Total Mercury (THg) Content in Red Mullet (Mullus barbatus) from Adriatic Sea (Central Mediterranean Sea): Relation to Biological Parameters, Sampling Area and Human Health Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
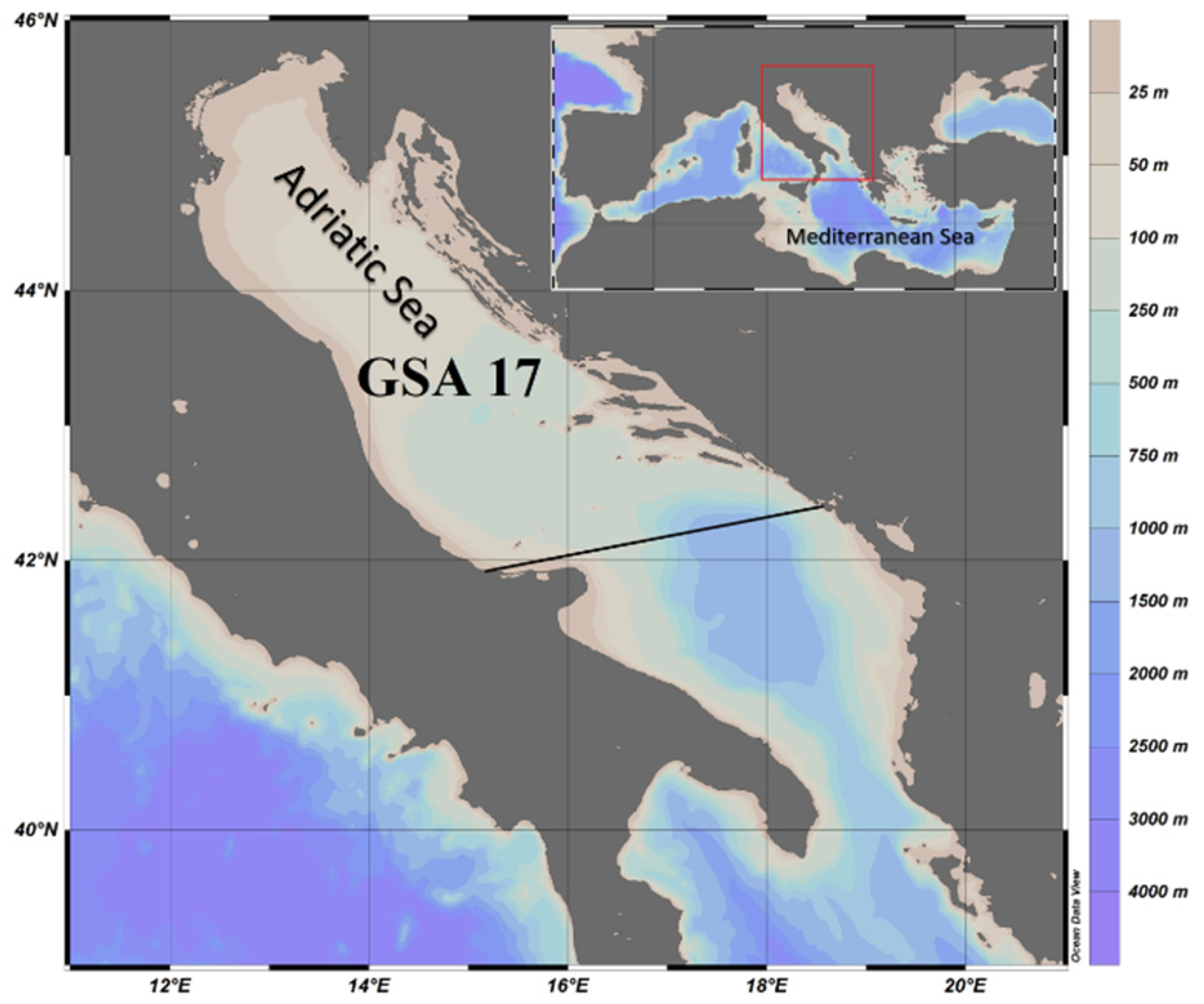
2.1. Fish Sampling
2.2. Laboratory Analysis
2.3. Quantification of Total Mercury Content
2.4. Lipid Content Determination
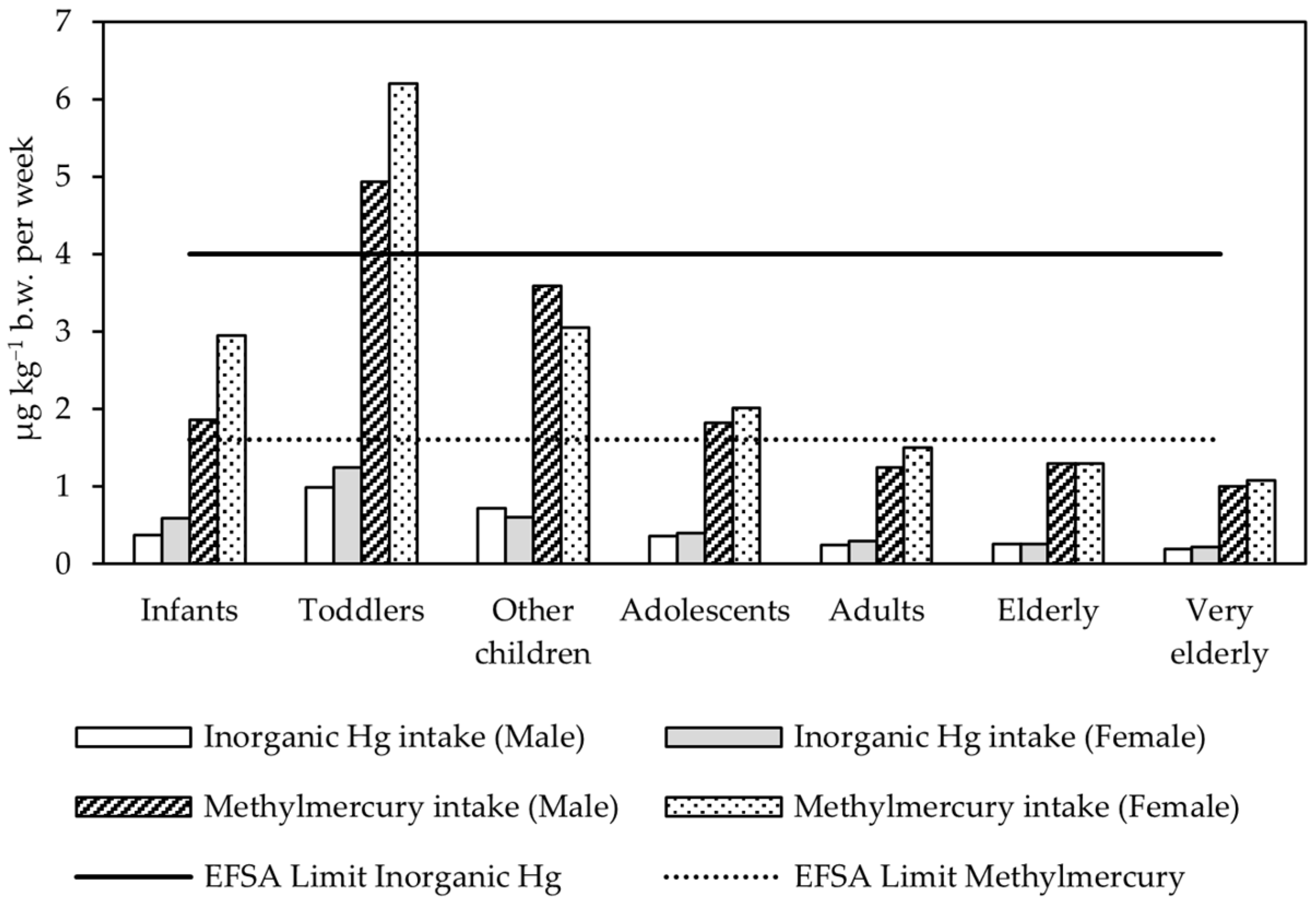
2.5. Risk Assessment
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavoie, R.A.; Jardine, T.D.; Chumchal, M.M.; Kidd, K.A.; Campbell, L.M. Biomagnification of Mercury in Aquatic Food Webs: A Worldwide Meta-Analysis. Environ. Sci. Technol. 2013, 47, 13385–13394. [Google Scholar] [CrossRef]
- Cossa, D.; Coquery, M. The Mediterranean Mercury Anomaly, a Geochemical or a BiologocalIssue. In The Mediterranean Sea. Handbook of Environmental Chemistry; Saliot, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 5K, pp. 177–208. [Google Scholar] [CrossRef]
- Covelli, S.; Faganeli, J.; Horvat, M.; Brambati, A. Mercury Contamination of Coastal Sediments as the Result of Long-Term Cinnabar Mining Activity (Gulf of Trieste, Northern Adriatic Sea). Appl. Geochem. 2001, 16, 541–558. [Google Scholar] [CrossRef]
- Spagnoli, F.; de Marco, R.; Dinelli, E.; Frapiccini, E.; Frontalini, F.; Giordano, P. Sources and Metal Pollution of Sediments from a Coastal Area of the Central Western Adriatic Sea (Southern Marche Region, Italy). Appl. Sci. 2021, 11, 1118. [Google Scholar] [CrossRef]
- Girolametti, F.; Fanelli, M.; Ajdini, B.; Truzzi, C.; Illuminati, S.; Susmel, S.; Celussi, M.; Šangulin, J.; Annibaldi, A. Dissolved Potentially Toxic Elements (PTEs) in Relation to Depuration Plant Outflows in Adriatic Coastal Waters: A Two Year Monitoring Survey. Water 2022, 14, 569. [Google Scholar] [CrossRef]
- Droghini, E.; Annibaldi, A.; Prezioso, E.; Tramontana, M.; Frapiccini, E.; de Marco, R.; Illuminati, S.; Truzzi, C.; Spagnoli, F. Mercury Content in Central and Southern Adriatic Sea Sediments in Relation to Seafloor Geochemistry and Sedimentology. Molecules 2019, 24, 4467. [Google Scholar] [CrossRef] [PubMed]
- Bilandžić, N.; Đokić, M.; Sedak, M. Metal Content Determination in Four Fish Species from the Adriatic Sea. Food Chem. 2011, 124, 1005–1010. [Google Scholar] [CrossRef]
- Annibaldi, A.; Truzzi, C.; Carnevali, O.; Pignalosa, P.; Api, M.; Scarponi, G.; Illuminati, S. Determination of Hg in Farmed and Wild Atlantic Bluefin Tuna (Thunnus thynnus L.) Muscle. Molecules 2019, 24, 1273. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.W. Neurotoxic Effects of Mercury—A Review. Environ. Res. 1977, 14, 329–373. [Google Scholar] [CrossRef]
- Esposito, V.; Andaloro, F.; Bianca, D.; Natalotto, A.; Romeo, T.; Scotti, G.; Castriota, L. Diet and Prey Selectivity of the Red Mullet, Mullus barbatus (Pisces: Mullidae), from the Southern Tyrrhenian Sea: The Role of the Surf Zone as a Feeding Ground. Mar. Biol. Res. 2014, 10, 167–178. [Google Scholar] [CrossRef]
- European Union (EU) Data Collection Framework. Italy—Annual Report for Data Collection in the Fisheries and Aquaculture Sectors; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Kargin, F. Seasonal Changes in Levels of Heavy Metals in Tissues of Mullus barbatus and Sparus aurata Collected from Iskenderun Gulf (Turkey). Water Air Soil Pollut. 1996, 90, 557–562. [Google Scholar] [CrossRef]
- Frapiccini, E.; Panfili, M.; Guicciardi, S.; Santojanni, A.; Marini, M.; Truzzi, C.; Annibaldi, A. Effects of Biological Factors and Seasonality on the Level of Polycyclic Aromatic Hydrocarbons in Red Mullet (Mullus barbatus). Environ. Pollut. 2020, 258, 113742. [Google Scholar] [CrossRef] [PubMed]
- Giani, D.; Baini, M.; Galli, M.; Casini, S.; Fossi, M.C. Microplastics Occurrence in Edible Fish Species (Mullus barbatus and Merluccius merluccius) Collected in Three Different Geographical Sub-Areas of the Mediterranean Sea. Mar. Pollut. Bull. 2019, 140, 129–137. [Google Scholar] [CrossRef]
- Corsi, I.; Mariottini, M.; Menchi, V.; Sensini, C.; Balocchi, C.; Focardi, S. Monitoring a Marine Coastal Area: Use of Mytilus galloprovincialis and Mullus barbatus as Bioindicators. Mar. Ecol. 2002, 23, 138–153. [Google Scholar] [CrossRef]
- Frapiccini, E.; Cocci, P.; Annibaldi, A.; Panfili, M.; Santojanni, A.; Grilli, F.; Marini, M.; Palermo, F.A. Assessment of Seasonal Relationship between Polycyclic Aromatic Hydrocarbon Accumulation and Expression Patterns of Oxidative Stress-Related Genes in Muscle Tissues of Red Mullet (M. barbatus) from the Northern Adriatic Sea. Environ. Toxicol. Pharmacol. 2021, 88, 103752. [Google Scholar] [CrossRef]
- Storelli, M.M.; Storelli, A.; Giacominelli-Stuffler, R.; Marcotrigiano, G.O. Mercury Speciation in the Muscle of Two Commercially Important Fish, Hake (Merluccius merluccius) and Striped Mullet (Mullus barbatus) from the Mediterranean Sea: Estimated Weekly Intake. Food Chem. 2005, 89, 295–300. [Google Scholar] [CrossRef]
- Storelli, M.M.; Marcotrigiano, G.O. Bioindicator Organisms: Heavy Metal Pollution Evaluation in the Ionian Sea (Mediterranean Sea—Italy). Environ. Monit. Assess. 2005, 102, 159–166. [Google Scholar] [CrossRef]
- Sulimanec Grgec, A.; Kljaković-Gašpić, Z.; Orct, T.; Tičina, V.; Sekovanić, A.; Jurasović, J.; Piasek, M. Mercury and Selenium in Fish from the Eastern Part of the Adriatic Sea: A Risk-Benefit Assessment in Vulnerable Population Groups. Chemosphere 2020, 261, 127742. [Google Scholar] [CrossRef]
- Copat, C.; Bella, F.; Castaing, M.; Fallico, R.; Sciacca, S.; Ferrante, M. Heavy Metals Concentrations in Fish from Sicily (Mediterranean Sea) and Evaluation of Possible Health Risks to Consumers. Bull. Environ. Contam. Toxicol. 2012, 88, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Naccari, C.; Cicero, N.; Ferrantelli, V.; Giangrosso, G.; Vella, A.; Macaluso, A.; Naccari, F.; Dugo, G. Toxic Metals in Pelagic, Benthic and Demersal Fish Species from Mediterranean FAO Zone 37. Bull. Environ. Contam. Toxicol. 2015, 95, 567–573. [Google Scholar] [CrossRef]
- Falcó, G.; Llobet, J.M.; Bocio, A.; Domingo, J.L. Daily Intake of Arsenic, Cadmium, Mercury, and Lead by Consumption of Edible Marine Species. J. Agric. Food Chem. 2006, 54, 6106–6112. [Google Scholar] [CrossRef]
- Martínez-Gómez, C.; Fernández, B.; Benedicto, J.; Valdés, J.; Campillo, J.A.; León, V.M.; Vethaak, A.D. Health Status of Red Mullets from Polluted Areas of the Spanish Mediterranean Coast, with Special Reference to Portmán (SE Spain). Mar. Environ. Res. 2012, 77, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Harmelin-Vivien, M.; Cossa, D.; Crochet, S.; Bănaru, D.; Letourneur, Y.; Mellon-Duval, C. Difference of Mercury Bioaccumulation in Red Mullets from the North-Western Mediterranean and Black Seas. Mar. Pollut. Bull. 2009, 58, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Perugini, M.; Visciano, P.; Manera, M.; Zaccaroni, A.; Olivieri, V.; Amorena, M. Heavy Metal (As, Cd, Hg, Pb, Cu, Zn, Se) Concentrations in Muscle and Bone of Four Commercial Fish Caught in the Central Adriatic Sea, Italy. Environ. Monit. Assess. 2014, 186, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Follesa, M.C.; Carbonara, P. Atlas of the Maturity Stages of Mediterranean Fishery Resources. Gen. Fish. Comm. Mediterr. Stud. Rev. 2019, 99, 1–259. [Google Scholar]
- Girolametti, F.; Panfili, M.; Colella, S.; Frapiccini, E.; Annibaldi, A.; Illuminati, S.; Marini, M.; Truzzi, C. Mercury Levels in Merluccius merluccius Muscle Tissue in the Central Mediterranean Sea: Seasonal Variation and Human Health Risk. Mar. Pollut. Bull. 2022, 176, 113461. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, C.; Illuminati, S.; Finale, C.; Annibaldi, A.; Lestingi, C.; Scarponi, G. Microwave-Assisted Solvent Extraction of Melamine from Seafood and Determination by Gas Chromatography–Mass Spectrometry: Optimization by Factorial Design. Anal. Lett. 2014, 47, 1118–1133. [Google Scholar] [CrossRef]
- Truzzi, C.; Illuminati, S.; Antonucci, M.; Scarponi, G.; Annibaldi, A. Heat Shock Influences the Fatty Acid Composition of the Muscle of the Antarctic Fish Trematomus bernacchii. Mar. Environ. Res. 2018, 139, 122–128. [Google Scholar] [CrossRef]
- Zarantoniello, M.; Randazzo, B.; Gioacchini, G.; Truzzi, C.; Giorgini, E.; Riolo, P.; Gioia, G.; Bertolucci, C.; Osimani, A.; Cardinaletti, G.; et al. Zebrafish (Danio rerio) Physiological and Behavioural Responses to Insect-Based Diets: A Multidisciplinary Approach. Sci. Rep. 2020, 10, 10648. [Google Scholar] [CrossRef] [PubMed]
- European Commission Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, L364, 5–24.
- Scientific Opinion on the Risk for Public Health Related to the Presence of Mercury and Methylmercury in Food. EFSA J. 2012, 10, 2985.
- Wold, S. Cross-Validatory Estimation of the Number of Components in Factor and Principal Components Models. Technometrics 1978, 20, 397–405. [Google Scholar] [CrossRef]
- di Lena, G.; Casini, I.; Caproni, R.; Fusari, A.; Orban, E. Total Mercury Levels in Commercial Fish Species from Italian Fishery and Aquaculture. Food Addit. Contam. Part B 2017, 10, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Carpentieri, P.; Colloca, F.; Cardinale, M.; Belluscio, A.; Ardizzone, G. Feeding Habits of European Hake (Merluccius merluccius) in the Central Mediterranean Sea. AquaDocs 2005, 103, 411–416. [Google Scholar]
- Storelli, M.M.; Stuffler, R.G.; Marcotrigiano, G.O. Total Mercury in Muscle of Benthic and Pelagic Fish from the South Adriatic Sea (Italy). Food Addit. Contam. 1998, 15, 876–883. [Google Scholar] [CrossRef]
- Bustamante, P.; Lahaye, V.; Durnez, C.; Churlaud, C.; Caurant, F. Total and Organic Hg Concentrations in Cephalopods from the North Eastern Atlantic Waters: Influence of Geographical Origin and Feeding Ecology. Sci. Total Environ. 2006, 368, 585–596. [Google Scholar] [CrossRef]
- Reñones, O.; Massutí, E.; Morales-Nin, B. Life History of the Red Mullet Mullus surmuletus from the Bottom-Trawl Fishery off the Island of Majorca (North-West Mediterranean). Mar. Biol. 1995, 123, 411–419. [Google Scholar] [CrossRef]
- Vassilopoulou, V.; Georgakopoulos-Gregoriades, E. Factors Influencing the Uptake of PCBs and DDTs in Red Mullet (Mullus barbatus) from Pagassitikos Gulf, Central Greece. Mar. Pollut. Bull. 1993, 26, 285–287. [Google Scholar] [CrossRef]
- Łuczyńska, J.; Paszczyk, B.; Nowosad, J.; Łuczyński, M. Mercury, Fatty Acids Content and Lipid Quality Indexes in Muscles of Freshwater and Marine Fish on the Polish Market. Risk Assessment of Fish Consumption. Int. J. Environ. Res. Public Health 2017, 14, 1120. [Google Scholar] [CrossRef]
- Soares, J.M.; Gomes, J.M.; Anjos, M.R.; Silveira, J.N.; Custódio, F.B.; Gloria, M.B.A. Mercury in Fish from the Madeira River and Health Risk to Amazonian and Riverine Populations. Food Res. Int. 2018, 109, 537–543. [Google Scholar] [CrossRef]
- European Parliament and the Council Commission Regulation (EU) No 420/2011 29 April 2011 Amending Regulation (EC) No 1881/2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Communities 2011, L111, 3–6.
- Use of the EFSA Comprehensive European Food Consumption Database in Exposure Assessment. EFSA J. 2011, 9, 2097. [CrossRef]

| Reproductive Stage | Sex | Sampling Area | n | Length, cm (Min–Max) | Weight, g (Min–Max) | Total Hg mg kg−1 ww (Min–Max) | Total Lipids g/100 g (Min–Max) |
|---|---|---|---|---|---|---|---|
| Juvenile | Female | Open sea | 6 | 12 ± 3 | 16 ± 1 | 0.25 ± 0.05 | 3.27 ± 0.05 |
| Coast | 9 | 11 ± 1 | 12 ± 2 | 0.03 ± 0.01 | 1.15 ± 0.09 | ||
| Male | Open sea | 4 | 10 ± 1 | 9 ± 3 | 0.15 ± 0.01 | 0.59 ± 0.02 | |
| Coast | 10 | 10 ± 1 | 13 ± 1 | 0.07 ± 0.01 | 1.12 ± 0.10 | ||
| Total | 29 | 11 ± 1 (10–12) | 13 ± 3 (9–16) | 0.13 ± 0.09 (0.03–0.25) | 1.53 ± 1.19 (0.59–3.27) | ||
| Adult | Female | Open sea | 19 | 17 ± 2 | 49 ± 12 | 0.41 ± 0.03 | 5.11 ± 0.49 |
| Coast | 44 | 16 ± 2 | 43 ± 13 | 0.19 ± 0.01 | 7.85 ± 0.74 | ||
| Male | Open sea | 30 | 13 ± 1 | 21 ± 5 | 0.23 ± 0.01 | 2.97 ± 0.17 | |
| Coast | 7 | 13 ± 1 | 26 ± 4 | 0.07 ± 0.01 | 6.49 ± 0.27 | ||
| Total | 100 | 15 ± 2 (13–17) | 35 ± 13 (21–49) | 0.22 ± 0.14 (0.07–0.41) | 5.61 ± 2.08 (2.97–7.85) | ||
| Spawning | Female | Open sea | 24 | 15 ± 2 | 36 ± 11 | 0.62 ± 0.02 | 1.62 ± 0.02 |
| Coast | 31 | 15 ± 2 | 39 ± 12 | 0.17 ± 0.01 | 4.23 ± 0.06 | ||
| Male | Open sea | 26 | 13 ± 1 | 26 ± 16 | 0.22 ± 0.01 | 1.85 ± 0.16 | |
| Coast | 8 | 13 ± 1 | 21 ± 7 | 0.11 ± 0.01 | 3.63 ± 0.30 | ||
| Total | 89 | 14 ± 1 (13–15) | 31 ± 8 (21–39) | 0.28 ± 0.23 (0.11–0.62) | 2.84 ± 1.29 (1.62–4.23) | ||
| Post-spawning | Female | Open sea | 31 | 16 ± 2 | 42 ± 17 | 0.12 ± 0.01 | 7.87 ± 0.07 |
| Coast | 80 | 15 ± 2 | 35 ± 13 | 0.05 ± 0.01 | 2.59 ± 0.14 | ||
| Male | Open sea | 58 | 13 ± 1 | 23 ± 9 | 0.23 ± 0.01 | 0.44 ± 0.03 | |
| Coast | 20 | 13 ± 1 | 22 ± 5 | 0.24 ± 0.01 | 1.82 ± 0.08 | ||
| Total | 189 | 14 ± 2 (13–16) | 31 ± 10 (22–42) | 0.16 ± 0.09 (0.05–0.24) | 3.18 ± 3.25 (0.44–7.87) | ||
| Grand total | 407 | 13 ± 2 (10–17) | 27 ± 12 (9–49) | 0.20 ± 0.15 (0.03–0.62) | 3.29 ± 2.43 (0.44–7.87) | ||
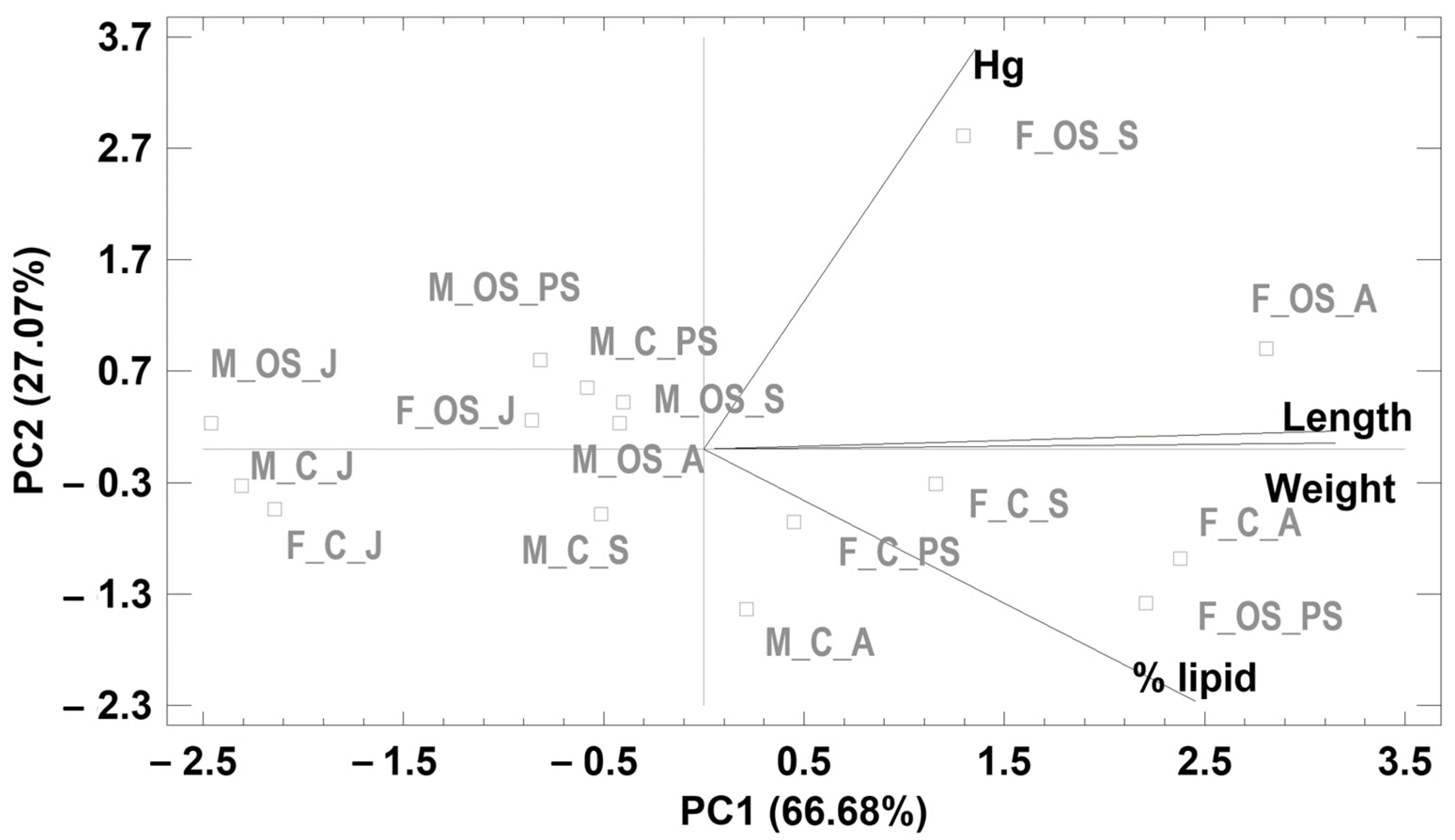
| Principal Components | ||
|---|---|---|
| 1 | 2 | |
| Variance explained | ||
| Eigenvalues | 2.6673 | 1.0828 |
| % of variance | 66.683 | 27.070 |
| Cumulative % | 66.683 | 93.754 |
| Factor loadings | ||
| Length | 0.5990 | 0.0387 |
| Weight | 0.5985 | 0.0127 |
| Hg | 0.2571 | 0.8452 |
| Lipid | 0.4657 | −0.5328 |
| Sampling Area | n | Total Length, cm (Weight, g) | THg, mg kg−1 ww (Min–Max) | Reference |
|---|---|---|---|---|
| Adriatic Sea | 407 (16 pool) | 10–17 (9–49) | 0.20 ± 0.15 (0.03–0.62) | This study |
| Adriatic Sea | (0.08–1.74) | [17] | ||
| Central Adriatic Sea | 14 | 13.75 ± 0.44 (34.96 ± 3.71) | 0.48 ± 0.9 | [25] |
| Eastern Adriatic Sea | 60 | 9.4–15.5 (10–62) | (0.178–0.996) | [19] |
| Central Adriatic Sea | 25 | 15 ± 0.4 (37 ± 5) | 0.236 ± 0.102 | [34] |
| Central Tyrrhenian Sea | 58 | 18 ± 2 (73 ± 29) | 0.702 ±0.834 | [34] |
| Spanish market | 20 | (0.14–0.36) | [22] | |
| Gulf of Lions | 132 | 8.5–24.5 | 0.244 ± 0.253 (0.004–1.962) | [24] |
| Sicily | 48 | 0.138 ± 0.107 | [21] | |
| Ionian Sea | (nd–1.50) | [17] | ||
| Ionian Sea | (nd–1.50) | [18] | ||
| Spanish Mediterranean | 36 | 13.5–14.5 | (0.074–0.121) | [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girolametti, F.; Frapiccini, E.; Annibaldi, A.; Illuminati, S.; Panfili, M.; Marini, M.; Santojanni, A.; Truzzi, C. Total Mercury (THg) Content in Red Mullet (Mullus barbatus) from Adriatic Sea (Central Mediterranean Sea): Relation to Biological Parameters, Sampling Area and Human Health Risk Assessment. Appl. Sci. 2022, 12, 10083. https://doi.org/10.3390/app121910083
Girolametti F, Frapiccini E, Annibaldi A, Illuminati S, Panfili M, Marini M, Santojanni A, Truzzi C. Total Mercury (THg) Content in Red Mullet (Mullus barbatus) from Adriatic Sea (Central Mediterranean Sea): Relation to Biological Parameters, Sampling Area and Human Health Risk Assessment. Applied Sciences. 2022; 12(19):10083. https://doi.org/10.3390/app121910083
Chicago/Turabian StyleGirolametti, Federico, Emanuela Frapiccini, Anna Annibaldi, Silvia Illuminati, Monica Panfili, Mauro Marini, Alberto Santojanni, and Cristina Truzzi. 2022. "Total Mercury (THg) Content in Red Mullet (Mullus barbatus) from Adriatic Sea (Central Mediterranean Sea): Relation to Biological Parameters, Sampling Area and Human Health Risk Assessment" Applied Sciences 12, no. 19: 10083. https://doi.org/10.3390/app121910083
APA StyleGirolametti, F., Frapiccini, E., Annibaldi, A., Illuminati, S., Panfili, M., Marini, M., Santojanni, A., & Truzzi, C. (2022). Total Mercury (THg) Content in Red Mullet (Mullus barbatus) from Adriatic Sea (Central Mediterranean Sea): Relation to Biological Parameters, Sampling Area and Human Health Risk Assessment. Applied Sciences, 12(19), 10083. https://doi.org/10.3390/app121910083












