Field Experience for Determination of Formaldehyde in Stack Emissions
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. CEN TC 264 WG 40 “Emissions—Formaldehyde”
- Reproducibility
- Repeatability
- Sampling procedures
- Safety aspects
2.2. Experimental Investigations
- Agenzia Provinciale Protezione Ambiente (APPA) Trento Laboratory, Trento (TN), Italy;
- Ricerca sul Sistema Energetico (RSE) Laboratory, Milan (MI), Italy.
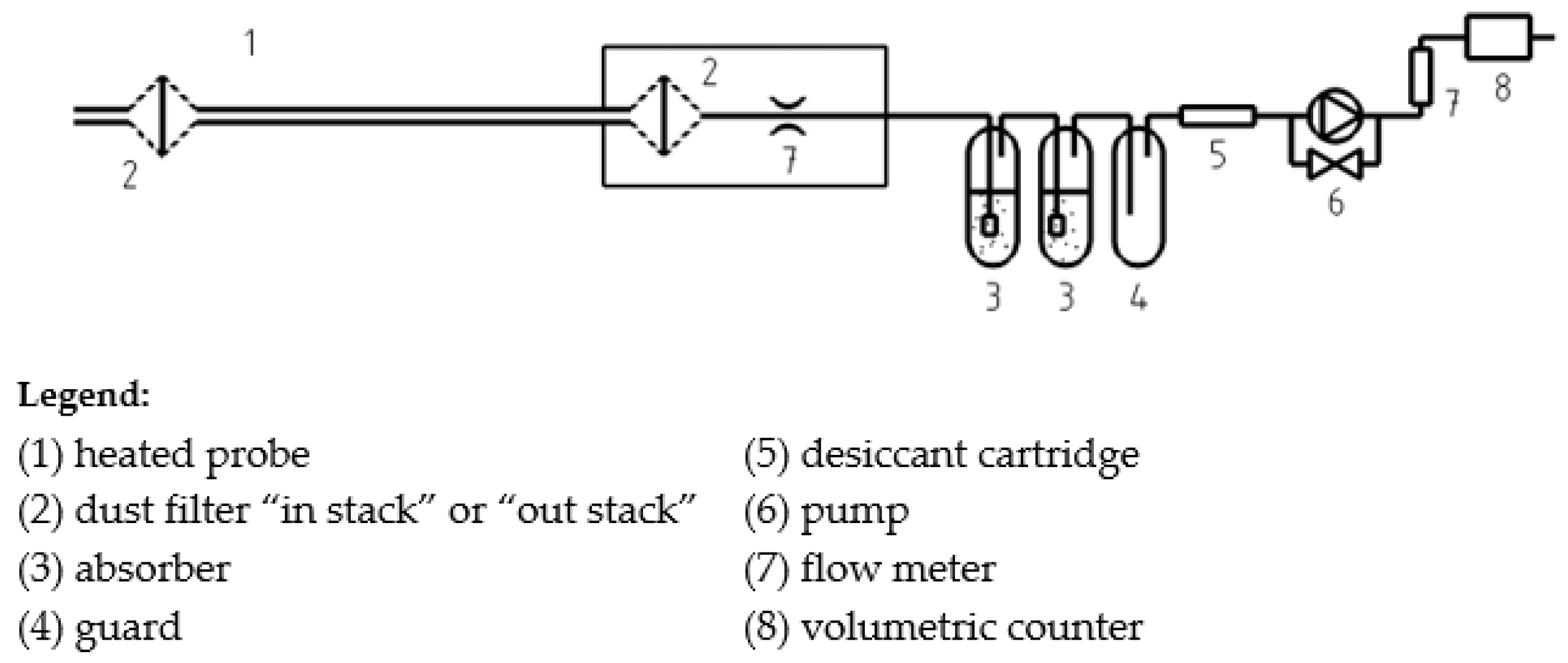
2.3. Sampling System
2.4. Instruments
2.5. Protocol Description
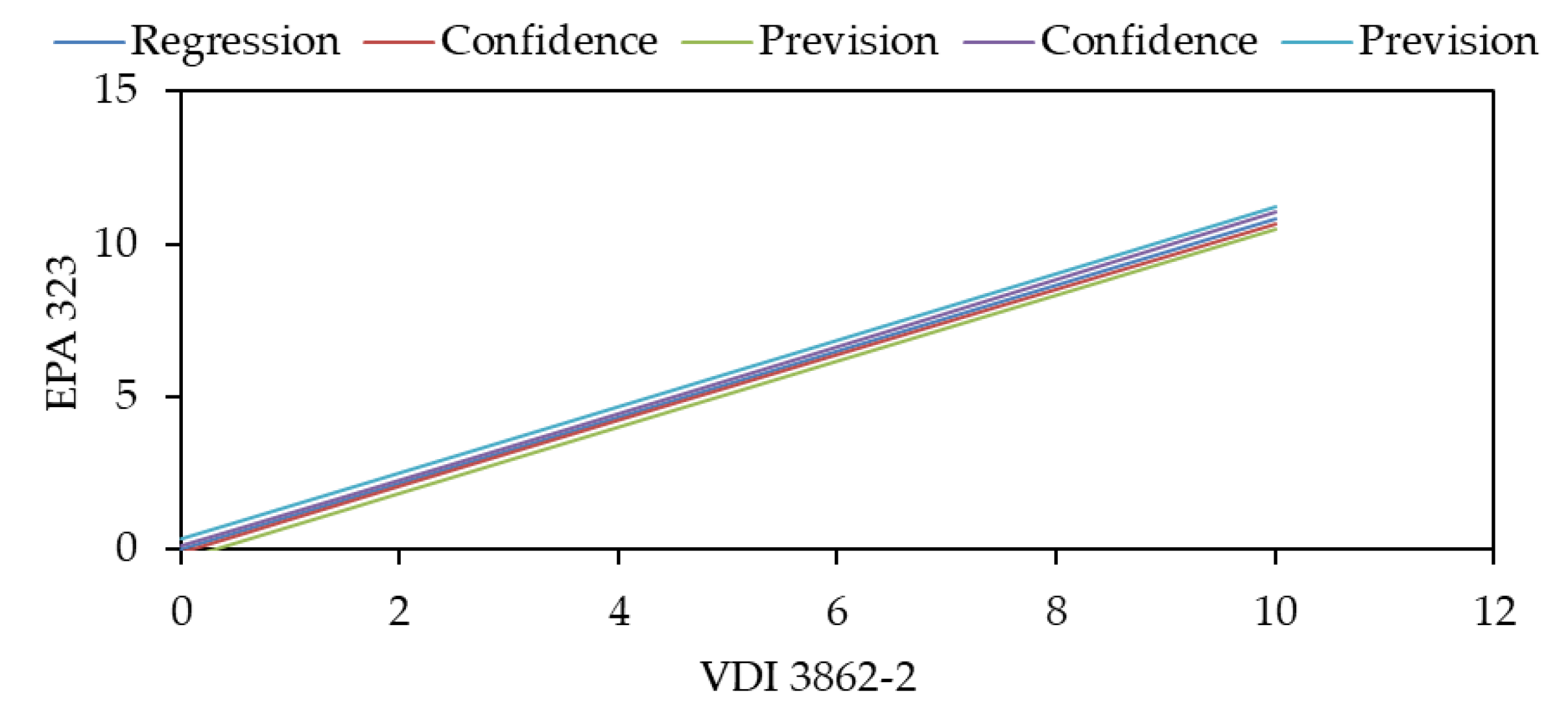
3. Results and Discussion
3.1. Sampling
3.2. Analysis
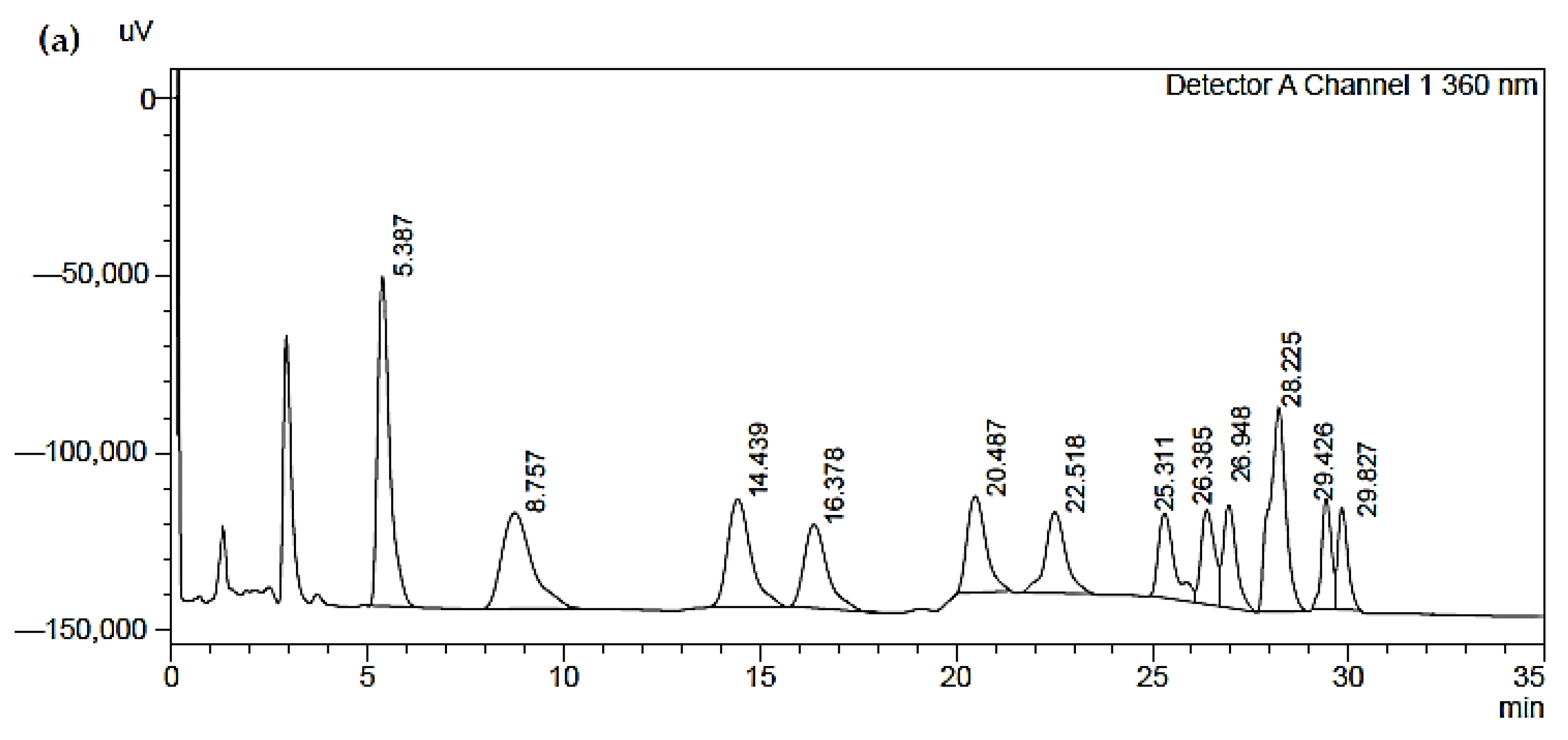
- The first (a) represents a reference standard used (the concentration values are guaranteed by the sample supplier). The peaks of the chromatograms are described in Table 8;
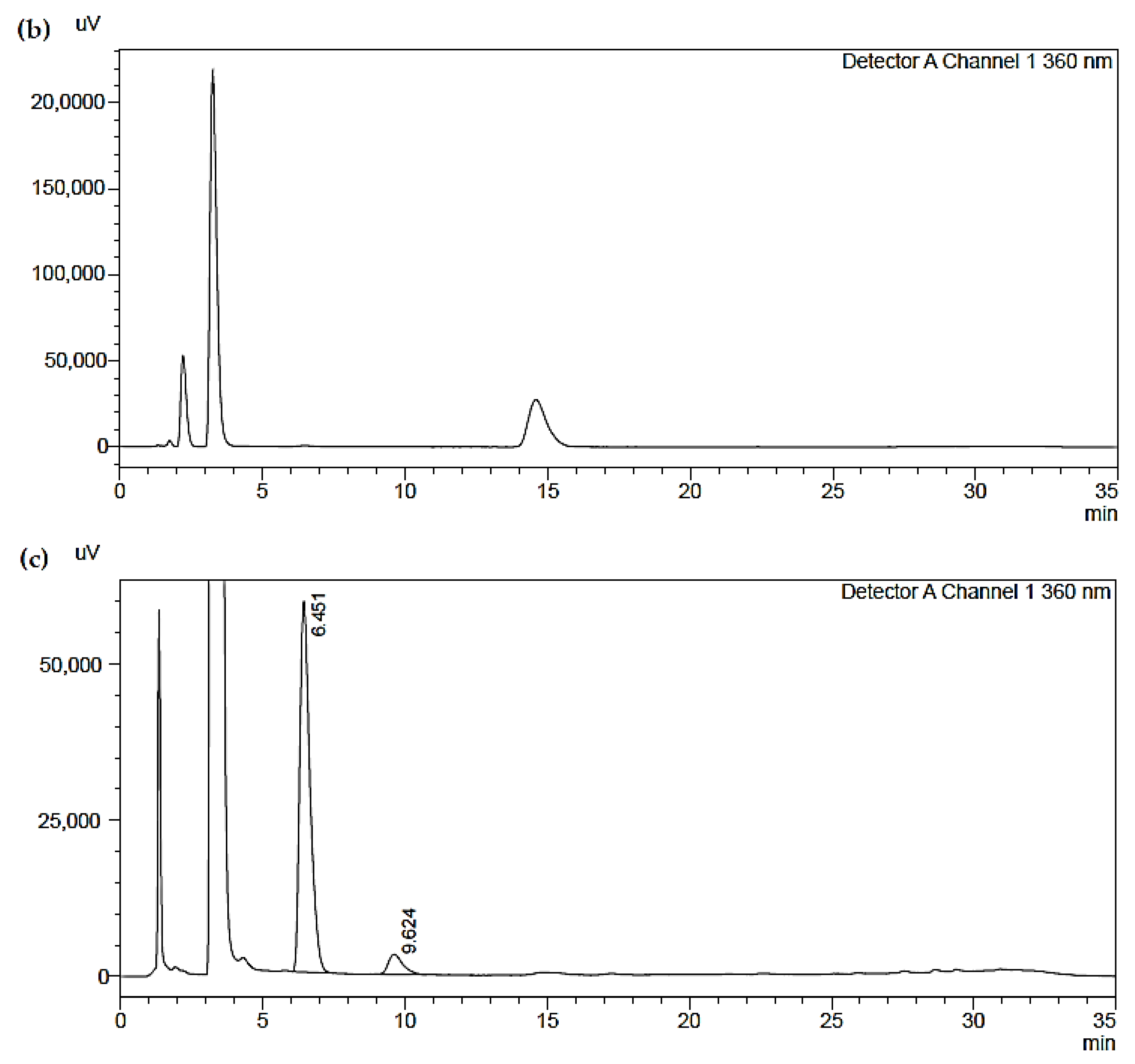
- The second (b) represents a blank sample, which shows the substantial suitability of the solutions used (the blank samples are real analytical samples not exposed to the fumes but which have been treated exactly like the others);
- The third (c) represents one of the analyzed samples: the sample contains only formaldehyde (H−CHO and acetaldehyde (CH3CHO), compatible with the analyzed analytes. The chromatogram results with a good baseline and very well identified peaks, which guarantees good accuracy of results, demonstrating the validity of the method.
- The first was analyzed by Lab. APPA Trento;
- The second complexed immediately and was analyzed after 5 days;
- The third complexed immediately and was analyzed after 15 days;
- The fourth complexed after 5 days and was analyzed after 5 days;
- The fifth complexed after 5 days and was analyzed after 15 days.
- An objective error in carrying out the analyzes;
- A modification of the plant conditions, which has led to the formation of an interferent.
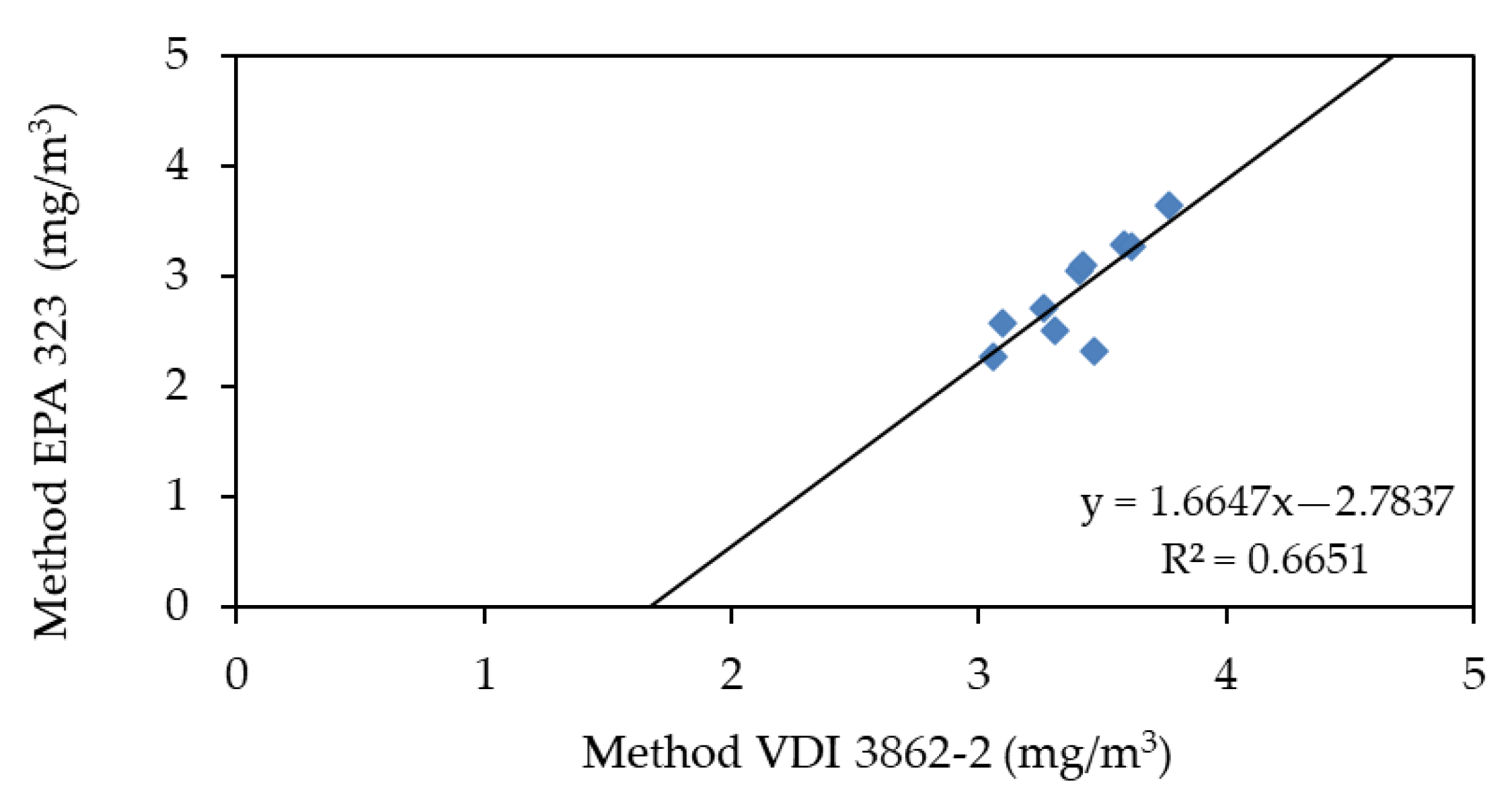
3.3. Performance of the Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Shao, M.; Li, L.Y.; Lu, S.H.; Chen, W.T.; Wang, C. Quantifying the ambient formaldehyde sources utilizing tracers. Chinese Chem. Lett. 2014, 25, 1489–1491. [Google Scholar] [CrossRef]
- Parrish, D.D.; Ryerson, T.B.; Mellqvist, J.; Johansson, J.; Fried, A.; Richter, D.; Walega, J.G.; Washenfelder, R.A.; de Gouw, J.A.; Peischl, J.; et al. Primary and secondary sources of formaldehyde in urban atmospheres: Houston Texas region. Atmos. Chem. Phys. 2012, 12, 3273–3288. [Google Scholar] [CrossRef] [Green Version]
- Lei, W.; Zavala, M.; de Foy, B.; Volkamer, R.; Molina, M.J.; Molina, L.T. Impact of primary formaldehyde on air pollution in the Mexico City Metropolitan Area. Atmos. Chem. Phys. 2009, 9, 2607–2618. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, S.M.; Arbilla, G. Formaldehyde and acetaldehyde associated with the use of natural gas as a fuel for light vehicles. Atmos. Environ. 2005, 39, 4513–4518. [Google Scholar] [CrossRef]
- Ho, S.S.H.; Ho, K.F.; Lee, S.C.; Cheng, Y.; Yu, J.Z.; Lam, K.M.; Feng, N.S.Y.; Huang, Y. Carbonyl emissions from vehicular exhausts sources in Hong Kong. J. Air Waste Manag. Assoc. 2012, 62, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Ho, K.F.; Chan, L.Y.; Zielinska, B.; Chow, J.C. Polycyclic Aromatic Hydrocarbons (pahs) and Carbonyl Compounds in Urban Atmosphere of Hong Kong. Atmos. Environ. 2001, 35, 5949–5960. [Google Scholar] [CrossRef]
- Guven, B.B.; Olaguer, E.P. Ambient formaldehyde source attribution in Houston during TexAQS II and TRAMP. Atmos. Environ. 2011, 45, 4272–4280. [Google Scholar] [CrossRef]
- Kaiser, J.; Wolfe, G.M.; Bohn, B.; Broch, S.; Fuchs, H.; Ganzeveld, L.N.; Gomm, S.; Häseler, R.; Hofzumahaus, A.; Holland, F.; et al. Evidence for an unidentified non-photochemical ground-level source of formaldehyde in the Po Valley with potential implications for ozone production. Atmos. Chem. Phys. 2015, 15, 1289–1298. [Google Scholar] [CrossRef] [Green Version]
- Seco, R.; Peñuelas, J.; Filella, I. Short-chain oxygenated VOCs: Emission and uptake by plants and atmospheric sources, sinks, and concentrations. Atmos. Environ. 2007, 41, 2477–2499. [Google Scholar] [CrossRef]
- Seinfeld, J.H.; Pandis, S.N. ATMOSPHERIC From Air Pollution to Climate Change, 2nd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Vlasenko, A.; MacDonald, A.M.; Sjostedt, S.J.; Abbatt, J.P.D. Formaldehyde measurements by Proton transfer reaction—Mass spectrometry (PTR-MS): Correction for humidity effects. Atmos. Meas. Tech. 2010, 3, 1055–1062. [Google Scholar] [CrossRef]
- Duan, Y.; He, K.; Zhang, G.; Hu, J. Photoresponsive micelles enabling codelivery of nitric oxide and formaldehyde for combinatorial antibacterial applications. Biomacromolecules 2021, 22, 2160–2170. [Google Scholar] [CrossRef] [PubMed]
- Birkett, N.; Al-Zoughool, M.; Bird, M.; Baan, R.A.; Zielinski, J.; Krewski, D. Overview of biological mechanisms of human carcinogens. J. Toxicol. Environ. Health Part B Crit. Rev. 2019, 22, 288–359. [Google Scholar] [CrossRef] [Green Version]
- Brandão, P.F.; Ramos, R.M.; Rodrigues, J.A. GDME-based methodology for the determination of free formaldehyde in cosmetics and hygiene products containing formaldehyde releasers. Anal. Bioanal. Chem. 2018, 410, 6873–6880. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Bai, Y.; Duong, A.; Smith, M.T.; Li, L.; Zhang, L. Formaldehyde in China: Production, consumption, exposure levels, and health effects. Environ. Int. 2009, 35, 1210–1224. [Google Scholar] [CrossRef] [PubMed]
- Park, B.-D.; Kim, J.-W. Dynamic Mechanical Analysis of Urea-Formaldehyde Resin Adhesives with Different Formaldehyde-To-Urea Molar Ratios. J. Appl. Polym. Sci. 2008, 108, 2045–2051. [Google Scholar] [CrossRef]
- He, Z.; Zhang, Y.; Wei, W. Formaldehyde and VOC emissions at different manufacturing stages of wood-based panels. Build. Environ. 2012, 47, 197–204. [Google Scholar] [CrossRef]
- Lui, K.H.; Ho, S.S.H.; Louie, P.K.; Chan, C.; Lee, S.; Hu, D.; Chan, P.; Lee, J.C.W.; Ho, K. Seasonal behavior of carbonyls and source characterization of formaldehyde (HCHO) in ambient air. Atmos. Environ. 2017, 152, 51–60. [Google Scholar] [CrossRef]
- Carlier, P.; Hannachi, H.; Mouvier, G. The chemistry of carbonyl compounds in the atmosphere-A review. Atmos. Environ. 1986, 20, 2079–2099. [Google Scholar] [CrossRef]
- Cheng, Y.; Lee, S.; Huang, Y.; Ho, K.; Ho, S.; Yau, P.; Louie, P.; Zhang, R. Diurnal and seasonal trends of carbonyl compounds in roadside, urban, and suburban environment of Hong Kong. Atmos. Environ. 2014, 89, 43–51. [Google Scholar] [CrossRef]
- Fappiano, L.; Carriera, F.; Iannone, A.; Notardonato, I.; Avino, P. A Review on Recent Sensing Methods for Determining Formaldehyde in Agri-Food Chain: A Comparison with the Conventional Analytical Approaches. Foods 2022, 11, 1351. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cui, X.; Fang, G. Rapid determination of formaldehyde and sulfur dioxide in food products and Chinese herbals. Food Chem. 2007, 103, 1487–1493. [Google Scholar] [CrossRef]
- Ahrenfeldt, J.; Jensen, T.K.; Henriksen, U.; Schramm, J. Investigation of continuous gas engine CHP operation on biomass producer gas. SAE Tech. Pap. 2005, 114, 1464–1475. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, S.; Liu, F.; Liu, J.; Zhu, Z.; Li, G. Formaldehyde and Methanol Emissions from a Methanol/Gasoline-Fueled Spark-Ignition (SI) Engine. Energy Fuels 2009, 23, 3313–3318. [Google Scholar] [CrossRef]
- Villanueva, F.; Lara, S.; Amo-Salas, M.; Cabañas, B.; Martín, P.; Salgado, S. Investigation of formaldehyde and other carbonyls in a small urban atmosphere using passive samplers. A comprehensive data analysis. Microchem. J. 2021, 167, 106270. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO: Lyon, France, 2006; Volume 88.
- European Commission Regulation. Part 3 of Annex VI of the EC Regulation 1272/2008; European Commission: Brussel, Belgium, 2014.
- Panda, N.; Kim, M.; Aoki, N.; Zhou, Z.; Shimosaka, T.; Kim, Y.; Lee, S.; Kim, D. Validation of primary formaldehyde gas standards prepared by dynamic thermogravimetry through a tri-national comparison of gaseous formaldehyde amount fraction. Accredit. Qual. Assur. 2016, 21, 295–304. [Google Scholar] [CrossRef]
- Hanoune, B.; LeBris, T.; Allou, L.; Marchand, C.; le Calvé, S. Formaldehyde measurements in libraries: Comparison between infrared diode laser spectroscopy and a DNPH-derivatization method. Atmos. Environ. 2006, 40, 5768–5775. [Google Scholar] [CrossRef]
- Dumas, T. Determination of formaldehyde in air by gas chromatography. J. Chromatogr. A 1982, 247, 289–295. [Google Scholar] [CrossRef]
- Mann, B.; Grayeski, M.L. New chemiluminescent derivatizing agent for the analysis of aldehydes and ketones by high-performance liquid chromatography with peroxyoxalate chemiluminescence. J. Chromatogr. A 1987, 386, 149–158. [Google Scholar] [CrossRef]
- Lorrain, J.M.; Fortune, C.R.; Dellinger, B. Sampling and Ion Chromatographic Determination of Formaldehyde and Acetaldehyde. Anal. Chem. 1981, 53, 1302–1305. [Google Scholar] [CrossRef]
- Wisthaler, A.; Apel, E.C.; Bossmeyer, J.; Hansel, A.; Junkermann, W.; Koppmann, R.; Meier, R.; Müller, K.; Solomon, S.J.; Steinbrecher, R.; et al. Technical Note: Intercomparison of formaldehyde measurements at the atmosphere simulation chamber SAPHIR. Atmos. Chem. Phys. 2008, 8, 2189–2200. [Google Scholar] [CrossRef]
- Hak, C.; Pundt, I.; Trick, S.; Kern, C.; Platt, U.; Dommen, J.; Ordóñez, C.; Prévôt, A.S.H.; Junkermann, W.; Astorga-Lloréns, C.; et al. Intercomparison of four different in-situ techniques for ambient formaldehyde measurements in urban air. Atmos. Chem. Phys. 2005, 5, 2881–2900. [Google Scholar] [CrossRef] [Green Version]
- Salthammer, T.; Mentese, S. Comparison of analytical techniques for the determination of aldehydes in test chambers. Chemosphere 2008, 73, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Giesen, R.; Schripp, T.; Markewitz, D.; Meyer, B.; Schwab, H.; Uhde, E.; Salthammer, T. Comparison of Methods for the Determination of Formaldehyde in Air. Anal. Lett. 2016, 49, 1613–1621. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J. Comparison of standard methods and gas chromatography method in determination of formaldehyde emission from MDF bonded with formaldehyde-based resins. Bioresour. Technol. 2005, 96, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Risholm-Sundman, M.; Larsen, A.; Vestin, E.; Weibull, A. Formaldehyde emission-Comparison of different standard methods. Atmos. Environ. 2007, 41, 3193–3202. [Google Scholar] [CrossRef]
- Kleindienst, T.E.; Corse, E.W.; Blanchard, F.T.; Lonneman, W.A. Evaluation of the performance of DNPH-coated silica gel and C18 cartridges in the measurement of formaldehyde in the presence and absence of ozone. Environ. Sci. Technol. 1998, 32, 124–130. [Google Scholar] [CrossRef]
- Cipriano, D.; Cefalì, A.M.; Allegrini, M. Experimenting with odour proficiency tests implementation using synthetic bench loops. Atmosphere 2021, 12, 761. [Google Scholar] [CrossRef]
- Cipriano, D.; Fialdini, L. Definition of reference values in synthetic emission monitoring bench loops. Accredit. Qual. Assur. 2018, 23, 125–132. [Google Scholar] [CrossRef]
- EPA. Method 323—Measurement of Formaldehyde Emissions from Natural Gas; U.S. Government Publishing Office: Washington, DC, USA, 2020.
- VDI 3862; Gaseous Emission Measurement; Measurement of Aliphatic and Aromatic Aldehydes and Ketones by DNPH Method. Verlag des Vereins Deutscher Ingenieure: Berlin, Germany, 2000.
- CEN/TS 17638:2021; Stationary Source Emissions—Manual Method for the Determination of the Mass Concentration of Formaldehyde—Reference Method. European Commission: Brussel, Belgium, 2021.
- ISO 10155; Stationary Source Emission—Automated Monitoring of Mass Concentrations of Particles—Performance Characteristics, Test Methods and Specifications. ISO: Geneva, Switzerland, 1995.





| Main Characteristics of the Methods | ||
|---|---|---|
| Method | EPA 323—VDI 3862-6 | VDI 3862-2 |
| Principle | Not isokinetical integral sampling | Not isokinetical integral sampling |
| Absorbing Solution | H2O | H2O + H2SO4 + DNPH |
| Measurement principle | Colorimetric | HPLC |
| Main reagent | Acetylacetone | 2,4-Dinitrophenylhydrazine hydrochloric acid solution (DNPH) |
| Characteristic Sampling | Lab. APPA Trento | Lab. APPA Trento | Lab. RSE |
|---|---|---|---|
| Method | Not isokinetic extraction | Not isokinetic extraction | Not isokinetic extraction |
| Number of samplings | 14 | 14 | 14 |
| Absorbing solution | Pure water | 0.01N H2SO4 + DNPH | 0.01 H2SO4 |
| Treatment of absorbing solution | None | None | DNPH addition |
| Washing solutions | Washed with water, added to sampling solution | Washed with water, added to sampling solution | None |
| Characteristic Analysis | Lab. APPA Trento | Lab. APPA Trento | Lab. RSE |
|---|---|---|---|
| Method implemented | EPA 323—VDI 3862-6 | VDI 3862-2 | VDI 3862-2 |
| Time of analysis | Day after sampling | Day after sampling | Solutions were divided into 4 aliquots: #1 added with DNPH and analyzed by Lab. APPA, 1 day after sampling #2 added with DNPH, stored for 2 days and analyzed #3 added with DNPH, stored for 15 days, and then analyzed #4 stored for 15 days and after added with DNPH and analyzed after 2 days |
| Characteristic Sampling | Lab. APPA Trento | Lab. APPA Trento | Lab. RSE |
|---|---|---|---|
| Method | Not isokinetic extraction | Not isokinetic extraction | Not isokinetic extraction |
| Number of samplings | 10 | 10 | 10 |
| Absorbing solution | Pure water | 0.01N H2SO4 + DNPH | 0.01 H2SO4 |
| Treatment of absorbing solution | None | None | DNPH addition |
| Washing solutions | Washed with water, added to sampling solution | Washed with water, added to sampling solution | None |
| Characteristic Analysis | Lab. APPA Trento | Lab. APPA Trento | Lab. RSE |
|---|---|---|---|
| Method implemented | EPA 323—VDI 3862-6 | VDI 3862-2 | VDI 3862-2 |
| Time of analysis | Day after sampling | Day after sampling | Solutions were divided into 5 aliquots: #1 added with DNPH just after sampling and analyzed by Lab. APPA #2 added with DNPH just after sampling and analyzed after 5 days #3 added with DNPH just after sampling and analyzed after 15 days #4 added with DNPH after 5 days and analyzed after 5 days #5 added with DNPH after 5 days and analyzed after 15 days |
| Sampling | Humidity (%) | H−CHO (mg/m3) |
|---|---|---|
| 1 | 12.7 | 33.4 |
| 2 | 6.6 | 1.2 |
| 3 | 14.4 | 47.4 |
| 4 | 5.0 | 3.1 |
| 5 | 16.5 | 14.5 |
| 6 | 11.2 | 3.0 |
| 7 | 11.2 | 2.9 |
| 8 | 11.5 | 2.9 |
| 9 | 10.6 | 10.6 |
| 10 | 12.0 | 8.7 |
| 11 | 12.2 | 7.5 |
| 12 | 10.9 | 3.0 |
| 13 | 10.9 | 3.4 |
| 14 | 10.3 | 12.8 |
| Sampling | Humidity (%) | H−CHO (mg/m3) |
|---|---|---|
| 1 | 2.4 | 11.7 |
| 2 | 1.7 | 11.4 |
| 3 | 1.8 | 9.3 |
| 4 | 1.7 | 11.4 |
| 5 | 1.9 | 11.0 |
| 6 | 1.6 | 10.8 |
| 7 | 1.5 | 10.2 |
| 8 | 1.6 | 11.4 |
| 9 | 1.6 | 11.0 |
| 10 | 1.6 | 11.1 |
| ID | Name | Retention Time | Area | Concentration (mg/mL) |
|---|---|---|---|---|
| 1 | Formaldehyde | 5.387 | 1,980,978 | 1.274 |
| 2 | Acetaldehyde | 8.757 | 1,447,755 | 1.177 |
| 3 | Acrolein | 14.439 | 1,253,045 | 0.969 |
| 4 | Propionaldehyde | 16.378 | 963,194 | 1.033 |
| 5 | Crotonaldehyde | 20.487 | 908,469 | 1.082 |
| 6 | Butyraldehyde | 22.518 | 828,587 | 1.033 |
| 7 | Benzaldehyde | 25.311 | 688,809 | 1.192 |
| 8 | Isovaleraldehyde | 26.385 | 63,514 | 1.053 |
| 9 | Valeraldehyde | 26.948 | 675,408 | 1.062 |
| 10 | Otolualdehyde | 28.225 | 1,659,591 | 1.177 |
| 11 | Hexanaldehyde | 29.426 | 534,402 | 1.049 |
| 12 | 2,5-Dimethylbenzaldehyde | 29.827 | 499,612 | 1.070 |
| 13 | Acetone | - | - | - |
| Samplings Lab. APPA Trento | Samplings Lab. RSE | |||||
|---|---|---|---|---|---|---|
| Samplings | Solution | VDI 3862-2 (Lab. APPA Trento) mg/L | EPA 323—VDI 3862-6 (Lab. APPA Trento) mg/L | Solution | VDI 3862-2 (Lab. RSE) mg/L | VDI 3862-2 (Lab. APPA Trento) mg/L |
| 1 | H2O | 0.711 | 2.293 | H2SO4 | 12.137 | 13.204 |
| 2 | H2O | 0.474 | 1.289 | H2SO4 | 7.787 | 1.488 |
| 3 | H2O | 2.873 | 6.572 | H2SO4 | 25.593 | 27.426 |
| 4 | H2O | 0.064 | 0.396 | H2SO4 | 3.811 | 3.977 |
| 5 | H2O | 0.048 | 0.336 | H2SO4 | 6.351 | 7.469 |
| 6 | H2O | 0.048 | 0.320 | H2SO4 | 3.680 | 3.678 |
| 7 | H2O | 0.057 | 0.323 | H2SO4 | 4.028 | 4.588 |
| 8 | H2O | 0.077 | 0.359 | H2SO4 | 3.898 | 4.294 |
| 9 | H2O | 1.344 | 1.535 | H2SO4 | 5.494 | 5.548 |
| 10 | H2O | 1.356 | 1.213 | H2SO4 | 4.974 | 4.908 |
| 11 | H2SO4 | 1.235 | 1.052 | H2SO4 | 4.770 | 4.801 |
| 12 | H2SO4 | 0.998 | 0.830 | H2SO4 | 4.462 | 4.278 |
| 13 | H2SO4 | 1.281 | 1.013 | H2SO4 | 4.614 | 4.427 |
| 14 | H2SO4 | 2.619 | 2.452 | H2SO4 | 10.670 | 10.828 |
| Samplings | Derivatized Sample and Analyzed on Arrival (mg/L) | Derivatized Sample at T0 and Analyzed after 15 days (mg/L) | Derivatized Sample after 15 Days and Analyzed Immediately (mg/L) |
|---|---|---|---|
| 1 | 12.62 | 13.25 | 6.70 |
| 2 | 1.08 | 1.03 | 0.81 |
| 3 | 30.29 | 33.79 | 18.27 |
| 4 | 2.33 | 2.47 | 2.05 |
| 5 | 4.71 | 5.45 | 2.38 |
| 6 | 2.20 | 2.50 | 1.90 |
| 7 | 2.27 | 2.56 | 2.00 |
| 8 | 2.22 | 2.42 | 1.91 |
| 9 | 4.04 | 4.79 | 2.28 |
| 10 | 3.08 | 3.74 | 1.72 |
| 11 | 2.55 | 3.10 | 1.44 |
| 12 | 2.29 | 2.69 | 2.39 |
| 13 | 2.67 | 2.82 | 2.42 |
| 14 | 10.38 | 12.03 | 6.24 |
| Samplings | Volume of Sampled (L) | Dry Volume of Sampled (L) | H−CHO (VDI 3862-2) mg/L | H−CHO (EPA 323 VDI 3862-6) mg/L |
|---|---|---|---|---|
| 1 | 32.20 | 31.10 | 0.647 | 0.435 |
| 2 | 30.50 | 29.30 | 0.566 | 0.423 |
| 3 | 31.00 | 29.70 | 0.735 | 0.561 |
| 4 | 31.00 | 29.70 | 0.694 | 0.578 |
| 5 | 31.00 | 29.50 | 0.716 | 0.598 |
| 6 | 30.50 | 28.90 | 0.799 | 0.717 |
| 7 | 67.40 | 63.90 | 1.674 | 1.523 |
| 8 | 31.00 | 29.30 | 0.846 | 0.777 |
| 9 | 31.00 | 29.20 | 0.819 | 0.739 |
| 10 | 31.50 | 29.60 | 0.926 | 0.897 |
| Sampling | H−CHO (Lab. APPA Trento) mg/L | H−CHO (Lab. RSE) mg/L |
|---|---|---|
| 1 | 2.389 | 2.196 |
| 2 | 1.653 | 1.682 |
| 3 | 1.774 | 1.742 |
| 4 | 1.658 | 1.663 |
| 5 | 1.857 | 1.763 |
| 6 | 1.561 | 1.551 |
| 7 | 1.456 | 1.483 |
| 8 | 1.643 | 1.667 |
| 9 | 1.583 | 1.610 |
| 10 | 1.624 | 1.715 |
| Sampling | Volume of Sampled (L) | Relative Humidity (%) | H−CHO (mg/L) |
|---|---|---|---|
| 1 | 34.449 | 11.712 | 2.954 |
| 2 | 29.228 | 11.366 | 1.802 |
| 3 | 32.199 | 9.327 | 2.162 |
| 4 | 32.271 | 11.356 | 2.072 |
| 5 | 34.519 | 11.011 | 2.316 |
| 6 | 32.229 | 10.788 | 1.870 |
| 7 | 71.547 | 10.247 | 3.272 |
| 8 | 30.724 | 11.417 | 2.006 |
| 9 | 30.877 | 11.017 | 1.888 |
| 10 | 30.725 | 11.129 | 1.902 |
| Complex Immediately | Complex after Three Days | |||
|---|---|---|---|---|
| Sampling | Analyzed after 5 Days (mg/L) | Analyzed after 15 Days (mg/L) | Analyzed after 5 Days (mg/L) | Analyzed after 15 Days (mg/L) |
| 1 | 2.954 | 2.946 | 2.080 | 1.812 |
| 2 | 1.802 | 1.714 | 1.338 | 1.072 |
| 3 | 2.162 | 2.118 | 1.652 | 1.610 |
| 4 | 2.072 | 1.846 | 1.524 | 1.462 |
| 5 | 2.316 | 2.322 | 1.734 | 1.756 |
| 6 | 1.870 | 1.802 | 1.238 | 1.208 |
| 7 | 3.272 | 3.072 | 2.424 | 2.464 |
| 8 | 2.006 | 1.930 | 1.418 | 1.430 |
| 9 | 1.888 | 1.688 | 1.222 | 1.204 |
| 10 | 1.902 | 1.490 | 1.222 | 1.158 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cefalì, A.M.; Bolzacchini, E.; Ferrero, L.; Clauser, G.; Dallapiccola, C.; Maggi, S.; Cipriano, D. Field Experience for Determination of Formaldehyde in Stack Emissions. Appl. Sci. 2022, 12, 10150. https://doi.org/10.3390/app121910150
Cefalì AM, Bolzacchini E, Ferrero L, Clauser G, Dallapiccola C, Maggi S, Cipriano D. Field Experience for Determination of Formaldehyde in Stack Emissions. Applied Sciences. 2022; 12(19):10150. https://doi.org/10.3390/app121910150
Chicago/Turabian StyleCefalì, Amedeo M., Ezio Bolzacchini, Luca Ferrero, Giuseppe Clauser, Christian Dallapiccola, Stefano Maggi, and Domenico Cipriano. 2022. "Field Experience for Determination of Formaldehyde in Stack Emissions" Applied Sciences 12, no. 19: 10150. https://doi.org/10.3390/app121910150
APA StyleCefalì, A. M., Bolzacchini, E., Ferrero, L., Clauser, G., Dallapiccola, C., Maggi, S., & Cipriano, D. (2022). Field Experience for Determination of Formaldehyde in Stack Emissions. Applied Sciences, 12(19), 10150. https://doi.org/10.3390/app121910150









