Preservation of Inner Ear Functions: Extending Glucocorticoid Therapy by Tissue-Protective α1-Antitrypsin
Abstract
:1. Introduction
1.1. Glucocorticoid Use in Inner Ear Pathologies
1.2. Glucocorticoids Are Not Tissue-Protective
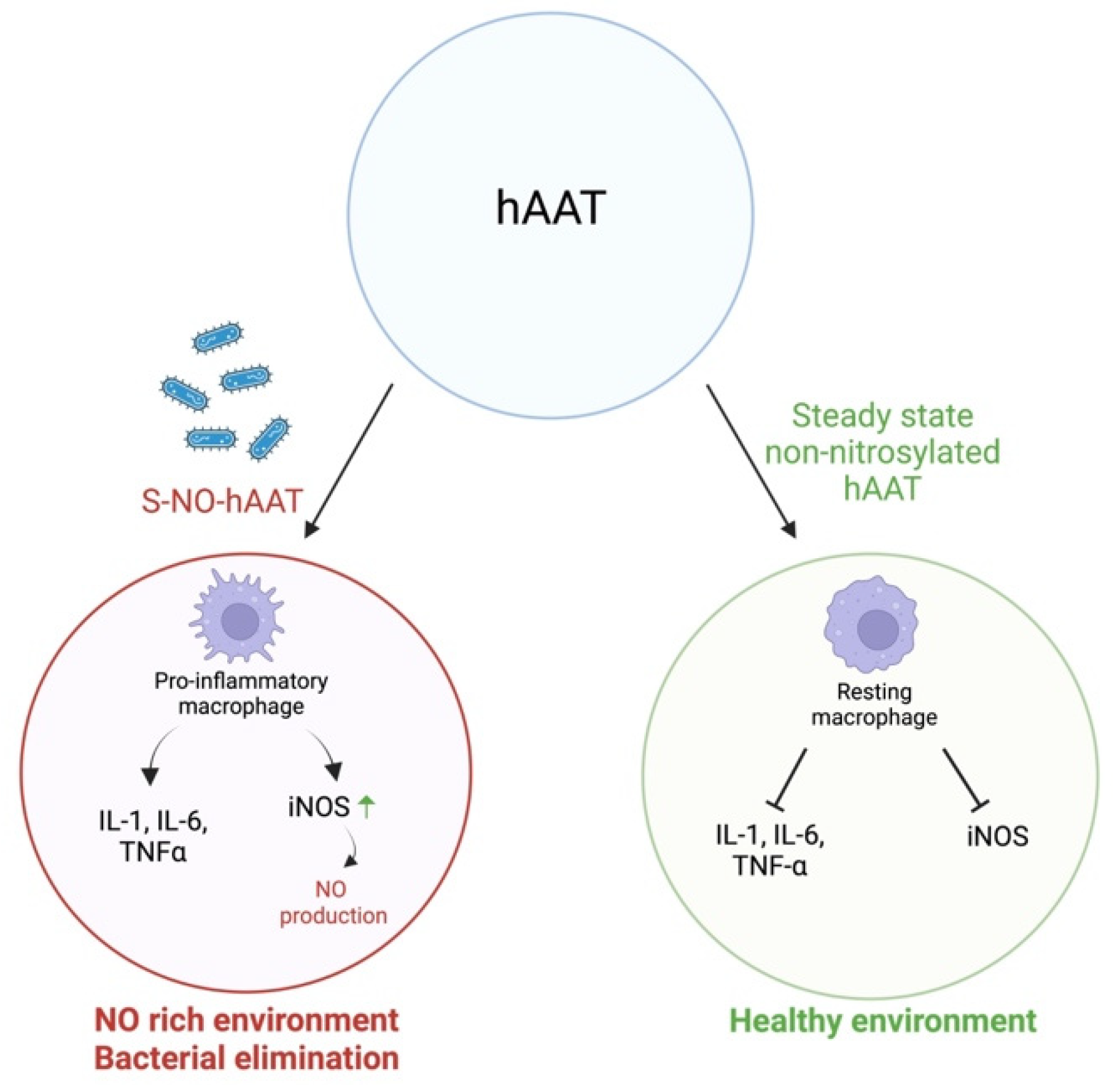
1.3. Alpha1-Antitrypsin Is Tissue-Protective
2. Inner Ear Injury and Glucocorticoid Therapy
2.1. Cochlear Implantation and Glucocorticoid Therapy
2.2. Stapedectomy and Glucocorticoid Therapy
2.3. Perilymphatic Fistula and Glucocorticoid Therapy
2.4. Post-Surgical Facial Paralysis and Glucocorticoid Therapy
3. Unmet Medical Needs in the Context of a Glucocorticoid Therapy
4. Combining AAT and Glucocorticoid Therapies
4.1. Reduction in the Bacterial Burden
4.2. Protection from Free Radicals and Reactive Oxygen Species
4.3. Accelerated Inflammatory Resolution and Improved Tissue Repair
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryan, A.F.; Harris, J.P.; Keithley, E.M. Immune-mediated hearing loss: Basic mechanisms and options for therapy. Acta Otolaryngol. Suppl. 2002, 122, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Derebery, M.J. Allergic and immunologic aspects of Meniere’s disease. Otolaryngol. Head Neck Surg. 1996, 114, 360–365. [Google Scholar] [CrossRef]
- Yoo, T.J.; Du, X.; Kwon, S.S. Molecular mechanism of autoimmune hearing loss. Acta Otolaryngol. Suppl. 2002, 122, 3–9. [Google Scholar] [CrossRef]
- Parnes, L.S.; Sun, A.H.; Freeman, D.J. Corticosteroid pharmacokinetics in the inner ear fluids: An animal study followed by clinical application. Laryngoscope 1999, 109, 1–17. [Google Scholar] [CrossRef]
- Jackson, L.E.; Silverstein, H. Chemical perfusion of the inner ear. Otolaryngol. Clin. N. Am. 2002, 35, 639–653. [Google Scholar] [CrossRef]
- David, M.; Douglas, D. A Comprehensive Review of the Adverse Effects of Systemic Corticosteroid. Otolaryngol. Clin. N. Am. 2010, 43, 753–768. [Google Scholar]
- Goforth, P.; Gudas, C.J. Effects of steroids on wound healing: A review of the literature. J. Foot Surg. 1980, 19, 22–28. [Google Scholar] [PubMed]
- Marr, K.A.; Carter, R.A.; Boeckh, M.; Martin, P.; Corey, L. Invasive aspergillosis in allogeneic stem cell transplant recipients: Changes in epidemiology and risk factors. Blood 2002, 100, 4358–4366. [Google Scholar] [CrossRef]
- Lenco, W.; Mcknight, M.; MacDonald, A.S. Effects of cortisone acetate, methylprednisolone and medroxyprogesterone on wound contracture and epithelialization rabbits. Plast. Reconstr. Surg. 1975, 56, 470–471. [Google Scholar] [CrossRef]
- Kaner, Z.; Ochayon, D.E.; Shahaf, G.; Baranovski, B.M.; Bahar, N.; Mizrahi, M.; Lewis, E.C. Acute phase protein α1-antitrypsin reduces the bacterial burden in mice by selective modulation of innate cell responses. J. Infect. Dis. 2015, 211, 1489–1498. [Google Scholar] [CrossRef]
- Guttman, O.; Baranovski, B.M.; Schuster, R.; Kaner, Z.; Freixo-Lima, G.S.; Bahar, N.; Kalay, N.; Mizrahi, M.I.; Brami, I.; Ochayon, D.E.; et al. Acute-phase protein α1-anti-trypsin: Diverting injurious innate and adaptive immune responses from non-authentic threats. Clin. Exp. Immunol. 2015, 179, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozeri, E.; Rider, P.; Rigbi, S.; Shahaf, G.; Nita, I.I.; Sekler, I.; Lewis, E.C.; Schuster, R. Differential signaling patterns of stimulated bone marrow-derived dendritic cells under α1-antitrypsin-enriched conditions. Cell. Immunol. 2021, 361, 104281. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.C. Expanding the Clinical Indications for α1-Antitrypsin Therapy. Mol. Med. 2012, 18, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Baranovski, B.M.; Schuster, R.; Nisim, O.; Brami, I.; Lior, Y.; Lewis, E.C. Alpha-1 Antitrypsin Substitution for Extrapulmonary Conditions in Alpha-1 Antitrypsin Deficient Patients. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 5, 267–276. [Google Scholar] [CrossRef]
- Abbate, A.; Van Tassell, B.W.; Christopher, S.; Abouzaki, N.A.; Sonnino, C.; Oddi, C.; Carbone, S.; Melchior, R.D.; Gambill, M.L.; Roberts, C.S.; et al. Effects of prolastin C (plasma-derived alpha-1 antitrypsin) on the acute inflammatory response in patients with ST-segment elevation myocardial infarction (from the VCU-alpha 1-RT pilot study). Am. J. Cardiol. 2015, 115, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E. A1-antitrypsin therapy for non-deficient individuals: Integrating and mitigating cross-pathology inflammatory and immune responses to the injured cell. Intern. Med. Rev. 2017, 3, e451. [Google Scholar] [CrossRef]
- Seidman, M.D. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope 2000, 110, 727–738. [Google Scholar] [CrossRef]
- Mora, R.; Barbieri, M.; Mora, F.; Mora, M.; Yoo, T.J. Intravenous infusion of recombinant tissue plasminogen activator for the treatment of patients with sudden and/or chronic hearing loss. Ann. Otol. Rhinol. Laryngol. 2003, 112, 665–670. [Google Scholar] [CrossRef]
- Miller, J.M.; Chi, D.H.; O’Keeffe, L.J.; Kruszka, P.; Raphael, Y.; Altschuler, R.A. Neurotrophins can enhance spiral ganglion cell survival after inner hair cell loss. Int. J. Dev. Neurosci. 1997, 15, 631–643. [Google Scholar] [CrossRef]
- Lalwani, A.K.; Walsh, B.J.; Reilly, P.G.; Muzyczka, N.; Mhatre, A.N. Development of in vivo gene therapy for hearing disorders: Introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996, 3, 588–592. [Google Scholar]
- Wang, Y.; Liberman, M.C. Restraint stress and protection from acoustic injury in mice. Hear. Res. 2002, 165, 96–102. [Google Scholar] [CrossRef]
- Alexiou, C.; Arnold, W.; Fauser, C.; Schratzenstaller, B.; Gloddek, B.; Fuhrmann, S.; Lamm, K. Sudden sensorineural hearing loss: Does application of glucocorticoids make sense? Arch. Otolaryngol. Head Neck Surg. 2001, 127, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekhar, S.S. Intratympanic dexamethasone for sudden sensorineural hearing loss: Clinical and laboratory evaluation. Otol. Neurotol. 2001, 22, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Hillman, T.M.; Arriaga, M.A.; Chen, D.A. Intratympanic steroids: Do they acutely improve hearing in cases of cochlear hydrops? Laryngoscope 2003, 113, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Nadol, J.B.; Eddington, D.K. Histopathology of the inner ear relevant to cochlear implantation. Adv. Otorhinolaryngol. 2006, 64, 31–49. [Google Scholar]
- Roland, P.S.; Wright, C.G. Surgical aspects of cochlear implantation: Mechanisms of insertional trauma. Adv. Otorhinolaryngol. 2006, 64, 11–30. [Google Scholar] [PubMed]
- Eshraghi, A.A.; He, J.; Mou, C.H.; Polak, M.; Zine, A.; Bonny, C.; Balkany, T.J.; Van De Water, T.R. D-JNKI-1 treatment prevents the progression of hearing loss in a model of cochlear implantation trauma. Otol. Neurotol. 2006, 27, 504–511. [Google Scholar] [CrossRef]
- Dinh, C.T.; Van De Water, T.R. Blocking pro-cell-death signal pathways to conserve hearing. Audiol. Neurootol. 2009, 14, 383–392. [Google Scholar] [CrossRef]
- Haake, S.M.; Dinh, C.T.; Chen, S.; Eshraghi, A.A.; Van De Water, T.R. Dexamethasone protects auditory hair cells against TNFalpha-initiated apoptosis via activation of PI3K/Akt and NFkappaB signaling. Hear. Res. 2009, 255, 22–32. [Google Scholar] [CrossRef]
- Dinh, C.T.; Bas, E.; Chan, S.S.; Dinh, J.N.; Vu, L.; Van De Water, T.R. Dexamethasone treatment of tumor necrosis factor-alpha challenged organ of Corti explants activates nuclear factor kappa B signaling that induces changes in gene expression that favor hair cell survival. Neuroscience 2011, 188, 157–167. [Google Scholar] [CrossRef]
- Van De Water, T.R.; Abi Hachem, R.N.; Dinh, C.T.; Bas, E.; Haake, S.M.; Hoosien, G.; Vivero, R.; Chan, S.; He, J.; Eshraghi, A.A.; et al. Conservation of hearing and protection of auditory hair cells against trauma-induced losses by local dexamethasone therapy: Molecular and genetic mechanisms. Cochlear Implant. Int. 2010, 11 (Suppl. S1), 42–55. [Google Scholar] [CrossRef] [PubMed]
- Dhanasingh, A.; Hochmair, I. Drug delivery in cochlear implantation. Acta Otolaryngol. 2021, 141, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Causon, A.; Verschuur, C.; Newman, T.A. A retrospective analysis of the contribution of reported factors in cochlear implantation on hearing preservation outcomes. Otol. Neurotol. 2015, 36, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, S.; Cloutier, F.; Philippon, D.; Côté, M.; Bussières, R.; Backous, D.D. Outcomes review of modern hearing preservation technique in cochlear implant. Auris Nasus Larynx 2016, 43, 485–488. [Google Scholar] [CrossRef]
- Skarżyńska, M.B.; Skarżyński, P.H.; Król, B.; Kozieł, M.; Osińska, K.; Gos, E.; Skarżyński, H. Preservation of hearing following cochlear implantation using different steroid therapy regimens: A prospective clinical study. Med. Sci. Monit. 2018, 24, 2437–2445. [Google Scholar] [CrossRef]
- O’Leary, S.J.; Choi, J.; Brady, K.; Matthews, S.; Ozdowska, K.B.; Payne, M.; McLean, T.; Rousset, A.; Lo, J.; Creber, N.; et al. Systemic methylprednisolone for hearing preservation during cochlear implant surgery: A double blinded placebo-controlled trial. Hear. Res. 2021, 404, 108224. [Google Scholar] [CrossRef] [PubMed]
- Cortés Fuentes, I.A.; Videhult Pierre, P.; Engmér Berglin, C. Improving clinical outcomes in cochlear implantation using glucocorticoid therapy: A review. Ear Hear. 2020, 41, 17–24. [Google Scholar] [CrossRef]
- Rajan, G.P.; Kuthubutheen, J.; Hedne, N.; Krishnaswamy, J. The role of preoperative, intratympanic glucocorticoids for hearing preservation in cochlear implantation: A prospective clinical study. Laryngoscope 2012, 122, 190–195. [Google Scholar] [CrossRef]
- Ramos, B.F.; Tsuji, R.K.; Bento, R.F.; Goffi-Gomez, M.V.; Ramos, H.F.; Samuel, P.A.; Brito, R. Hearing preservation using topical dexamethasone alone and associated with hyaluronic acid in cochlear implantation. Acta Otolaryngol. 2015, 135, 473–477. [Google Scholar] [CrossRef]
- Enticott, J.C.; Eastwood, H.T.; Briggs, R.J.; Dowell, R.C.; O’Leary, S.J. Methylprednisolone applied directly to the round window reduces dizziness after cochlear implantation: A randomized clinical trial. Audiol. Neurootol. 2011, 16, 289–303. [Google Scholar] [CrossRef]
- Paasche, G.; Tasche, C.; Stöver, T.; Lesinski-Schiedat, A.; Lenarz, T. The long-term effects of modified electrode surfaces and intracochlear corticosteroids on postoperative impedances in cochlear implant patients. Otol. Neurotol. 2009, 30, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Prenzler, N.K.; Salcher, R.; Timm, M.; Gaertner, L.; Lenarz, T.; Warnecke, A. Intracochlear administration of steroids with a catheter during human cochlear implantation: A safety and feasibility study. Drug Deliv. Transl. Res. 2018, 8, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, A.D.; Carlson, M.L.; Zuniga, M.G.; Bennett, M.L.; Wanna, G.B.; Haynes, D.S.; Rivas, A. Impact of perioperative oral steroid use on low-frequency hearing preservation after cochlear implantation. Otol. Neurotol. 2015, 36, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Kuthubutheen, J.; Joglekar, S.; Smith, L.; Friesen, L.; Smilsky, K.; Millman, T.; Ng, A.; Shipp, D.; Coates, H.; Arnoldner, C.; et al. The role of preoperative steroids for hearing preservation cochlear implantation: Results of a randomized controlled trial. Audiol. Neurootol. 2017, 22, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Bas, E.; Bohorquez, J.; Goncalves, S.; Perez, E.; Dinh, C.T.; Garnham, C.; Hessler, R.; Eshraghi, A.A.; Van De Water, T.R. Electrode array-eluted dexamethasone protects against electrode insertion trauma induced hearing and hair cell losses, damage to neural elements, increases in impedance and fibrosis: A dose response study. Hear. Res. 2016, 337, 12–24. [Google Scholar] [CrossRef]
- Wilk, M.; Hessler, R.; Mugridge, K.; Jolly, C.; Fehr, M.; Lenarz, T.; Scheper, V. Impedance changes and fibrous tissue growth after cochlear implantation are correlated and can be reduced using a dexamethasone eluting electrode. PLoS ONE 2016, 11, e0147552. [Google Scholar] [CrossRef]
- Wiet, R.J.; Harvey, S.A.; Bauer, G.P. Complications in stapes surgery. Options for prevention and management. Otolaryngol. Clin. N. Am. 1993, 26, 471–490. [Google Scholar] [CrossRef]
- Chavanis, P.-H.; Sire, C. Virial theorem and dynamical evolution of self-gravitating Brownian particles in an unbounded domain. I. Overdamped models. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2006, 73, 066103. [Google Scholar] [CrossRef]
- Kaufman, R.S.; Schuknecht, H.F. Reparative granuloma following stapedectomy: A clinical entity. Ann. Otol. Rhinol. Laryngol. 1967, 76, 1008–1017. [Google Scholar] [CrossRef]
- Shambaugh, G.E., Jr.; Causse, J.; Petrovic, A.; Chevance, L.G.; Valvassori, G.E. New concepts in management of otospongiosis. Arch Otolaryngol. 1974, 100, 419–426. [Google Scholar] [CrossRef]
- Causse, J.B. Etiology and therapy of cochlear drops following stapedectomy. Am. J. Otol. 1980, 1, 221–224. [Google Scholar]
- Causse, J.B.; Causse, J.R. Minimizing cochlear loss during and after stapedectomy. Otolaryngol. Clin. N. Am. 1982, 15, 813–835. [Google Scholar] [CrossRef]
- Dornhoffer, J.L.; Milewski, C. Management of the open labyrinth. Otolaryngol. Head Neck Surg. 1995, 112, 410–414. [Google Scholar] [CrossRef]
- Riechelmann, H.; Tholen, M.; Keck, T.; Rettinger, G. Perioperative glucocorticoid treatment does not influence early post-laser stapedotomy hearing thresholds. Am. J. Otol. 2000, 21, 809–812. [Google Scholar] [PubMed]
- Deveze, A.; Matsuda, H.; Elziere, M.; Ikezono, T. Diagnosis and treatment of perilymphatic fistula. Adv. Otorhinolaryngol. 2018, 81, 133–145. [Google Scholar] [PubMed]
- Goto, F.; Ogawa, K.; Kunihiro, T.; Kurashima, K.; Kobayashi, H.; Kanzaki, J. Perilymph fistula—45 case analysis. Auris Nasus Larynx 2001, 28, 29–33. [Google Scholar] [CrossRef]
- Vrabec, J.T. Delayed facial palsy after tympanomastoid surgery. Am. J. Otol. 1999, 20, 26–30. [Google Scholar]
- Althaus, S.R.; House, H.P. Delayed post-stapedectomy facial paralysis: A report of five cases. Laryngoscope 1973, 83, 1234–1240. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen toxicity and reactive oxygen species: The devil is in the details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef]
- Petrache, I.; Hajjar, J.; Campos, M. Safety and efficacy of alpha-1-antitrypsin augmentation therapy in the treatment of patients with alpha-1-antitrypsin deficiency. Biologics 2009, 3, 193–204. [Google Scholar] [CrossRef]
- Lieberman, J. Augmentation therapy reduces frequency of lung infections in antitrypsin deficiency: A new hypothesis with supporting data. Chest 2000, 118, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Kaminska, A.M.; Gill, W.; Chmura, K.; Feldman, N.E.; Bai, X.; Floyd, C.M.; Fulton, K.E.; Huitt, G.A.; Strand, M.; et al. Alpha-1-antitrypsin (AAT) anomalies are associated with lung disease due to rapidly growing mycobacteria and AAT inhibits Mycobacterium abscessus infection of macrophages. Scand. J. Infect. Dis. 2007, 39, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Griese, M.; Latzin, P.; Kappler, M.; Weckerle, K.; Heinzlmaier, T.; Bernhardt, T.; Hartl, D. Alpha1-Antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur. Respir. J. 2007, 29, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Cantin, A.M.; Woods, D.E. Aerosolized prolastin suppresses bacterial proliferation in a model of chronic Pseudomonas aeruginosa lung infection. Am. J. Respir. Crit. Care Med. 1999, 160, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Pott, G.B.; Beard, K.S.; Bryan, C.L.; Merrick, D.T.; Shapiro, L. Alpha-1 antitrypsin reduces severity of pseudomonas pneumonia in mice and inhibits epithelial barrier disruption and pseudomonas invasion of respiratory epithelial cells. Front. Public Health 2013, 1, 19. [Google Scholar] [CrossRef] [Green Version]
- Kaner, Z.; Engelman, R.; Schuster, R.; Rider, P.; Greenberg, D.; Av-Gay, Y.; Benhar, M.; Lewis, E.C. S-nitrosylation of α1-antitrypsin triggers macrophages toward inflammatory phenotype and enhances intra-cellular bacteria elimination. Front. Immunol. 2019, 10, 590. [Google Scholar] [CrossRef]
- Murphy, M.P.; McEnery, T.; McQuillan, K.; McElvaney, O.F.; McElvaney, O.J.; Landers, S.; Coleman, O.; Bussayajirapong, A.; Hawkins, P.; Henry, M.; et al. α1 Antitrypsin therapy modulates the neutrophil membrane proteome and secretome. Eur. Respir. J. 2020, 55, 1901678. [Google Scholar] [CrossRef]
- Hawkins, P.; McEnery, T.; Gabillard-Lefort, C.; Bergin, D.A.; Alfawaz, B.; Shutchaidat, V.; Meleady, P.; Henry, M.; Coleman, O.; Murphy, M.; et al. In vitro and in vivo modulation of NADPH oxidase activity and reactive oxygen species production in human neutrophils by α1-antitrypsin. ERJ Open Res. 2021, 7, 00234–02021. [Google Scholar] [CrossRef]
- El-Saied, S.; Zaknoun, M.; Alatawna, O.; Joshua, B.-Z.; Kabahaa, N.; Kaplan, D.M.; Lewis, E.C. Trauma-induced vestibular dysfunction: Possible functional repair under α1-antitrypsin-rich conditions. Cell Immunol. 2020, 356, 10415. [Google Scholar] [CrossRef]
- Gimmon, A.; Sherker, L.; Kojukarov, L.; Zaknoun, M.; Lior, Y.; Fadel, T.; Schuster, R.; Lewis, E.C.; Silberstein, E. Accelerated wound border closure using a microemulsion containing non-inhibitory recombinant α1-antitrypsin. Int. J. Mol. Sci. 2022, 23, 7364. [Google Scholar] [CrossRef]
- Bellacen, K.; Kalay, N.; Ozeri, E.; Shahaf, G.; Lewis, E.C. Revascularization of pancreatic islet allografts is enhanced by α-1-antitrypsin under anti-inflammatory conditions. Cell Transplant. 2013, 22, 2119–2133. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.; Bar-Nathan, O.; Tiosano, A.; Lewis, E.C.; Silberstein, E. Enhanced Survival and Accelerated Perfusion of Skin Flap to Recipient Site Following Administration of Human α1-Antitrypsin in Murine Models. Adv. Wound Care 2019, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.R.; Morell, B.; Biedermann, L.; Schreiner, P. Protein-losing enteropathy as precursor of inflammatory bowel disease: A review of the literature. BMJ Case Rep. 2021, 14, e238802. [Google Scholar] [CrossRef]
- Braamskamp, M.J.A.M.; Dolman, K.M.; Tabbers, M.M. Clinical practice. Protein-Losing Enteropathy Child. Eur. J. Pediatr. 2010, 169, 1179–1185. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, R.M.; Antin, J.H. Therapeutic options for steroid-refractory acute and chronic GVHD: An evolving landscape. Expert Rev. Hematol. 2020, 13, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, L.; Morin, F.; Robin, M.; Peyneau, M.; Schlageter, M.H.; Desmier, D.; Pagliuca, S.; Del Galy, A.S.; de Fontbrune, F.S.; Xhaard, A.; et al. Human-derived α1-antitrypsin is still efficacious in heavily pretreated patients with steroid-resistant gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant. 2020, 26, 1620–1626. [Google Scholar] [CrossRef]
- El-Saied, S.; Schmitt, H.; Durisin, M.; Joshua, B.Z.; Abu Tailakh, M.; Prenzler, N.; Lenarz, T.; Kaplan, D.M.; Lewis, E.C.; Warnecke, A. Endogenous α1-antitrypsin levels in the perilymphatic fluid correlates with severity of hearing loss. Clin. Otolaryngol. 2020, 45, 495–499. [Google Scholar] [CrossRef]
- Causse, J.; Chevance, L.G.; Bretlau, P.; Jorgensen, M.B.; Uriel, J.; Berges, J. Enzymatic concept of otospongiosis and cochlear otospongiosis. Clin. Otolaryngol. Allied. Sci. 1977, 2, 23–32. [Google Scholar] [CrossRef]
- Schuster, R.; Motola-Kalay, N.; Baranovski, B.M.; Bar, L.; Tov, N.; Stein, M.; Lewis, E.C.; Ayalon, M.; Sagiv, Y. Distinct anti-inflammatory properties of alpha1-antitrypsin and corticosteroids reveal unique underlying mechanisms of action. Cell. Immunol. 2020, 356, 104177. [Google Scholar] [CrossRef]
- Tilg, H.; Vannier, E.; Vachino, G.; Dinarello, C.A.; Mier, J.W. Antiinflammatory properties of hepatic acute phase proteins: Preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J. Exp. Med. 1993, 178, 1629–1636. [Google Scholar] [CrossRef]
- Hammond, G.L.; Smith, C.L.; Paterson, N.A.; Sibbald, W.J. A role for corticosteroid-binding globulin in delivery of cortisol to activated neutrophils. J. Clin. Endocrinol. Metab. 1990, 71, 34–39. [Google Scholar] [CrossRef]
- Bai, A.; Feng, Z.; Kadiyala, V.; Aerts, S.; Honda, J.R.; Li, L. Alpha-1-Antitrypsin Binds to Glucocorticoid Receptor to Induce Angiopoietin-Like Protein 4 Expression. In C32 COPD: Translational and Mechanistic Studies; American Thoracic Society: New York, NY, USA, 2019. [Google Scholar]
- Kadiyala, V.; Sasse, S.K.; Altonsy, M.O.; Berman, R.; Chu, H.W.; Phang, T.L. Cistrome-based Cooperation between Airway Epithelial Glucocorticoid Receptor and NF-kappaB Orchestrates Anti-inflammatory Effects. J. Biol. Chem. 2016, 291, 12673–12687. [Google Scholar] [CrossRef]
- Scheper, V.; Schmidtheisler, M.; Lasch, F.; von der Leyen, H.; Koch, A.; Schwieger, J.; Büchner, A.; Lesinski-Schiedat, A.; Lenarz, T. Randomized placebo-controlled clinical trial investigating the effect of antioxidants and a vasodilator on overall safety and residual hearing preservation in cochlear implant patients. Trials 2020, 21, 643. [Google Scholar] [CrossRef]
- Mauro, A.G.; Mezzaroma, E.; Marchetti, C.; Narayan, P.; Del Buono, M.G.; Capuano, M.; Prestamburgo, A.; Catapano, S.; Salloum, F.N.; Abbate, A.; et al. A Preclinical Translational Study of the Cardioprotective Effects of Plasma-Derived Alpha-1 Anti-trypsin in Acute Myocardial Infarction. J. Cardiovasc. Pharmacol. 2017, 69, 273. [Google Scholar] [CrossRef]
- Simats, A.; Ramiro, L.; Valls, R.; de Ramón, H.; García-Rodríguez, P.; Orset, C.; Artigas, L.; Sardon, T.; Rosell, A.; Montaner, J. Ceruletide and alpha-1 antitrypsin as a novel combination therapy for ischemic stroke. Neurotherapeutics 2022, 19, 513–527. [Google Scholar] [CrossRef]
- Jeong, K.H.; Lim, J.H.; Lee, K.H.; Kim, M.J.; Jung, H.Y.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, Y.L.; Kim, C.D. Protective Effect of Alpha 1-Antitrypsin on Renal Ischemia-Reperfusion Injury. Transplant. Proc. 2019, 51, 2814–2822. [Google Scholar] [CrossRef]
- Antonelli, P.J.; Schultz, G.S.; Sundin, D.J.; Pemberton, P.A.; Barr, P.J. Protease inhibitors alpha1-antitrypsin and ilomastat are not ototoxic in the chinchilla. Laryngoscope 2003, 113, 1764–1769. [Google Scholar] [CrossRef]
- Antonelli, P.J.; Schultz, G.S.; Sundin, D.J.; Pemberton, P.A.; Barr, P.J. Alpha1-antitrypsin single dose adjuvant therapy for acute otitis media. Otolaryngol. Head Neck Surg. 2006, 135, 111–115. [Google Scholar]
- Antonelli, P.J.; Schultz, G.S.; Kim, K.M.; Cantwell, J.S.; Sundin, D.J.; Pemberton, P.A.; Barr, P.J. Alpha 1-antitrypsin and ilomastat inhibit inflammatory proteases present in human middle ear effusions. Laryngoscope 2003, 113, 1347–1351. [Google Scholar] [CrossRef]
- Moshizi, S.A.; Pastras, C.J.; Sharma, R.; Mahmud, M.P.; Ryan, R.; Razmjou, A.; Asadnia, M. Recent advancements in bioelectronic devices to interface with the peripheral vestibular system. Biosens. Bioelectron. 2022, 214, 114521. [Google Scholar] [CrossRef]

| Glucocorticoids | α1-Antitrypsin | |
|---|---|---|
| Mechanism of action | Comprehensive inhibition of inflammatory-associated molecules inhibiting the inflammatory induction of anti-inflammatory pathways | Immune modulation diverting excessive inflammation towards an anti-inflammatory resolution and tissue repair |
| Clinical indication | Ear surgery (e.g., cochlear implants, stapes and inner ear fistulas), inner ear disease (e.g., vestibular neuronitis and sudden sensorineural hearing loss) | Genetic AAT deficiency |
| Dosage | The protocols vary | Lifelong weekly 60–80 mg/kg slow drip i.v. infusions |
| Potential benefit to hearing system | Inhibition of excessive inflammatory damage and minimization of damage to hair cells | Facilitation of early inner ear tissue repair, inhibition of excessive inflammatory damage and preservation of hair cell viability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amar, A.; Lewis, E.C.; Kaplan, D.M.; El-Saied, S. Preservation of Inner Ear Functions: Extending Glucocorticoid Therapy by Tissue-Protective α1-Antitrypsin. Appl. Sci. 2022, 12, 9359. https://doi.org/10.3390/app12189359
Amar A, Lewis EC, Kaplan DM, El-Saied S. Preservation of Inner Ear Functions: Extending Glucocorticoid Therapy by Tissue-Protective α1-Antitrypsin. Applied Sciences. 2022; 12(18):9359. https://doi.org/10.3390/app12189359
Chicago/Turabian StyleAmar, Amit, Eli C. Lewis, Daniel M. Kaplan, and Sabri El-Saied. 2022. "Preservation of Inner Ear Functions: Extending Glucocorticoid Therapy by Tissue-Protective α1-Antitrypsin" Applied Sciences 12, no. 18: 9359. https://doi.org/10.3390/app12189359





