infiltration of the visceral pleura (n = 1) Lung auscultation (n = 1): bilateral decreased air entry Mean (n = 3) HR (130 bmp); SaO2 (95.3%); MAP (62.6); RR (53.6); BT (37.7 °C) Therapy Nystatin (n = 3) Fluconazole iv (6 mg/kgc) (n = 1) Progression: MD |
| Falah, 2020 Cureus [22] Retrospective study No funding | Sample size: n = 10 (n = 9 with oral lesions) Mean age: 6.1 y.o (from 4 month to 11 y.o) Gender ratio: 7M/2F Comorbidities: None Ongoing treatments: MD | Severity 6 hospitalizations Treatment 8 IVIG, 6 acetylsalicylic acid, 3 oxygen, 2 steroids, 5 antibiotics, 1 tocilizumab, 1 anticoagulant, 1 antiviral therapy, 1 plasma therapy, 3 inotropic support Time to oral lesions onset: MD | Others n = 9 N/D changes n = 9 Number: N/D Distribution: N/D Location: N/D oral cavity, lips Cyto/Histopathology: MD | Diagnosis Others: KD n = 9 Diagnostic procedure(s) (+) 7 RT-PCR (+) 1 SARS-CoV-2 serology Chest CT: pulmonary opacity n = 3; cardiomegaly n = 2 Serological exam: (↑) IL-6 (n = 1); LDH (n = 2); D-dimer (n = 3); Troponin (n = 1); ESR (n = 4); Fibrinogen (n = 1); Ferritin (n = 5); Procalcitonin (n = 1); CRP (n = 9); White cell (↓) Platelets (n = 3); Hemoglobin (n = 5); Albumin (n = 6); Lymphopenia (n = 2) Therapy: MD Progression: MD |
| Jones, 2020 Hospital Pediatrics [17] Case Report No fundings | Sample size: n = 1 Mean age: 6 month-old Gender ratio: 1F Comorbidities: MD Ongoing treatments: MD | Severity hospitalization Treatment single dose of 2 g/kg IVIG, acetylsalicylic acid Time to oral lesions onset: MD | Others n = 2 Fissures n = 1 Number: N/D Distribution: N/D Location: lips Cyto/Histopathology: MD Hypertrophic papilla n = 1 Number: N/D Distribution: N/D Location: tongue Cyto/Histopathology: MD | Diagnosis Others: KD n = 1 Diagnostic procedure(s) (+) RT-PCR BT: 38.8 °C Urine culture: negative HR: 200 bpm SaO2: 100% Serological exam: normocytic anemia ↑ CRP (13.3 mg/dL); ESR (118) ↓ Na+ (133 mmol/L); Albumin (2.8 g/dL) Echocardiographic: normal Chest X-ray: opacity in the left midlung zone Therapy: MD Progression: MD |
| Labé, 2020 JEADV [18] Case series No funding | Sample size: n = 2 Mean age: 4.5 y.o (3 to 6 y.o.) Gender ratio: 2M Comorbidities: MD Ongoing treatments:1 None, 1 MD | Severity 2 hospitalizations Treatment 1 IVIG (2 g/kg) Time to oral lesions onset: MD | Erosion and Ulcers n = 1 Erosion n = 1 Number: N/D Distribution: Multiple Location: lips, gingiva Cyto/Histopathology: MD Others n = 3 Haemorrhagic crusts n = 1 Number: Multiple Distribution: N/D Location: lips Cyto/Histopathology: MD N/D changes n = 2 Number: N/D Distribution: N/D Location: N/D oral cavity, lips, tongue Cyto/Histopathology: MD | Diagnosis EM n = 1 Others: KD n = 1 Diagnostic procedure(s) (+) 1 RT-PCR 1 CT: ground-glass opacities and consolidation in the right posterobasal area Serological exam: (-) 1 Mycoplasma pneumoniae; HSV (↑) CRP (195 mg/L); Leucocytes (17.400/mm3) Therapy: MD Progression: MD |
| Marinescu, 2022 Infection and Drug Resistance [23] Case Report No funding | Sample size: n = 1 Mean age: 8 y.o Gender ratio: 1F Comorbidities: ITP Ongoing treatments: None | Severity Hospitalization Treatment IVIG 1 g/kg/d for 2 d, intravenous methylprednisolone 1 mg/kg/d Time to oral lesions onset: MD | Maculae and Petechiae n = 3 Petechiae n = 1 Number: Multiple Distribution: Bilateral asymmetrical Location: N/D oral mucosa, lips Cyto/Histopathology: MD Purpura n = 1 Number: Multiple Distribution: Bilateral asymmetrical Location: N/D oral mucosa, lips Cyto/Histopathology: MD Ecchymoses n = 1 Number: Multiple Distribution: Bilateral asymmetrical Location: N/D oral mucosa, lips Cyto/Histopathology: MD | Diagnosis ITP n = 1 Diagnostic procedure(s) (+) RT-PCR BT: 36.4 °C HR: 90 bpm BP: 115/70 mmHg RR: 19 SaO2: 100% Serological exam: Severe thrombocytopenia (0 × 103 μ/L) (↓) Leukopenia (2500 μ/L) (↑) ALT (233.3 U/L); AST (108.7 U/L); Creatinine; CRP; Ferritin; D-dimer (-) EBV; HAV; HBV; HCV; VZV; CMV; HIV; HSV; parvovirus B19; seasonal influenza; adenovirus; Escherichia coli; Bordetella pertussis; Chlamyda pneumoniae; Mycoplasma pneumoniae; Staphylococcus; Streptococcus Chest X-ray, Abdominal Ultrasound, Doppler sonography, echocardiography: normal Therapy: MD Progression: MD |
| Studies | Population | COVID-19 Vaccine | Reported Primary Oral Lesions | Oral Lesions Diagnosis, Therapy and Progression |
|---|---|---|---|---|
| Bogs, 2022 Vaccines [24] Case report No funding | Sample size: n = 1 Mean age: 15 y.o Gender ratio: 1M Comorbidities: aseptic meningitis Ongoing treatments: MD History of COVID-19: No | Vaccine type COVID-19 mRNA BNT162b2 Comirnaty (Pfizer-BioNTech) Vaccine dose n = 2nd Time to oral lesions onset: 13d | Erosion and Ulcers n = 1 Herp. n = 1 Number: Multiple Distribution: Bilateral asymmetrical Location: lips; cheeks Cyto/Histopathology: MD | Diagnosis Others:Behçet’s disease n = 1 Diagnostic procedure(s) Serological exam: (↑) Mild leukocytosis (10,920/µL) with predominant neutrophils (8980/µL); C-reactive protein (5.34 mg/dL); IgA and IgM CSF/serum (-) rheumatoid factor; ANA; pANCA; cANCA; anti-dsDNA; HLA-B51 CSF analysis: Pleocytosis of 242/µL white blood cells consisting of both granulocytes (109/µL) and lymphomononuclear cells (133/µL) Liquor: lactate (2.92 mmol/L); glucose (65 mg/dL); proteins (69.4 mg/dL) (-) Bacterial cultures: 16S ribosomal DNA-PCR Virological PCR analyses for: HSV-1/2; HHV-6/7; parvovirus B19; EBV; VZV; CMV; entero-; parecho-; adeno-; noro-; measles; mump; FSME-; B. burgdorferi. (-) PCR and serological test for SARS-CoV-2 (-) DISQVER® Ultrasound: echo-free joint effusion OCT: inhomogeneities in the posterior vitreous Cranial MRI: slight pachy and leptomeningeal enhancement Therapy Ibuprofen Cefotaxime for 8 d Aciclovir for 2 d Progression: MD |
| Petruzzi, 2022 BMC Oral Health [25] Case series No funding | Sample size: n = 4 (pediatric subjects n = 1) Mean age: 15 y.o Gender ratio: 1M Comorbidities: West Syndrome Ongoing treatments: Valproate History of COVID-19: MD | Vaccine type COVID-19 mRNA BNT162b2 Comirnaty (Pfizer-BioNTech) Vaccine dose n = 1st Time to oral lesions onset: 7 d | Erosion and Ulcers n = 1 Maculae and Petechiae n = 1 Erythema n = 1 Number: Multiple Distribution: N/D Location: N/D oral mucosa; lips; vermilion Cyto/Histopathology: MD Others n= 2 Squamous crusted n = 1 Number: Multiple Distribution: N/D Location: lips; vermilion Cyto/Histopathology: MD Pseudo-membranes n = 1 Number: Multiple Distribution: N/D Location: N/D oral mucosa Cyto/Histopathology: MD | Diagnosis EM n = 1 Diagnostic procedure(s): MD Therapy 4 mg of betamethasone and antihistamine medication Prednisone 25 mg/die tapering the dosage, in association to topical 0.05% clobetasol propionate ointment Progression After 7d of treatment regression of mucosal lesions |
| Studies | Confounding | Selection of Participants | Measurement Classification of Interventions | Deviations from Intended Intervention | Missing Data | Measurement of Outcomes | Selection of the Reported Results |
|---|---|---|---|---|---|---|---|
| Akca, 2020 [15] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/ PY/PN/ N/NI | Y/ PY/PN/ N/NI |
| Al Ameer, 2020 [19] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI |
| Bardellini, 2021 [20] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI |
| Bogs, 2020 [24] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI |
| Cant, 2020 [16] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI |
| Dima, 2020 [21] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI |
| Falah, 2020 [22] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI |
| Jones, 2020 [17] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI |
| Labé, 2020 [18] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI |
| Marinescu, 2022 [23] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI |
| Petruzzi, 2022 [25] | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI | Y/PY/ PN/ N/NI | Y/PY/PN/ N/NI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Spirito, F.; Caggiano, M.; Di Palo, M.P.; Contaldo, M.; D’Ambrosio, F.; Martina, S.; Amato, A. Oral Lesions in Pediatric Subjects: SARS-CoV-2 Infection and COVID-19 Vaccination. Appl. Sci. 2022, 12, 8995. https://doi.org/10.3390/app12188995
Di Spirito F, Caggiano M, Di Palo MP, Contaldo M, D’Ambrosio F, Martina S, Amato A. Oral Lesions in Pediatric Subjects: SARS-CoV-2 Infection and COVID-19 Vaccination. Applied Sciences. 2022; 12(18):8995. https://doi.org/10.3390/app12188995
Chicago/Turabian StyleDi Spirito, Federica, Mario Caggiano, Maria Pia Di Palo, Maria Contaldo, Francesco D’Ambrosio, Stefano Martina, and Alessandra Amato. 2022. "Oral Lesions in Pediatric Subjects: SARS-CoV-2 Infection and COVID-19 Vaccination" Applied Sciences 12, no. 18: 8995. https://doi.org/10.3390/app12188995
APA StyleDi Spirito, F., Caggiano, M., Di Palo, M. P., Contaldo, M., D’Ambrosio, F., Martina, S., & Amato, A. (2022). Oral Lesions in Pediatric Subjects: SARS-CoV-2 Infection and COVID-19 Vaccination. Applied Sciences, 12(18), 8995. https://doi.org/10.3390/app12188995











