A Machine Learning Approach to Support Treatment Identification for Chiari I Malformation
Abstract
:1. Introduction
- CM type I (CM-I): herniation of one or both cerebellar tonsils at least 5 mm below the foramen magnum, often asymptomatic. Hydrocephalus and anterior flattening of the midbrain, pons and medulla oblongata may occur. It is further sub-classified into CM I-A, when associated with syringomyelia, and CM I-B, when no syringomyelic cavity is present [6].
- CM type II (CM-II): caudal migration of the brainstem, cerebellum and fourth ventricle through the foramen magnum together with downward displacement of the cervical spinal cord. It is always associated with open spinal dysraphism or cystic spina bifida (myelomeningocele or myelocele) and also with syringomyelia and hydrocephalus.
- CM type 0 (CM-0): syringomyelia with no associated tonsilla rhombencephalic herniation or minimal (less than 3 mm long).
- CM type 1.5 or “Chiari Complex” (CM 1.5): tonsillar prolapse and brainstem kinking in the context of CVJ malformation. Although CM-I and CM 1.5 share morphological and anatomical similarities, an accurate radiological distinction needs to be made because patients with CM 1.5 have more probability to undergo initial decompressive surgery failure and there is usually persistence of syringomyelia [4].
2. Methods
2.1. Database
2.1.1. Inclusion Criteria
- CM-I patients with or without syringomyelia and undergoing posterior cranial fossa decompression surgery;
- cerebellar tonsil ≥5 mm below McRae’s line (basion-to-opisthion) on T1-weighted sagittal image and at least one of the following symptoms: posterior headache worsened with the Valsalva manoeuvre, mixed cranial nerve disorders (dysphagia, dysphonia, hiccups), cranial oto-vestibular disorders (dizziness, tinnitus), long way disorders (motor and sensory problems), cerebellar signs (ataxia, dysmetria, tremors), sphincter disorders and scoliosis;
- patients undergoing osteo-ligamentous decompression surgery or posterior fossa decompression with dural plastic with or without tonsillar coarctation;
- period of surgery between January 2010 and December 2020;
- presence of clinical and instrumental follow-up data at 1 year;
- signature of informed consent to the surgery and use of clinical data for research purposes.
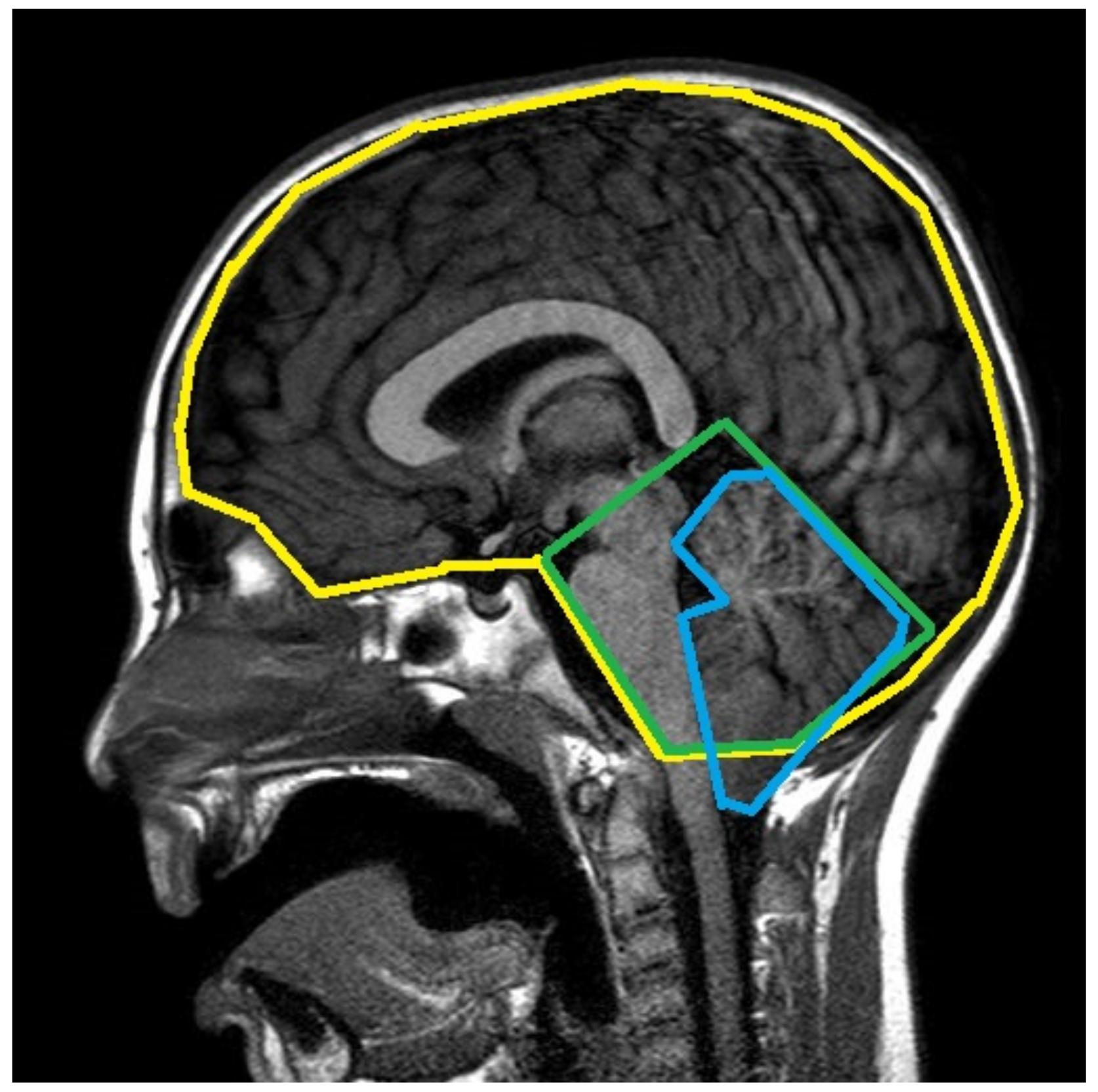
2.1.2. Clinical and Radiological Data
- cerebellar tonsil descent (length of hernia—LenH);
- length of the tentorium (LenT);
- angle of the tentorium (AngT);
- ratio of cerebellum area to posterior cranial fossa area (C/PF);
- ratio of posterior cranial fossa area to brain area (PF/B);
- antero-posterior diameter of posterior fossa (DiaAntP);
- height of posterior cranial fossa (H_PF);
- length of the clivus (LenC);
- length of the foramen magnum (LenFM);
- distance between corpus callosum and foramen magnum (DCCFM—only measured manually);
- distance between pons and foramen magnum (DPoFM—only measured manually);
- distance between fastigium and foramen magnum (DFFM—only measured manually).
2.1.3. Properties of Recruited Patients
2.1.4. Different Groups of Patients Considered
- Group 0 includes symptomatic patients without syringomyelia at diagnosis who, following surgery, had a clinical and radiological improvement within the first year, characterized by a reduction in symptoms and tonsillar hernia (19 patients, 32.7%).
- Group 1 is constituted by symptomatic patients with syringomyelia at diagnosis who, following surgery, had clinical and radiological improvement within the first year, with a reduction of the syringomyelic cavity (17 patients, 29.3%).
- Group 2 is formed by symptomatic patients with syringomyelia at diagnosis who, after surgery, had a clinical and radiological worsening, either with an increase in the size of the syringomyelic cavity or with persistence of symptoms (13 patients, 22.4%).
- Group 3 is the group of symptomatic patients without syringomyelia at diagnosis who, following decompression surgery, developed syringomyelia within the first year (9 patients, 15.6%).
2.2. Registration
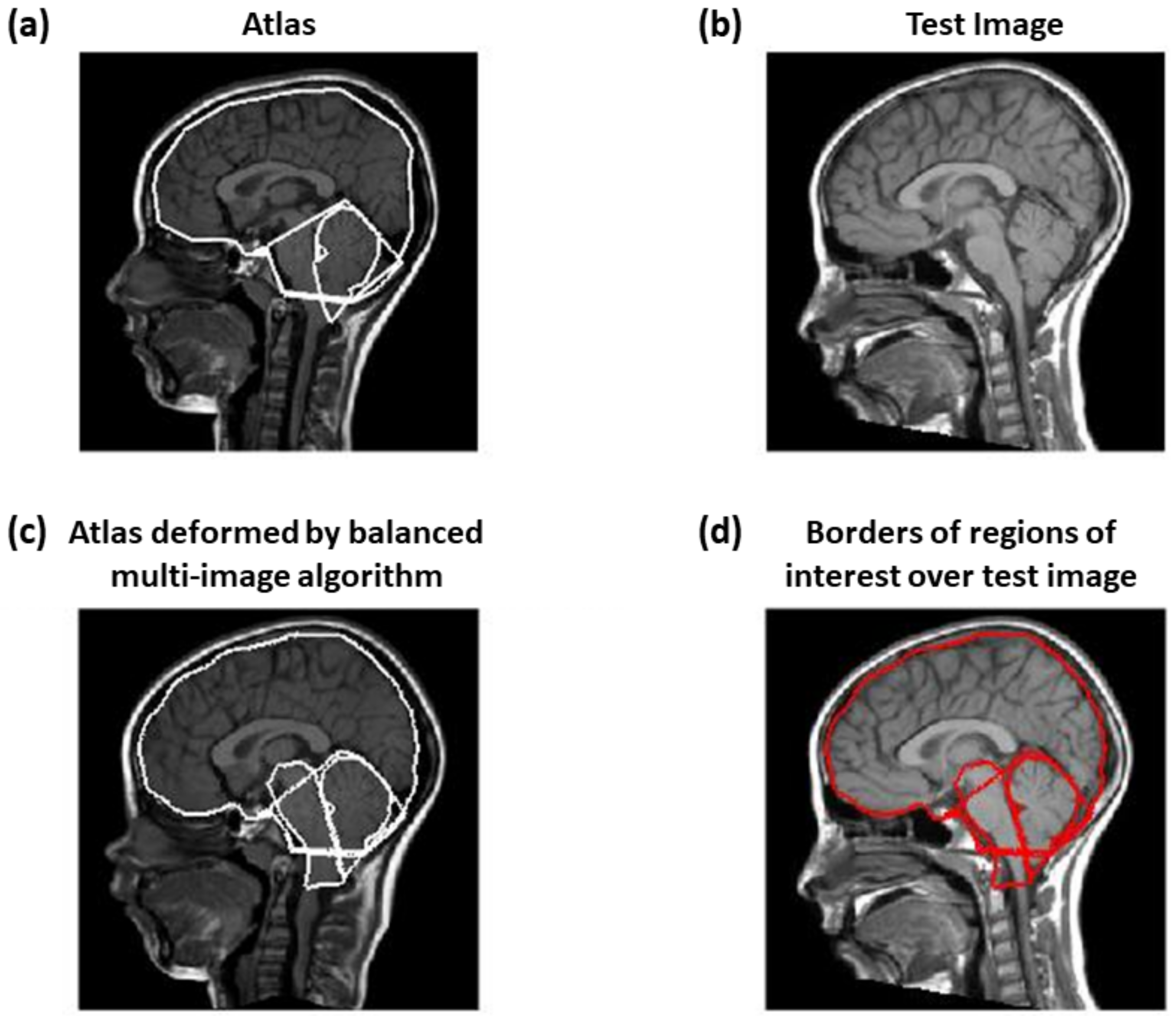
2.2.1. Balanced Multi-Image Demons
- Different images from the atlas and the test MRI were matched by a single optimal deformation, found using the following update for the linearized problem mentioned abovewhere and are N images obtained from the atlas and test image (as detailed below), is the Jacobian matrix of and .
- The iterations were performed in alternation to the atlas and to the test sets of images ( and , respectively).
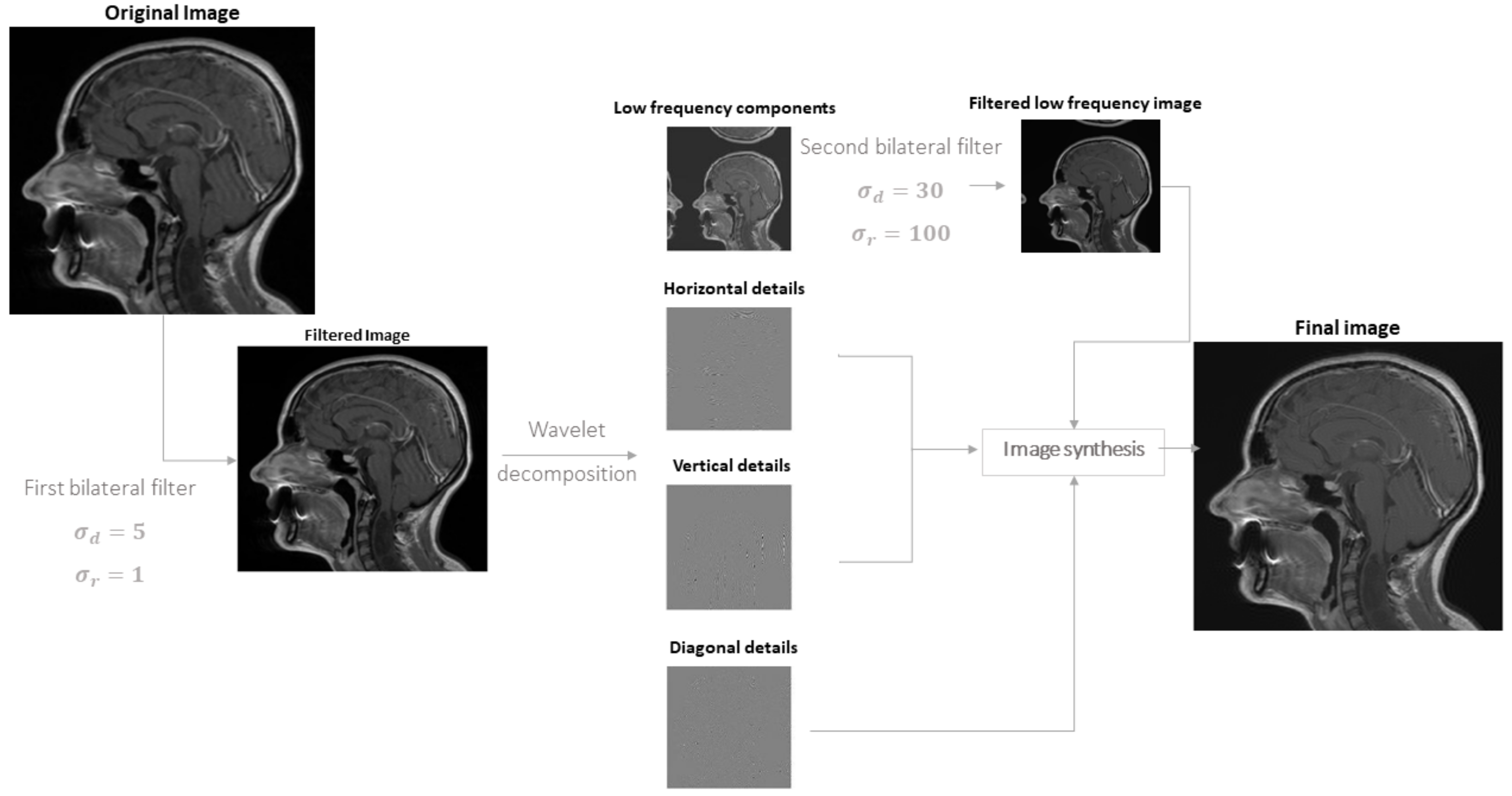
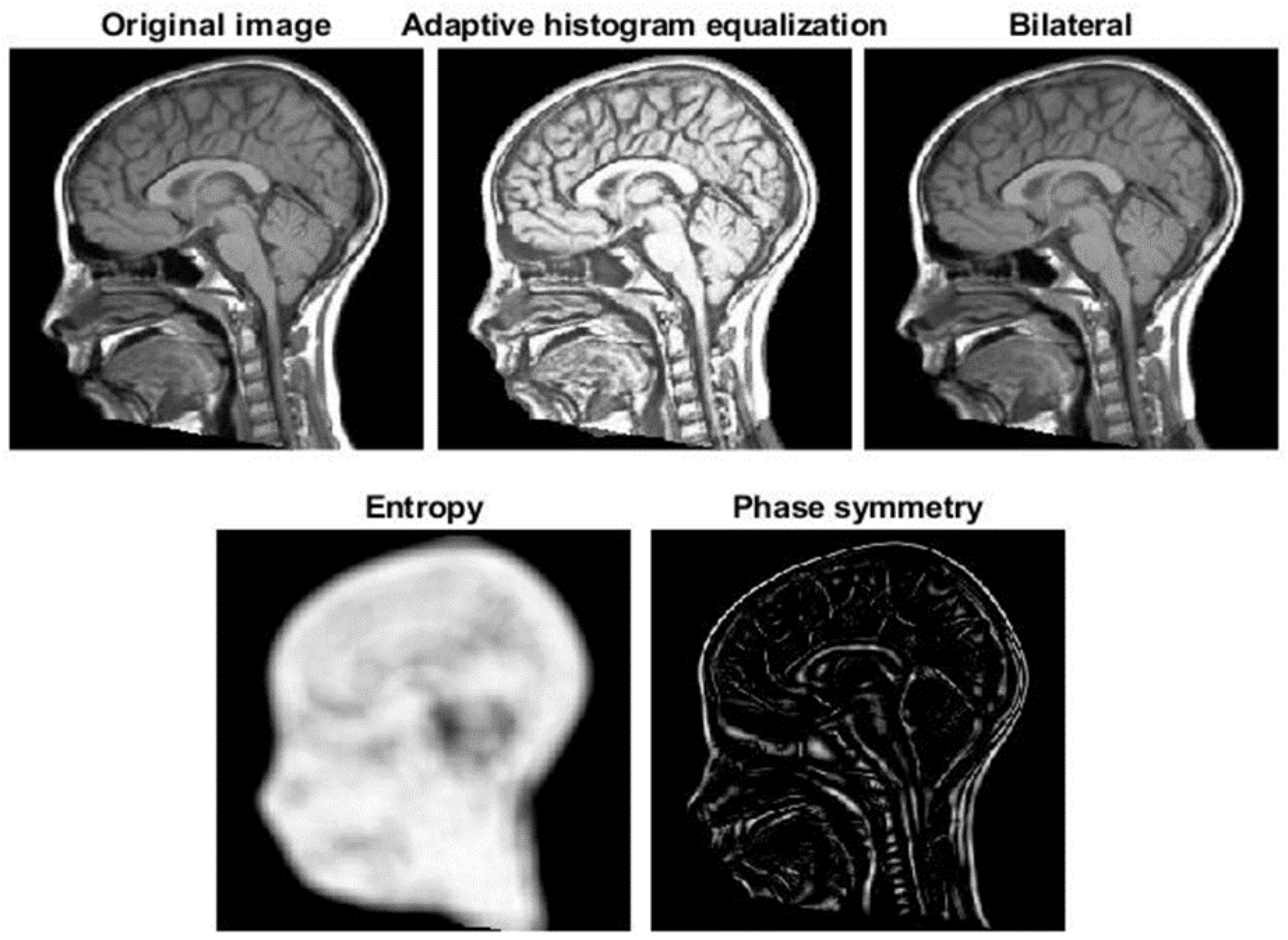
2.2.2. Correction by Active Contour
- is the internal energy, defined aswhere and are the first and second derivatives of , respectively; = 0.05 controls the tension and = 0.5 is related to the rigidity of the active contour.
- is the energy due to the image and is the weighted sum of 3 terms, allowing the snake to be attracted on dark regions, edges and termination points, respectivelywhere is defined by a Gaussian smoothing of the original image (with standard deviation 3 pixels), is the norm of the image (again with Gaussian smoothing on the derivatives) and depends on the curvature of level lines [40] and = 5, = 20, = 10.Then we included also the Gradient Vector Flow (GVF) [41], which is the vector field minimizing the energy functionalwhere is the edge map and = 0.2; notice that it is close to the gradient of f (and thus it forces the snake to go in the direction opposite to the gradient of the MRI) when such a gradient is large, otherwise a smooth field is obtained. This contribution allows us to help the snake to follow the borders of concave regions [41].
- are external forces, pushing the snake toward low intensity edge points in the image. It is defined as the sum of two terms: the distance transform from low intensity points (identified by image binarization with a two-class k-means clustering, as in [30]) and the balloon force, in direction normal to the snake and allowing it to contract when the other contributions are small [42]; the two contributions had weights and , respectively.
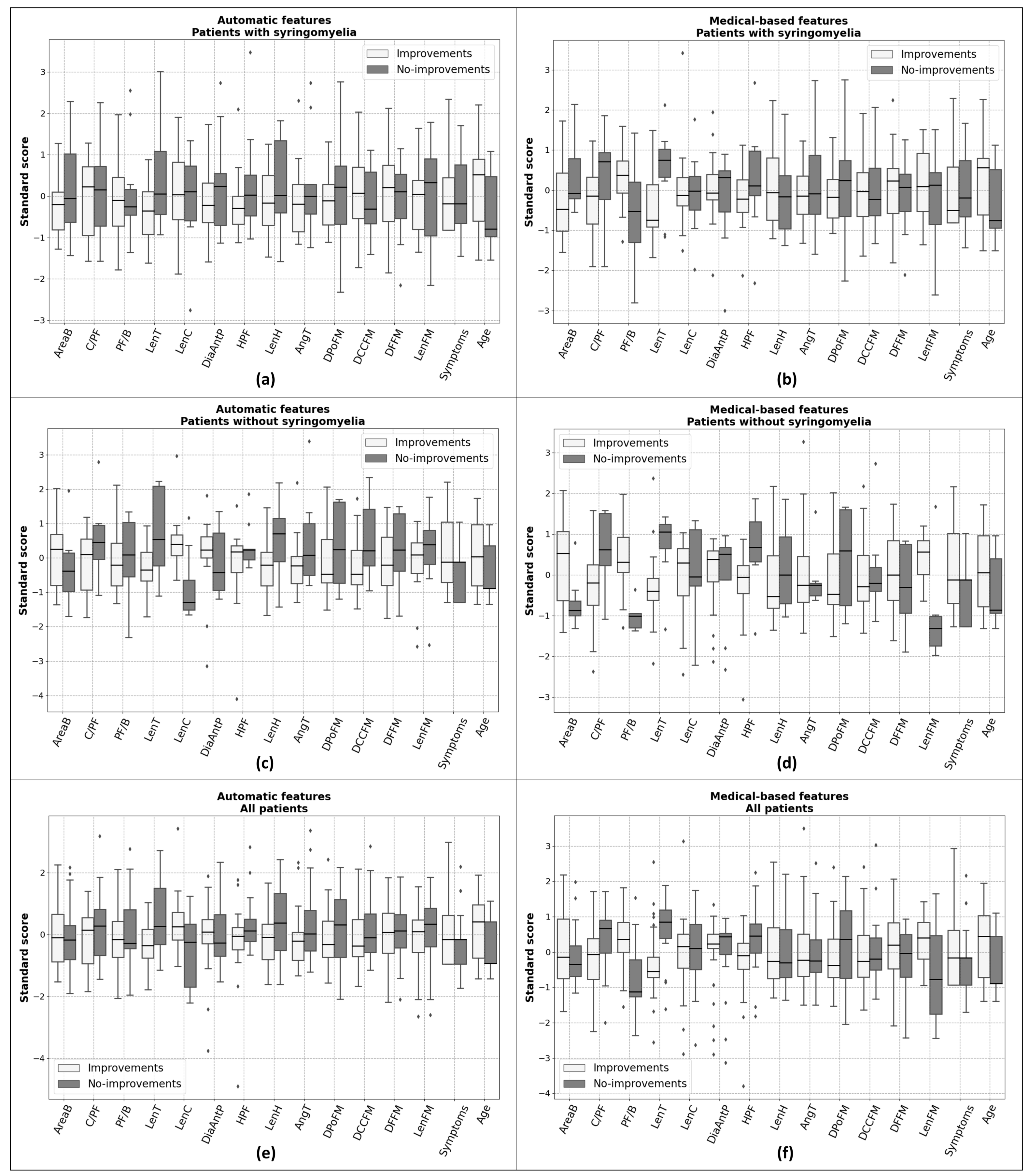
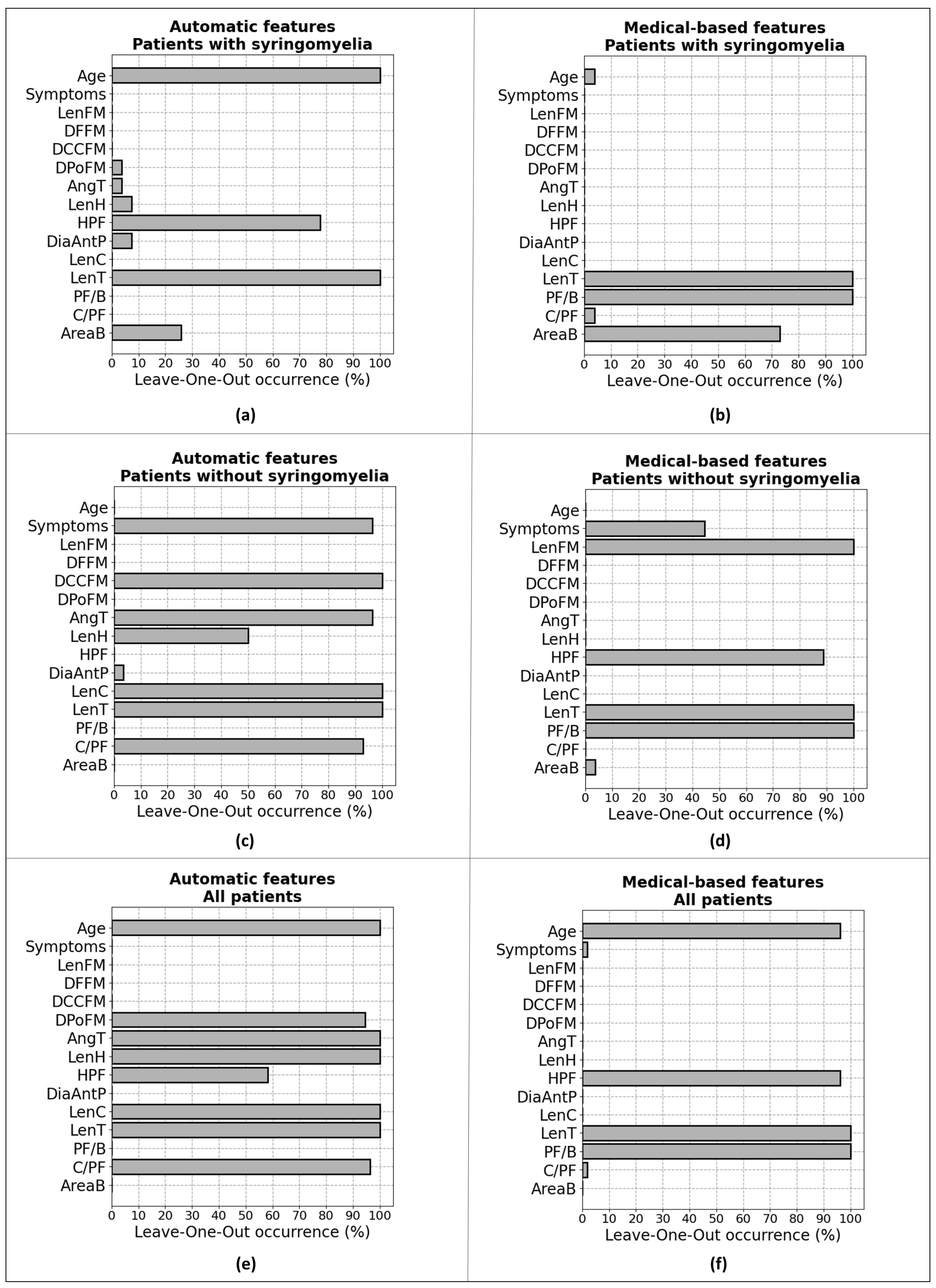
2.3. Patients’ Group Identification
2.3.1. Investigation of the Relevance of Features
- nuchal headache;
- mixed cranial nerve disorders (dysphagia, dysphonia, hiccups);
- disorders of the oto-vestibular cranial nerves (vertigo, tinnitus);
- motor/sensory problems;
- cerebellar signs (ataxia, dysmetria, tremors);
- sphincter disorders;
- scoliosis;
- other symptoms.
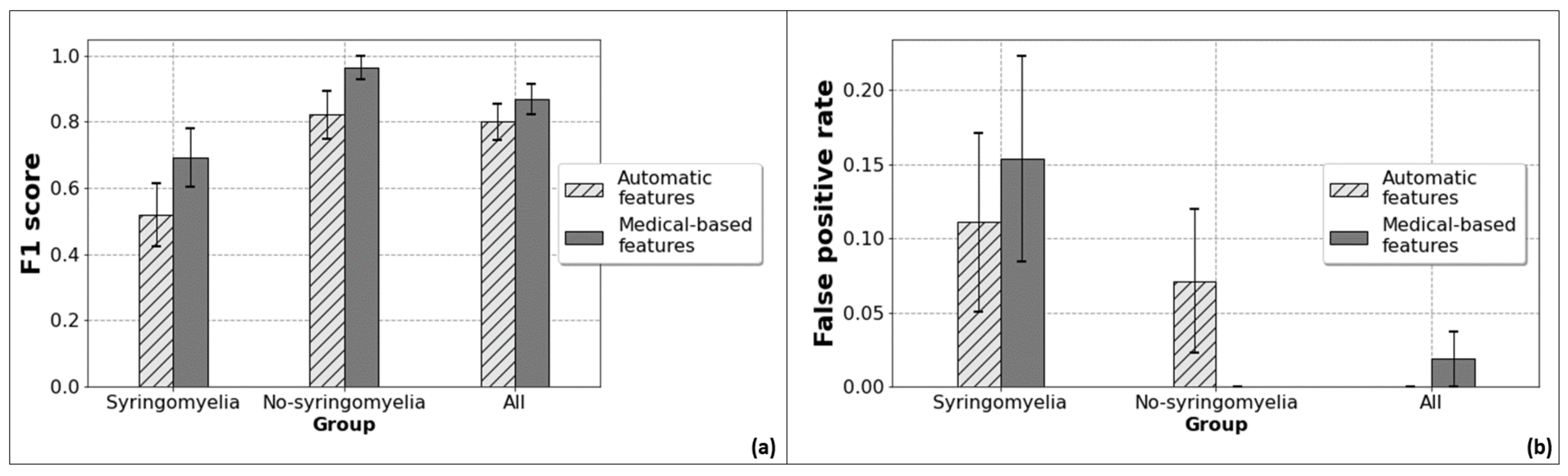
2.3.2. Binary Classification
- features automatically obtained from the patients who have syringomyelia before treatment;
- features measured automatically from the patients who did not show syringomyelia;
- features estimated automatically, including all the patients;
- manual measurements from patients with syringomyelia;
- features measured manually on patients without syringomyelia;
- manual measurements of all patients.
3. Results
4. Discussion
5. Conclusions and Further Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AngT | angle of the tentorium |
| C/PF | ratio of cerebellum area to posterior cranial fossa area |
| CVJ | craniocervical junction |
| CM-I | Chiari Malformation Type I |
| CSF | cerebro-spinal fluid |
| DiaAntP | antero-posterior diameter of posterior fossa |
| DCCFM | distance between corpus callosum and foramen magnum |
| DFFM | distance between fastigium and foramen magnum |
| DPoFM | distance between pons and foramen magnum |
| GVF | Gradient Vector Flow |
| H_PF | height of posterior cranial fossa |
| LASSO | least absolute shrinkage and selection operator |
| LenC | length of the clivus |
| LenFM | length of the foramen magnum |
| LenH | length of hernia |
| LenT | length of tentorium |
| LOO | leave-one-out |
| MRI | magnetic resonance imaging |
| PF/B | Ratio of posterior cranial fossa area to brain area |
| REDCap | Research Electronic Data Capture |
References
- Massimi, L.; Peretta, P.; Erbetta, A.; Solari, A.; Farinotti, M.; Ciaramitaro, P.; Saletti, V.; Caldarelli, M.; Canheu, A.C.; Celada, C.; et al. Diagnosis and treatment of Chiari malformation type 1 in children: The International Consensus Document. Neurol. Sci. 2022, 43, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Dhar, A.; Elsanafiry, M.S.M.; Goel, A. Chiari malformation: Has the dilemma ended? J. Craniovertebr. Junction Spine 2017, 8, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Gad, K.A.; Yousem, D.M. Syringohydromyelia in Patients with Chiari I Malformation: A Retrospective Analysis. Am. J. Neuroradiol. 2017, 38, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Bordes, S.; Jenkins, S.; Tubbs, R.S. Defining, diagnosing, clarifying, and classifying the Chiari I malformations. Child’s Nerv. Syst. 2019, 35, 1785–1792. [Google Scholar] [CrossRef]
- Curone, M.; Valentini, L.G.; Vetrano, I.; Beretta, E.; Furlanetto, M.; Chiapparini, L.; Erbetta, A.; Bussone, G. Chiari malformation type 1-related headache: The importance of a multidisciplinary study. Neurol. Sci. 2017, 38, 91–93. [Google Scholar] [CrossRef]
- Hiremath, S.B.; Fitsiori, A.; Boto, J.; Torres, C.; Zakhari, N.; Dietemann, J.L.; Meling, T.R.; Vargas, M.I. The Perplexity Surrounding Chiari Malformations—Are We Any Wiser Now? AJNR Am. J. Neuroradiol. 2020, 41, 1975–1981. [Google Scholar] [CrossRef]
- Fric, R.; Ringstad, G.; Eide, P.K. Chiari-malformasjon type 1—Diagnostikk og behandling. Tidsskr. Den Nor. Legeforening 2019, 139. [Google Scholar] [CrossRef]
- Hansberry, D.; Agarwal, N.; Tomei, K.; Goldstein, I. Posterior reversible encephalopathy syndrome in a patient with a Chiari I malformation. Surg. Neurol. Int. 2013, 4, 130. [Google Scholar]
- Markunas, C.A.; Tubbs, R.S.; Moftakhar, R.; Ashley-Koch, A.E.; Gregory, S.G.; Oakes, W.J.; Speer, M.C.; Iskandar, B.J. Clinical, radiological, and genetic similarities between patients with Chiari Type I and Type 0 malformations. J. Neurosurg. Pediatr. 2012, 9, 372–378. [Google Scholar] [CrossRef]
- Urbizu, A.; Toma, C.; Poca, M.A.; Sahuquillo, J.; Cuenca-León, E.; Cormand, B.; Macaya, A. Chiari Malformation Type I: A Case-Control Association Study of 58 Developmental Genes. PLoS ONE 2013, 8, e57241. [Google Scholar] [CrossRef]
- Chatrath, A.; Marino, A.; Taylor, D.; Elsarrag, M.; Soldozy, S.; Jane, J.A. Chiari I malformation in children-the natural history. Child’s Nerv. Syst. 2019, 35, 1793–1799. [Google Scholar] [CrossRef]
- Balasa, A.; Kunert, P.; Dziedzic, T.; Bieleci, M.; Kujawski, S.; Marchel, A. Comparison of dural grafts and methods of graft fixation in Chiari malformation type I decompression surgery. Sci. Rep. 2021, 11, 14801. [Google Scholar] [CrossRef]
- Genitori, L.; Peretta, P.; Nurisso, C. Chiari type I anomalies in children and adolescents: Minimally invasive management in a series of 53 cases. Child’s Nerv. Syst. 2000, 16, 707–718. [Google Scholar] [CrossRef]
- Caldarelli, M.; Novegno, F.; Vassimi, L. The role of limited posterior fossa craniectomy in the surgical treatment of Chiari malformation type I: Experience with a pediatric series. J. Neurosurg. 2007, 106, 187–195. [Google Scholar] [CrossRef]
- Kennedy, B.C.; Kelly, K.M.; Phan, M.Q. Outcomes after suboccipital decompression without dural opening in children with Chiari malformation type I. J. Neurosurg. Ped. 2015, 16, 150–158. [Google Scholar] [CrossRef]
- Limonadi, F.M.; Selden, N.R. Dura-splitting of the craniocervical junction: Reduced operative time, hospital stay, and cost with equivalent early outcome. J. Neurosurg. 2004, 101 (Suppl. 2), 184–188. [Google Scholar] [CrossRef]
- Siasios, J.; Kapsalaki, E.Z.; Fountas, K.N. Surgical management of patients with Chiari I malformation. Int. J. Ped. 2012, 2012, 640127. [Google Scholar] [CrossRef]
- Baisden, J. Controversies in Chiari I malformations. Surg Neurol. Int. 2012, 3 (Suppl. 3), S232–S237. [Google Scholar] [CrossRef]
- Urbizu, A.; Poca, M.A.; Vidal, X.; Rovira, A.; Sahuquillo, J.; Macaya, A. MRI-based morphometric analysis of posterior cranial fossa in the diagnosis of Chiari malformation type I. J. Neuroimaging 2014, 24, 250–256. [Google Scholar] [CrossRef]
- Bagci, A.M.; Lee, S.H.; Nagornaya, N. Automated posterior cranial fossa volumetry by MRI: Applications to Chiari malformation type I. AJNR Am. J. Neuroradiol. 2013, 34, 1758–1763. [Google Scholar] [CrossRef]
- Furtado, S.V.; Reddy, K.; Hedge, A.S. Posterior fossa morphometry in symptomatic pediatric and adult Chiari I malformation. J. Clin. Neurosci. 2009, 16, 1449–1454. [Google Scholar] [CrossRef]
- Nishikawa, M.; Sakamoto, H.; Hakuba, A. Pathogenesis of Chiari malformation: A morphometric study of the posterior cranial fossa. J. Neurosurg. 1997, 86, 40–47. [Google Scholar] [CrossRef]
- Trigylidas, T.; Baronia, B.; Vassilyadi, M. Posterior fossa dimension and volume estimates in pediatric patients with Chiari I malformation. Child’s Nerv. Syst. 2008, 24, 329–336. [Google Scholar] [CrossRef]
- Despotovic, I.; Goossens, B.; Philips, W. MRI Segmentation of the Human Brain: Challenges, Methods, and Applications. Comput. Math. Methods Med. 2015, 2015, 450341. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, L.; Nie, D.; Cao, X.; Rekik, I.; Lee, S.; He, H.; Shen, D. Automatic brain labeling via multi-atlas guided fully convolutional networks. Med. Image Anal. 2019, 51, 157–168. [Google Scholar] [CrossRef]
- Tetik, B.; Mert Doğan, G.; Paşahan, R.; Durak, M.A.; Güldoğan, E.; Saraç, K.; Önal, Ç.; Yıldırım, İ.O. Multi-parameter-based radiological diagnosis of Chiari Malformation using Machine Learning Technology. Int. J. Clin. Pract. 2021, 75, e14746. [Google Scholar] [CrossRef]
- Tanaka, K.W.; Russo, C.; Liu, S.; Stoodley, M.A.; Di Ieva, A. Use of deep learning in the MRI diagnosis of Chiari malformation type I. Neuroradiology 2022, 64, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Urbizu, A.; Martin, B.A.; Moncho, D.; Rovira, A.; Poca, M.A.; Sahuquillo, J.; Macaya, A.; Español, M.I. Machine learning applied to neuroimaging for diagnosis of adult classic Chiari malformation: Role of the basion as a key morphometric indicator. J. Neurosurg. 2018, 129, 779–791. [Google Scholar] [CrossRef]
- Mesin, L.; Mokabberi, F.; Carlino, C.F. Identification of optimal surgical intervention for Chiari I malformation. In Proceedings of the IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB), Siena, Italy, 9–11 July 2019; pp. 1–5. [Google Scholar]
- Mesin, L.; Mokabberi, F.; Carlino, C.F. Automated Morphological Measurements of Brain Structures and Identification of Optimal Surgical Intervention for Chiari I Malformation. IEEE J. Biomed. Health Inform. 2020, 24, 3144–3153. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harris, P.A.; Taylor, R.; Minor, B.L.; Elliott, V.; Fernandez, M.; O’Neal, L.; McLeod, L.; Delacqua, G.; Delacqua, F.; Kirby, J.; et al. REDCap Consortium, The REDCap consortium: Building an international community of software partners. J. Biomed. Inform. 2019, 95, 103208. [Google Scholar] [CrossRef] [PubMed]
- Mesin, L. Balanced multi-image demons for non-rigid registration of magnetic resonance images. Magn. Reson. Imaging 2020, 74, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Sotiras, A.; Davatzikos, C.; Paragios, N. Deformable medical image registration: A survey. IEEE Trans. Med. Imaging 2013, 32, 1153–1190. [Google Scholar] [CrossRef] [PubMed]
- Thirion, J.P. Image matching as a diffusion process: An analogy with Maxwell’s demons. Med. Image Anal. 1998, 2, 243–260. [Google Scholar] [CrossRef]
- Vercauteren, T.; Pennec, X.; Perchant, A.; Ayache, N. Diffeomorphic demons: Efficient non-parametric image registration. NeuroImage 2009, 45, 61–72. [Google Scholar] [CrossRef]
- Tomasi, C.; Manduchi, R. Bilateral filtering for gray and color images. In Proceedings of the Sixth International Conference on Computer Vision (IEEE Cat. No.98CH36271), Bombay, India, 7 January 1998; pp. 839–846. [Google Scholar]
- Mishro, P.K.; Agrawal, S.; Panda, R.; Hansdah, K.T. MR Image Enhancement using Stationary Wavelet Transform based Approach. In Proceedings of the 11th International Conference on Computing, Communication and Networking Technologies (ICCCNT), Kharagpur, India, 1–3 July 2020; pp. 1–6. [Google Scholar]
- Kovesi, P. Symmetry and Asymmetry from Local Phase. In Proceedings of the Tenth Australian Joint Converence on Artificial Intelligence, Perth, Australia, 30 November–4 December 1997; pp. 2–4. [Google Scholar]
- Kass, M.; Witkin, A.; Terzopoulos, D. Snakes: Active contour models. Int. J. Comput. Vis. 1988, 1, 321–331. [Google Scholar] [CrossRef]
- Xu, C.; Prince, J.L. Gradient vector flow: A new external force for snakes. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition, San Juan, Puerto Rico, 17–19 June 1997; pp. 66–71. [Google Scholar]
- Cohen, L.D. Note: On active contour models and balloons. CVGIP (Image Underst.) 1991, 53, 211–218. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection via the lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Baraniuk, R.G. Compressive sensing [lecture notes]. IEEE Signal Process. Mag. 2007, 24, 118–121. [Google Scholar] [CrossRef]
- Cortes, C.; Vapnik, V.N. Support-Vector Networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Kalb, S.; Perez-Orribo, L.; Mahan, M.; Theodore, N.; Nakaji, P.; Bristol, R.E. Evaluation of operative procedures for symptomatic outcome after decompression surgery for Chiari type I malformation. J. Clin. Neur. 2012, 19, 1268–1272. [Google Scholar] [CrossRef]
- Shenoy, V.S.; Sampath, R. Syringomyelia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Massimi, L.; Frassanito, P.; Bianchi, F.; Tamburrini, G.; Caldarelli, M. Bony decompression vs duraplasty for Chiari I malformation: Does the eternal dilemma matter? Child’s Nerv. Syst. 2019, 35, 1827–1838. [Google Scholar] [CrossRef]
| Measurement | Manual | Automatic | Difference | Paired Difference |
|---|---|---|---|---|
| Brain Area | 15,990 ± 1523 | 15,632 ± 1251 | 0.4% (p = 0.27) | 6.5% (p < 0.05) |
| PF Area | 3343 ± 702 | 3003 ± 415 | 16.6% (p < 0.05) | 20.7% (p < 0.05) |
| Cerebellum Area | 2043 ± 392 | 1895 ± 363 | 7.0% (p < 0.05) | 16.4% (p < 0.05) |
| C/PF | 0.62 ± 0.11 | 0.63 ± 0.06 | −0.7% (p = 0.92) | 12.9% (p = 0.49) |
| PF/B | 0.21 ± 0.03 | 0.19 ± 0.02 | 12.4% (p < 0.05) | 16.7% (p < 0.05) |
| LenT | 42.1 ± 15.7 | 40.5 ± 6.1 | −0.5% (p = 0.82) | 23.1% (p = 0.47) |
| LenC | 46.6 ± 12.6 | 46.3 ± 8.5 | 0.8% (p = 0.52) | 13.3% (p = 0.46) |
| LenFM | 42.7 ± 10.4 | 40.7 ± 9.9 | 5.9% (p = 0.30) | 25.5% (p = 0.18) |
| DiaAntP | 74.8 ± 20.3 | 75.5 ± 6.5 | 7.2% (p < 0.05) | 12.1% (p < 0.05) |
| H_PF | 56.6 ± 12.4 | 52.3 ± 7.9 | 6.9% (p < 0.05) | 14.1% (p < 0.05) |
| LenH | 15.9 ± 7.7 | 11.8 ± 4.3 | 14.4% (p < 0.05) | 31.9% (p < 0.05) |
| AngT | 1.13 ± 0.23 | 1.08 ± 0.16 | 1.3% (p = 0.24) | 15.1% (p = 0.18) |
| DPoFM | 38.3 ± 6.5 | |||
| DCCFM | 62.3 ± 6.6 | |||
| DFFM | 27.9 ± 4.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesin, L.; Ponzio, F.; Carlino, C.F.; Lenge, M.; Noris, A.; Leo, M.C.; Sica, M.; McGreevy, K.; Fabrik, E.L.A.; Giordano, F. A Machine Learning Approach to Support Treatment Identification for Chiari I Malformation. Appl. Sci. 2022, 12, 9039. https://doi.org/10.3390/app12189039
Mesin L, Ponzio F, Carlino CF, Lenge M, Noris A, Leo MC, Sica M, McGreevy K, Fabrik ELA, Giordano F. A Machine Learning Approach to Support Treatment Identification for Chiari I Malformation. Applied Sciences. 2022; 12(18):9039. https://doi.org/10.3390/app12189039
Chicago/Turabian StyleMesin, Luca, Francesco Ponzio, Christian Francesco Carlino, Matteo Lenge, Alice Noris, Maria Carmela Leo, Michela Sica, Kathleen McGreevy, Erica Leila Ahngar Fabrik, and Flavio Giordano. 2022. "A Machine Learning Approach to Support Treatment Identification for Chiari I Malformation" Applied Sciences 12, no. 18: 9039. https://doi.org/10.3390/app12189039





