Factorial Design to Stimulate Biomass Development with Chemically Modified Starch
Abstract
:1. Introduction
2. Materials and Methods
3. Results
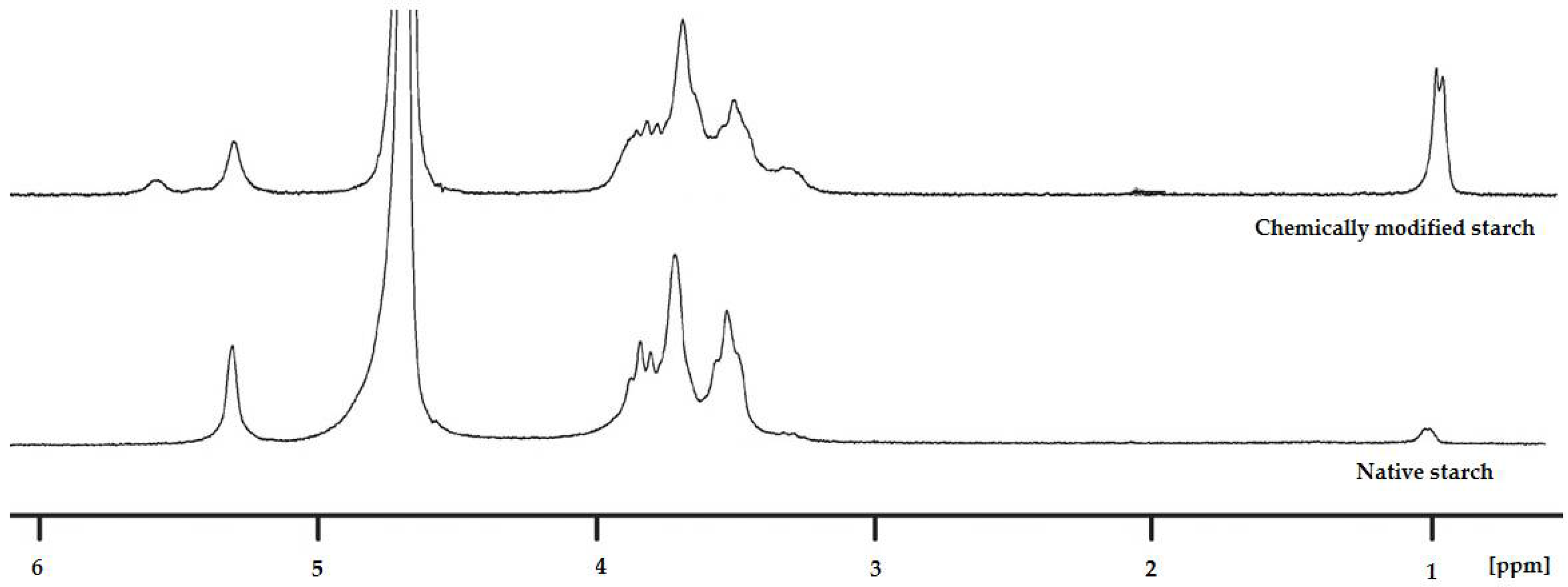
3.1. Chemical Modification of Potato Starch
3.2. Elaboration of the Mathematical Model
- X1, X2, X3—the actual optimal values;
- x1, x2, x3—adimensional optimal values;
- ΔX1, ΔX2, ΔX3—the step of each field of variation;
- X1med, X2med, X3med—the average value reality of the parameters.
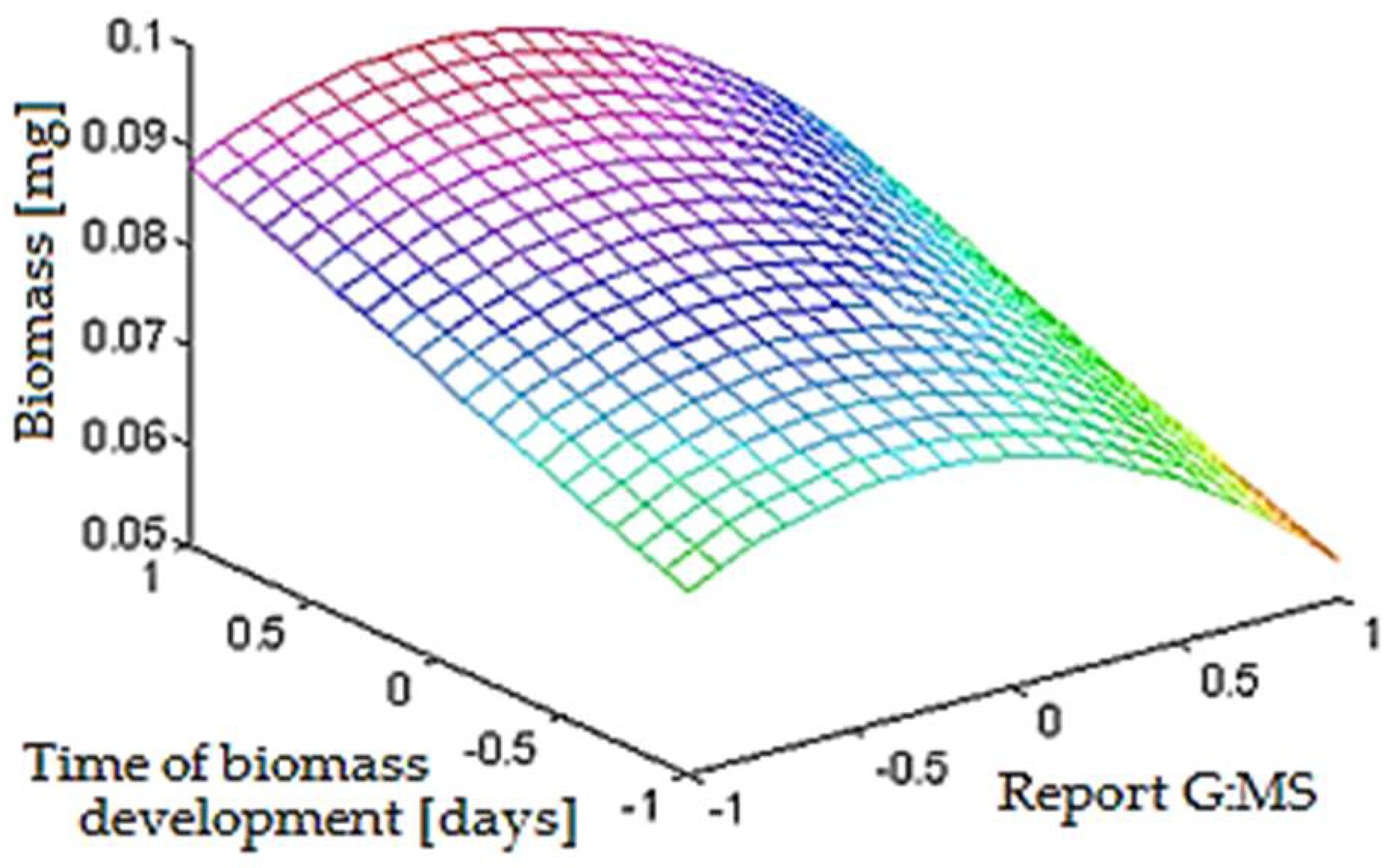
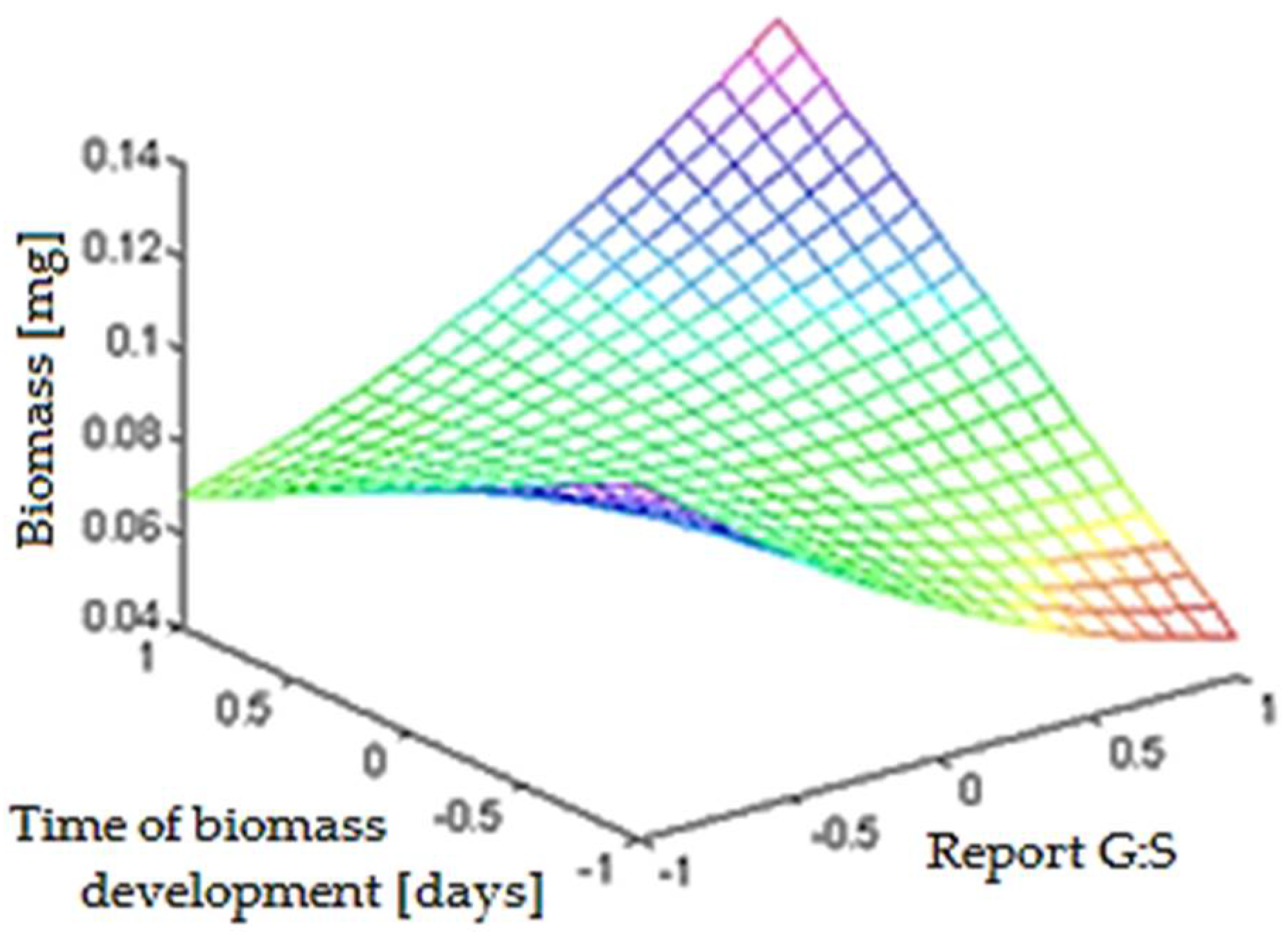
- the optimal time of development of biomass is 5.67 days ≈ 136 h;
- the optimal report between glucose and starch is 1:1.35;
- the optimal report between glucose and modified starch is 1:1.27.
4. Discussion
- reactions that alter the molecular weight of polymer reactions of degradation and crosslinking reactions;
- reactions that change the properties (without major changes in their molecular weight): stabilization reactions and reactions functionalities.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hong, Y.-E.; Kweon, M. Optimization of the Formula and Processing Factors for Gluten-Free Rice Bread with Tamarind Gum. Foods 2020, 9, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristiawan, M.; Della Valle, G.; Berzin, F. Extrusion Simulation for the Design of Cereal and Legume Foods. Foods 2022, 11, 1780. [Google Scholar] [CrossRef]
- Tian, Z.; Hou, L.; Hu, M.; Gao, Y.; Li, D.; Fan, B.; Wang, F.; Li, S. Optimization of Sporulation Conditions for Bacillus subtilis BSNK-5. Processes 2022, 10, 1133. [Google Scholar] [CrossRef]
- Costa, D.S.d.; Takeuchi, K.P.; Silva, R.M.d.; Oliveira Filho, J.G.d.; Bertolo, M.R.V.; Belisário, C.M.; Egea, M.B.; Plácido, G.R. Cassava-Starch-Based Films Incorporated with Buriti (Mauritia flexuosa L.) Oil: A New Active and Bioactive Material for Food Packaging Applications. Polysaccharides 2022, 3, 121–135. [Google Scholar] [CrossRef]
- Franco, E.; Dussán, R.; Navia, D.P.; Amú, M. Study of the Annealing Effect of Starch/Polyvinyl Alcohol Films Crosslinked with Glutaraldehyde. Gels 2021, 7, 249. [Google Scholar] [CrossRef]
- Rosu, A.M.; Veignie, E.; Surpateanu, G.; Brabie, G.; Miron, D.N.; Rafin, C. Synthesis and evaluation of hydroxypropylated potato starch as polymeric support for benzo[a]pyrene degradation by Fenton reaction. Carbohydr. Polym. 2011, 83, 1486–1491. [Google Scholar] [CrossRef]
- de Souza, A.F.; Galindo, H.M.; de Lima, M.A.B.; Ribeaux, D.R.; Rodríguez, D.M.; da Silva Andrade, R.F.; Gusmão, N.B.; de Campos-Takaki, G.M. Biotechnological Strategies for Chitosan Production by Mucoralean Strains and Dimorphism Using Renewable Substrates. Int. J. Mol. Sci. 2020, 21, 4286. [Google Scholar] [CrossRef]
- Dudeney, A.W.L.; Narayanan, A.; Tarasova, I.I. Removal Of Iron From Silica Sand: Integrated Effluent Treatment By Sulfate Reduction, Photochemical Reduction And Reverse Osmosis. In Biohydrometallurgy and the Environment toward the Mining of the 21st Century; Process Metallurgy 9A; Elsevier: Amsterdam, The Netherlands, 1999; pp. 617–625. [Google Scholar]
- Edwards, K.J.; Bond, P.L.; Gihring, T.M.; Banfield, J.F. An Archaeal Iron-Oxidising Extreme Acidophile Important In Acid Mine Drainage. Science 2000, 287, 1796–1799. Available online: https://www.science.org/doi/10.1126/science.287.5459.1796 (accessed on 9 August 2022). [CrossRef] [Green Version]
- Philus, C.D.; Mahanty, B. Dynamic modelling of tetrazolium-based microbial toxicity assay—A parametric proxy of traditional dose-response relationship. Environ. Sci. Pollut. Res. 2021, 28, 45390–45401. [Google Scholar] [CrossRef]
- Johnson, D.B. Importance Of Microbial Ecology In The Development Of New Mineral Technologies. Hydrometallurgy 2001, 59, 147–158. [Google Scholar] [CrossRef]
- Johnson, D.B. Biological Removal Of Sulfurous Compounds From Inorganic Wastewaters. In Environmental Technologies to Treat Sulfur Pollution: Principles and Engineering; Lens, P., Hulshoff Pol, L., Eds.; International Association on Water Quality: London, UK, 2000; pp. 175–206. [Google Scholar]
- Johnson, D.B.; Hallberg, K.B. Pitfalls of passive mine water treatment. Rev. Environ. Sci. Biotechnol. 2002, 1, 335–343. [Google Scholar] [CrossRef]
- Norris, P.R.; Burton, N.P.; Foulis, A.M. Acidophiles In Bioreactor Mineral Processing. Extremophiles 2000, 4, 71–76. [Google Scholar] [CrossRef]
- Okibe, N.; Gericke, M.; Hallberg, K.B.; Johnson, D.B. Enumeration And Characterization Of Acidophilic Microorganisms Isolated From A Pilot Plant Stirred Tank Bioleaching Operation. Appl. Environ. Microb. 2003, 69, 1936–1943. [Google Scholar] [CrossRef] [Green Version]
- Georgescu, A.M.; Brabie, G.; Nistor, I.D.; Nardou, F.; Penot, C. Utilization Of Experimental Design For Specific Area Optimization Of A Pillared Bentonite. J. Food Saf. 2013, XII, 284–290. [Google Scholar]
- Georgescu, A.M.; Muntianu, G.; Nistor, I.D.; Nardou, F. Modeling And Optimization of Al-Pillaring Process Using Experimental Design Procedure. J. Agroaliment. Process. Technol. 2017, 23, 212–216. [Google Scholar]
- Cerasola, V.A.; Perlotti, L.; Pennisi, G.; Orsini, F.; Gianquinto, G. Potential Use of Superabsorbent Polymer on Drought-Stressed Processing Tomato (Solanum lycopersicum L.) in a Mediterranean Climate. Horticulturae 2022, 8, 718. [Google Scholar] [CrossRef]
- Ajbar, A.; Alqahtani, R.T.; Musa, S.S. Static and Dynamic Analysis of a Continuous Bioreactor Model for the Production of Biofuel from Refinery Wastewater Using Rhodococcus opacus. Mathematics 2022, 10, 2842. [Google Scholar] [CrossRef]
- Kubit, A.; Trzepieciński, T.; Kluz, R.; Ochałek, K.; Slota, J. Multi-Criteria Optimisation of Friction Stir Welding Parameters for EN AW-2024-T3 Aluminium Alloy Joints. Materials 2022, 15, 5428. [Google Scholar] [CrossRef]
- Meengam, C.; Sillapasa, K. Evaluation of Optimization Parameters of Semi-Solid Metal 6063 Aluminum Alloy from Friction Stir Welding Process Using Factorial Design Analysis. J. Manuf. Mater. Process. 2020, 4, 123. [Google Scholar] [CrossRef]
- Nejrotti, S.; Mannu, A.; Blangetti, M.; Baldino, S.; Fin, A.; Prandi, C. Optimization of Nazarov Cyclization of 2,4-Dimethyl-1,5-diphenylpenta-1,4-dien-3-one in Deep Eutectic Solvents by a Design of Experiments Approach. Molecules 2020, 25, 5726. [Google Scholar] [CrossRef]
- Fotovvati, B.; Balasubramanian, M.; Asadi, E. Modeling and Optimization Approaches of Laser-Based Powder-Bed Fusion Process for Ti-6Al-4V Alloy. Coatings 2020, 10, 1104. [Google Scholar] [CrossRef]
- Kiss, E.L.; Berkó, S.; Gácsi, A.; Kovács, A.; Katona, G.; Soós, J.; Csányi, E.; Gróf, I.; Harazin, A.; Deli, M.A.; et al. Design and Optimization of Nanostructured Lipid Carrier Containing Dexamethasone for Ophthalmic Use. Pharmaceutics 2019, 11, 679. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Factorial Design as a Tool for the Optimization of PLGA Nanoparticles for the Co-Delivery of Temozolomide and O6-Benzylguanine. Pharmaceutics 2019, 11, 401. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.; Ahmad, M.; Ganat, T. Experimental Study of Bentonite-Free Water Based Mud Reinforced with Carboxymethylated Tapioca Starch: Rheological Modeling and Optimization Using Response Surface Methodology (RSM). Polymers 2021, 13, 3320. [Google Scholar] [CrossRef]
- Schmidt, O.; Magel, E.; Frühwald, A.; Glukhykh, L.; Erdt, K.; Kaschuro, S. Influence of sugar and starch content of palm wood on fungal development and prevention of fungal colonization by acid treatment. Holzforschung 2016, 70, 783–791. [Google Scholar] [CrossRef]
- Fannin, T.E.; Marcus, M.D.; Anderson, D.A.; Bergman, H.L. Use of a fractional factorial design to evaluate interactions of environmental factors affecting biodegradation rates. Appl. Environ. Microbiol. 1981, 42, 936–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanrahan, G.; Zhu, J.; Gibani, S.; Patil, D.G. Chemometrics And Statistics | Experimental Design. In Encyclopedia of Analytical Science, 2nd ed.; Worsfold, P., Townshend, A., Poole, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 8–13. ISBN 9780123693976. [Google Scholar] [CrossRef]
- Lin, C.J.; Zhang, R.; Waisner, S.A. Effects of process factors on the performance of electrochemical disinfection for wastewater in a continuous-flow cell reactor. Environ. Sci. Pollut. Res. 2021, 28, 36573–36584. [Google Scholar] [CrossRef]
- Kushwaha, O.; Uthayakumar, H.; Kumaresan, K. Modeling of carbon dioxide fixation by microalgae using hybrid artificial intelligence (AI) and fuzzy logic (FL) methods and optimization by genetic algorithm (GA). Environ. Sci. Pollut. Res. 2022; Online ahead of print. [Google Scholar] [CrossRef]

| Parameter | Reduced Variable | Minimum Value (−1) | Average Value (0) | Maximum Value (+1) |
|---|---|---|---|---|
| Time of development (days) | x1 | 3 | 5 | 7 |
| Report glucose:starch | x2 | 1:1 | 1:1.5 | 1:2 |
| Report glucose:modified starch | x3 | 1:1 | 1:1.5 | 1:2 |
| yk0 | y10 | y20 | y30 |
|---|---|---|---|
| Quantity of biomass [mg] | 0.142 | 0.158 | 0.079 |
| Nb. | Time of Development [days] | Report Glucose: Starch | Report Glucose: Modified Starch | Biomass [mg] |
|---|---|---|---|---|
| x1 | x2 | x3 | Y1 | |
| 1 | −1 (3) | −1 (1:1) | −1 (1:1) | 0.142 |
| 2 | 0 (1:1.5) | 0.144 | ||
| 3 | +1 (1:2) | 0.124 | ||
| 4 | 0 (1:1.5) | −1 (1:1) | 0.014 | |
| 5 | 0 (1:1.5) | 0.016 | ||
| 6 | +1 (1:2) | 0.095 | ||
| 7 | +1 (1:2) | −1 (1:1) | 0.113 | |
| 8 | 0 (1:1.5) | 0.012 | ||
| 9 | +1 (1:2) | 0.011 | ||
| 10 | 0 (5) | −1 (1:1) | −1 (1:1) | 0.143 |
| 11 | 0 (1:1.5) | 0.145 | ||
| 12 | +1 (1:2) | 0.013 | ||
| 13 | 0 (1:1.5) | −1 (1:1) | 0.096 | |
| 14 | 0 (1:1.5) | 0.014 | ||
| 15 | +1 (1:2) | 0.013 | ||
| 16 | +1 (1:2) | −1 (1:1) | 0.012 | |
| 17 | 0 (1:1.5) | 0.144 | ||
| 18 | +1 (1:2) | 0.146 | ||
| 19 | +1 (7) | −1 (1:1) | −1 (1:1) | 0.014 |
| 20 | 0 (1:1.5) | 0.099 | ||
| 21 | +1 (1:2) | 0.021 | ||
| 22 | 0 (1:1.5) | −1 (1:1) | 0.148 | |
| 23 | 0 (1:1.5) | 0.150 | ||
| 24 | +1 (1:2) | 0.146 | ||
| 25 | +1 (1:2) | −1 (1:1) | 0.095 | |
| 26 | 0 (1:1.5) | 0.098 | ||
| 27 | +1 (1:2) | 0.078 |
| Coefficients | Function of Response Y1 (Quantity of Biomass [mg]) |
|---|---|
| a0 | 0.083 |
| a1 | 9.889·10−3 |
| a2 | −7.556·10−3 |
| a3 | −7.222·10−3 |
| a11 | 3.778·10−3 |
| a22 | 9.444·10−3 |
| a33 | −0.012 |
| a12 | 0.034 |
| a13 | 2.25·10−3 |
| a23 | 0.013 |
| a123 | 7.5·10−3 |
| tj | t0 | t1 | t2 | t3 | t12 | t13 | t23 | t11 | t22 | t33 | t123 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Calculated value | 10.266 | 1.23 | 0.94 | 0.899 | 4.261 | 0.28 | 1.617 | 0.47 | 1.175 | 1.521 | 0.933 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popa, O.; Rosu, A.-M.; Nicuta, D.; Voicu, R.E.; Zichil, V.; Nistor, I.D. Factorial Design to Stimulate Biomass Development with Chemically Modified Starch. Appl. Sci. 2022, 12, 10069. https://doi.org/10.3390/app121910069
Popa O, Rosu A-M, Nicuta D, Voicu RE, Zichil V, Nistor ID. Factorial Design to Stimulate Biomass Development with Chemically Modified Starch. Applied Sciences. 2022; 12(19):10069. https://doi.org/10.3390/app121910069
Chicago/Turabian StylePopa, Olga, Ana-Maria Rosu, Daniela Nicuta, Roxana Elena Voicu, Valentin Zichil, and Ileana Denisa Nistor. 2022. "Factorial Design to Stimulate Biomass Development with Chemically Modified Starch" Applied Sciences 12, no. 19: 10069. https://doi.org/10.3390/app121910069







