X-ray Phase Contrast Imaging from Synchrotron to Conventional Sources: A Review of the Existing Techniques for Biological Applications
Abstract
:Featured Application
Abstract
1. Introduction
2. Physical Foundations of X-ray Phase Contrast and Dark-Field Imaging
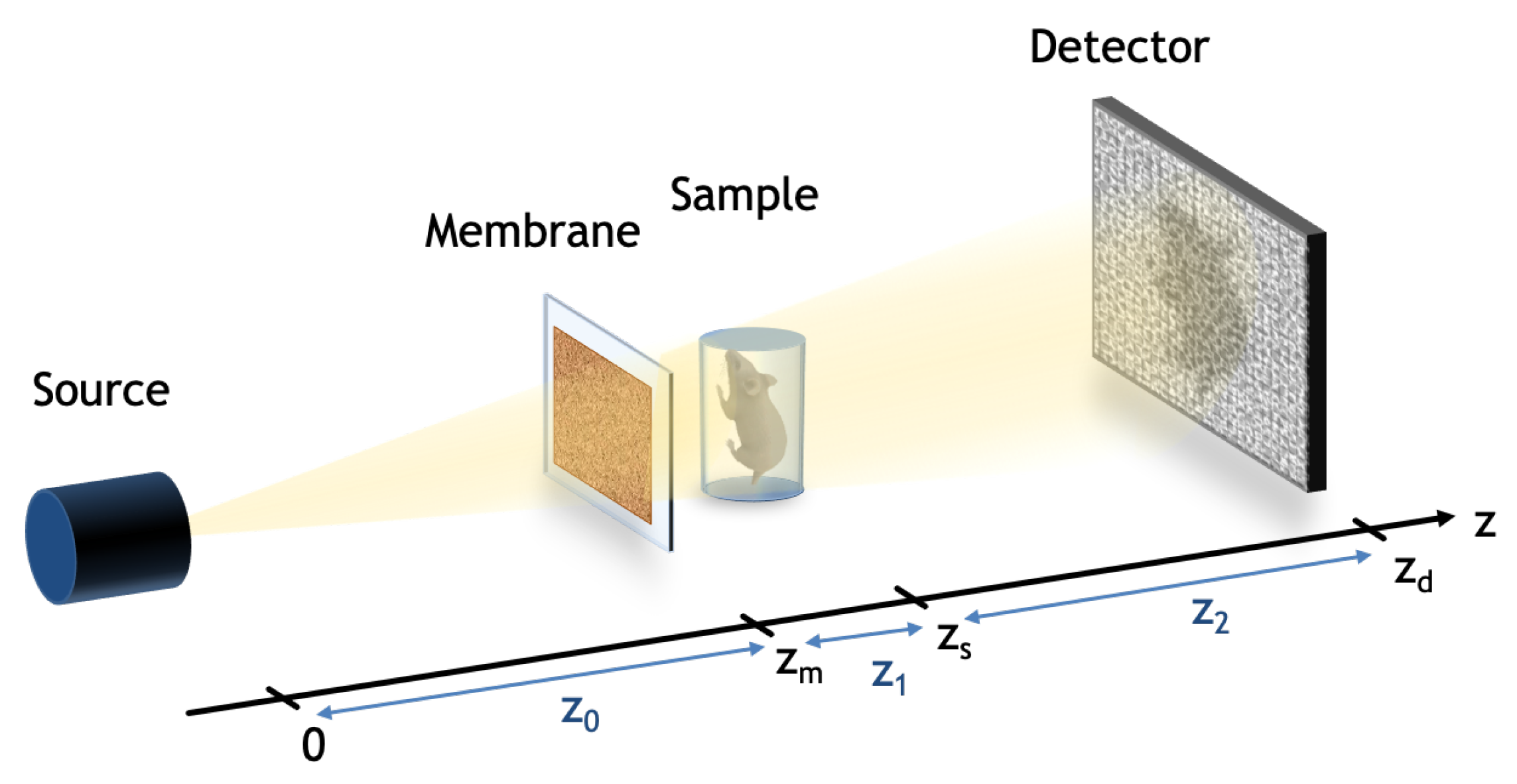
2.1. Theoretical Coherent Case
2.2. Partially Coherent Systems
2.3. Attenuation
2.4. Phase Contrast Imaging (PCI)
2.5. Dark-Field Imaging (DI)
3. Phase Contrast Imaging Techniques on Conventional Systems
3.1. Propagation-Based Imaging
3.2. Analyzer-Based Imaging
3.3. Grating Interferometry
- Its limited dose efficiency as several positions of the gratings are required to obtain each projection, and part of the flux going through the sample will not be used to produce the image, as it will be absorbed in G2;
- Its acquisition time which is still too long for tomography;
- Its fabrication which is tedious and expensive;
- Its ability to retrieve the refraction only in one direction, making it insensitive to variations that are parallel to the gratings and causes bad performance in terms of noise [46] (noise power spectrum diverging with low frequency) with tomographic reconstruction due to a bad integration for the phase.
3.4. Edge Illumination
3.5. Mesh-Based Imaging
3.6. Modulation-Based Imaging (Also Known as Speckle)
- No field of view limitation other than the detector (the random mask can be easy to manufacture).
- No resolution limitation other than the optical system.
- Being radiation-dose-efficient because no absorbing element is used between the sample and the detector, meaning that all photons passing through the sample eventually contribute to the image formation.
3.7. Recent Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bravin, A.; Coan, P.; Suortti, P. X-ray phase-contrast imaging: From pre-clinical applications towards clinics. Phys. Med. Biol. 2013, 58, R1–R35. [Google Scholar] [CrossRef]
- Willner, M.; Herzen, J.; Grandl, S.; Auweter, S.; Mayr, D.; Hipp, A.; Chabior, M.; Sarapata, A.; Achterhold, K.; Zanette, I.; et al. Quantitative breast tissue characterization using grating-based X-ray phase-contrast imaging. Phys. Med. Biol. 2014, 59, 1557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Brun, E.; Coan, P.; Huang, Z.; Sztrókay, A.; Diemoz, P.C.; Liebhardt, S.; Mittone, A.; Gasilov, S.; Miao, J.; et al. High-resolution, low-dose phase contrast X-ray tomography for 3D diagnosis of human breast cancers. Proc. Natl. Acad. Sci. USA 2012, 109, 18290–18294. [Google Scholar] [CrossRef] [PubMed]
- Rougé-Labriet, H.; Berujon, S.; Mathieu, H.; Bohic, S.; Fayard, B.; Ravey, J.N.; Robert, Y.; Gaudin, P.; Brun, E. X-ray Phase Contrast osteo-articular imaging: A pilot study on cadaveric human hands. Sci. Rep. 2020, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Nagashima, M.; Kido, K.; Hoshino, Y.; Kiyohara, J.; Makifuchi, C.; Nishino, S.; Nagatsuka, S.; Momose, A. Cadaveric and in vivo human joint imaging based on differential phase contrast by X-ray Talbot-Lau interferometry. Zeitschrift für Medizinische Physik 2013, 23, 222–227. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Bunk, O.; David, C.; Bech, M.; Le Duc, G.; Bravin, A.; Cloetens, P. High-resolution brain tumor visualization using three-dimensional X-ray phase contrast tomography. Phys. Med. Biol. 2007, 52, 6923. [Google Scholar] [CrossRef]
- Töpperwien, M.; van der Meer, F.; Stadelmann, C.; Salditt, T. Three-dimensional virtual histology of human cerebellum by X-ray phase-contrast tomography. Proc. Natl. Acad. Sci. USA 2018, 115, 6940–6945. [Google Scholar] [CrossRef]
- Chourrout, M.; Rositi, H.; Ong, E.; Hubert, V.; Paccalet, A.; Foucault, L.; Autret, A.; Fayard, B.; Olivier, C.; Bolbos, R.; et al. Brain virtual histology with X-ray phase-contrast tomography Part I: Whole-brain myelin mapping in white-matter injury models. Biomed. Opt. Express 2022, 13, 1620–1639. [Google Scholar] [CrossRef]
- Gassert, F.T.; Urban, T.; Frank, M.; Willer, K.; Noichl, W.; Buchberger, P.; Schick, R.; Koehler, T.; von Berg, J.; Fingerle, A.A.; et al. X-ray dark-field chest imaging: Qualitative and quantitative results in healthy humans. Radiology 2021, 301, 389–395. [Google Scholar] [CrossRef]
- Broche, L.; Pisa, P.; Porra, L.; Degrugilliers, L.; Bravin, A.; Pellegrini, M.; Borges, J.B.; Perchiazzi, G.; Larsson, A.; Hedenstierna, G.; et al. Individual airway closure characterized in vivo by phase-contrast CT imaging in injured rabbit lung. Crit. Care Med. 2019, 47, e774–e781. [Google Scholar] [CrossRef]
- Paganin, D. Coherent X-ray Optics; Number 6; Oxford University Press on Demand: Oxford, UK, 2006. [Google Scholar]
- Paganin, D.M.; Pelliccia, D. X-ray phase-contrast imaging: A broad overview of some fundamentals. Adv. Imaging Electron Phys. 2021, 218, 63–158. [Google Scholar]
- Morgan, K.S.; Siu, K.K.W.; Paganin, D. The projection approximation and edge contrast for X-ray propagation-based phase contrast imaging of a cylindrical edge. Opt. Express 2010, 18, 9865–9878. [Google Scholar] [CrossRef] [PubMed]
- Teague, M.R. Deterministic phase retrieval: A Green’s function solution. JOSA 1983, 73, 1434–1441. [Google Scholar] [CrossRef]
- Davis, T.; Gao, D.; Gureyev, T.; Stevenson, A.; Wilkins, S. Phase-contrast imaging of weakly absorbing materials using hard X-rays. Nature 1995, 373, 595–598. [Google Scholar] [CrossRef]
- Momose, A.; Takeda, T.; Itai, Y.; Hirano, K. Phase–contrast X–ray computed tomography for observing biological soft tissues. Nat. Med. 1996, 2, 473–475. [Google Scholar] [CrossRef]
- Schulz, G.; Weitkamp, T.; Zanette, I.; Pfeiffer, F.; Beckmann, F.; David, C.; Rutishauser, S.; Reznikova, E.; Müller, B. High-resolution tomographic imaging of a human cerebellum: Comparison of absorption and grating-based phase contrast. J. R. Soc. Interface 2010, 7, 1665–1676. [Google Scholar] [CrossRef]
- Kitchen, M.J.; Buckley, G.A.; Gureyev, T.E.; Wallace, M.J.; Andres-Thio, N.; Uesugi, K.; Yagi, N.; Hooper, S.B. CT dose reduction factors in the thousands using X-ray phase contrast. Sci. Rep. 2017, 7, 15953. [Google Scholar] [CrossRef]
- von Nardroff, R. Refraction of X-rays by small particles. Phys. Rev. 1926, 28, 240. [Google Scholar] [CrossRef]
- Yaroshenko, A.; Pritzke, T.; Koschlig, M.; Kamgari, N.; Willer, K.; Gromann, L.; Auweter, S.; Hellbach, K.; Reiser, M.; Eickelberg, O.; et al. Visualization of neonatal lung injury associated with mechanical ventilation using X-ray dark-field radiography. Sci. Rep. 2016, 6, 24269. [Google Scholar] [CrossRef]
- Snigirev, A.; Snigireva, I.; Kohn, V.; Kuznetsov, S.; Schelokov, I. On the possibilities of X-ray phase contrast microimaging by coherent high-energy synchrotron radiation. Rev. Sci. Instrum. 1995, 66, 5486–5492. [Google Scholar] [CrossRef]
- Weitkamp, T.; Diaz, A.; David, C.; Pfeiffer, F.; Stampanoni, M.; Cloetens, P.; Ziegler, E. X-ray phase imaging with a grating interferometer. Opt. Express 2005, 13, 6296–6304. [Google Scholar] [CrossRef] [PubMed]
- Olivo, A.; Speller, R. A coded-aperture technique allowing X-ray phase contrast imaging with conventional sources. Appl. Phys. Lett. 2007, 91, 074106. [Google Scholar] [CrossRef]
- Wen, H.; Bennett, E.E.; Hegedus, M.M.; Carroll, S.C. Spatial harmonic imaging of X-ray scattering—Initial results. IEEE Trans. Med. Imaging 2008, 27, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.S.; Paganin, D.M.; Siu, K.K. X-ray phase imaging with a paper analyzer. Appl. Phys. Lett. 2012, 100, 124102. [Google Scholar] [CrossRef]
- Berujon, S.; Wang, H.; Sawhney, K. X-ray multimodal imaging using a random-phase object. Phys. Rev. A 2012, 86, 063813. [Google Scholar] [CrossRef]
- Paganin, D.; Mayo, S.C.; Gureyev, T.E.; Miller, P.R.; Wilkins, S.W. Simultaneous phase and amplitude extraction from a single defocused image of a homogeneous object. J. Microsc. 2002, 206, 33–40. [Google Scholar] [CrossRef]
- Zuo, C.; Li, J.; Sun, J.; Fan, Y.; Zhang, J.; Lu, L.; Zhang, R.; Wang, B.; Huang, L.; Chen, Q. Transport of intensity equation: A tutorial. Opt. Lasers Eng. 2020, 135, 106187. [Google Scholar] [CrossRef]
- Guigay, J.P.; Langer, M.; Boistel, R.; Cloetens, P. Mixed transfer function and transport of intensity approach for phase retrieval in the Fresnel region. Opt. Lett. 2007, 32, 1617–1619. [Google Scholar] [CrossRef]
- Lundström, U.; Larsson, D.H.; Burvall, A.; Takman, P.A.; Scott, L.; Brismar, H.; Hertz, H.M. X-ray phase contrast for CO2 microangiography. Phys. Med. Biol. 2012, 57, 2603. [Google Scholar] [CrossRef]
- Variola, A. The THOMX project. In Proceedings of the 2nd International Particle Accelerator Conference (IPAC’11), Joint Accelerator Conferences Website, San Sebastian, Spain, 4–9 September 2011; pp. 1903–1905. [Google Scholar]
- Schleede, S.; Meinel, F.G.; Bech, M.; Herzen, J.; Achterhold, K.; Potdevin, G.; Malecki, A.; Adam-Neumair, S.; Thieme, S.F.; Bamberg, F.; et al. Emphysema diagnosis using X-ray dark-field imaging at a laser-driven compact synchrotron light source. Proc. Natl. Acad. Sci. USA 2012, 109, 17880–17885. [Google Scholar] [CrossRef]
- Gureyev, T.; Paganin, D.; Arhatari, B.; Taba, S.; Lewis, S.; Brennan, P.; Quiney, H. Dark-field signal extraction in propagation-based phase-contrast imaging. Phys. Med. Biol. 2020, 65, 215029. [Google Scholar] [CrossRef]
- Leatham, T.A.; Paganin, D.M.; Morgan, K.S. X-ray dark-field and phase retrieval without optics, via the Fokker–Planck equation. arXiv 2021, arXiv:2112.10999. [Google Scholar]
- Diemoz, P.; Bravin, A.; Coan, P. Theoretical comparison of three X-ray phase-contrast imaging techniques: Propagation-based imaging, analyzer-based imaging and grating interferometry. Opt. Express 2012, 20, 2789–2805. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Majidi, K.; Brankov, J.G. Analyzer-based phase-contrast imaging system using a micro focus X-ray source. Rev. Sci. Instruments 2014, 85, 085114. [Google Scholar] [CrossRef]
- Ronchi, V. Forty years of history of a grating interferometer. Appl. Opt. 1964, 3, 437–451. [Google Scholar] [CrossRef]
- Zanette, I.; David, C.; Rutishauser, S.; Weitkamp, T. 2D grating simulation for X-ray phase-contrast and dark-field imaging with a Talbot interferometer. In AIP Conference Proceedings; American Institute of Physics: New York, NY, USA, 2010; Volume 1221, pp. 73–79. [Google Scholar]
- Pinzek, S.; Beckenbach, T.; Viermetz, M.; Meyer, P.; Gustschin, A.; Andrejewski, J.; Gustschin, N.; Herzen, J.; Schulz, J.; Pfeiffer, F. Fabrication of X-ray absorption gratings via deep X-ray lithography using a conventional X-ray tube. J. Micro/Nanopatterning Mater. Metrol. 2021, 20, 043801. [Google Scholar] [CrossRef]
- Romano, L.; Vila-Comamala, J.; Jefimovs, K.; Stampanoni, M. High-Aspect-Ratio Grating Microfabrication by Platinum-Assisted Chemical Etching and Gold Electroplating. Adv. Eng. Mater. 2020, 22, 2000258. [Google Scholar] [CrossRef]
- Noda, D.; Tanaka, M.; Shimada, K.; Yashiro, W.; Momose, A.; Hattori, T. Fabrication of large area diffraction grating using LIGA process. Microsyst. Technol. 2008, 14, 1311–1315. [Google Scholar] [CrossRef]
- Pfeiffer, F.; Weitkamp, T.; Bunk, O.; David, C. Phase retrieval and differential phase-contrast imaging with low-brilliance X-ray sources. Nat. Phys. 2006, 2, 258–261. [Google Scholar] [CrossRef]
- Willer, K.; Fingerle, A.A.; Noichl, W.; De Marco, F.; Frank, M.; Urban, T.; Schick, R.; Gustschin, A.; Gleich, B.; Herzen, J.; et al. X-ray dark-field chest imaging for detection and quantification of emphysema in patients with chronic obstructive pulmonary disease: A diagnostic accuracy study. Lancet Digit. Health 2021, 3, e733–e744. [Google Scholar] [CrossRef]
- Urban, T.; Gassert, F.T.; Frank, M.; Willer, K.; Noichl, W.; Buchberger, P.; Schick, R.C.; Koehler, T.; Bodden, J.H.; Fingerle, A.A.; et al. Qualitative and quantitative assessment of emphysema using dark-field chest radiography. Radiology 2022, 303, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Viermetz, M.; Gustschin, N.; Schmid, C.; Haeusele, J.; von Teuffenbach, M.; Meyer, P.; Bergner, F.; Lasser, T.; Proksa, R.; Koehler, T.; et al. Dark-field computed tomography reaches the human scale. Proc. Natl. Acad. Sci. USA 2022, 119, e2118799119. [Google Scholar] [CrossRef] [PubMed]
- Köhler, T.; Jürgen Engel, K.; Roessl, E. Noise properties of grating-based X-ray phase contrast computed tomography. Med Phys. 2011, 38, S106–S116. [Google Scholar] [CrossRef]
- Olivo, A.; Arfelli, F.; Cantatore, G.; Longo, R.; Menk, R.; Pani, S.; Prest, M.; Poropat, P.; Rigon, L.; Tromba, G.; et al. An innovative digital imaging set-up allowing a low-dose approach to phase contrast applications in the medical field. Med Phys. 2001, 28, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Diemoz, P.; Hagen, C.; Endrizzi, M.; Olivo, A. Sensitivity of laboratory based implementations of edge illumination X-ray phase-contrast imaging. Appl. Phys. Lett. 2013, 103, 244104. [Google Scholar] [CrossRef]
- Hagen, C.K.; Endrizzi, M.; Towns, R.; Meganck, J.A.; Olivo, A. A preliminary investigation into the use of edge illumination X-ray phase contrast micro-CT for preclinical imaging. Mol. Imaging Biol. 2020, 22, 539–548. [Google Scholar] [CrossRef]
- Kallon, G.K.; Wesolowski, M.; Vittoria, F.A.; Endrizzi, M.; Basta, D.; Millard, T.P.; Diemoz, P.C.; Olivo, A. A laboratory based edge-illumination X-ray phase-contrast imaging setup with two-directional sensitivity. Appl. Phys. Lett. 2015, 107, 204105. [Google Scholar] [CrossRef]
- Wen, H.H.; Bennett, E.E.; Kopace, R.; Stein, A.F.; Pai, V. Single-shot X-ray differential phase-contrast and diffraction imaging using two-dimensional transmission gratings. Opt. Lett. 2010, 35, 1932–1934. [Google Scholar] [CrossRef]
- Wen, H.; Bennett, E.E.; Hegedus, M.M.; Rapacchi, S. Fourier X-ray scattering radiography yields bone structural information. Radiology 2009, 251, 910–918. [Google Scholar] [CrossRef]
- Bennett, E.E.; Kopace, R.; Stein, A.F.; Wen, H. A grating-based single-shot X-ray phase contrast and diffraction method for in vivo imaging. Med Phys. 2010, 37, 6047–6054. [Google Scholar] [CrossRef]
- Sun, W.; MacDonald, C.A.; Petruccelli, J.C. Propagation-based and mesh-based X-ray quantitative phase imaging with conventional sources. In Proceedings of the Computational Imaging IV, Baltimore, MD, USA, 14–15 April 2019; Volume 10990, p. 109900U. [Google Scholar]
- Sun, W.; Pyakurel, U.; MacDonald, C.A.; Petruccelli, J.C. Grating-free quantitative phase retrieval for X-ray phase-contrast imaging with conventional sources. Biomed. Phys. Eng. Express 2022, 8, 055016. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sun, W.; MacDonald, C.; Petruccelli, J.C. The application of harmonic techniques to enhance resolution in mesh-based X-ray phase imaging. J. Appl. Phys. 2019, 125, 233101. [Google Scholar] [CrossRef]
- Quenot, L.; Rougé-Labriet, H.; Bohic, S.; Berujon, S.; Brun, E. Implicit tracking approach for X-Ray Phase-Contrast Imaging with a random mask and a conventional system. Optica 2021, 8, 1412–1415. [Google Scholar] [CrossRef]
- Cieślak, M.J.; Gamage, K.A.; Glover, R. Coded-aperture imaging systems: Past, present and future development—A review. Radiat. Meas. 2016, 92, 59–71. [Google Scholar] [CrossRef]
- How, Y.Y.; Morgan, K.S. Quantifying the X-ray dark-field signal in single-grid imaging. Opt. Express 2022, 30, 10899–10918. [Google Scholar] [CrossRef]
- Morgan, K.S.; Paganin, D.M. Applying the fokker–planck equation to grating-based X-ray phase and dark-field imaging. Sci. Rep. 2019, 9, 17465. [Google Scholar] [CrossRef]
- Pavlov, K.M.; Paganin, D.M.; Li, H.T.; Berujon, S.; Rougé-Labriet, H.; Brun, E. X-ray multi-modal intrinsic-speckle-tracking. J. Opt. 2020, 22, 125604. [Google Scholar] [CrossRef]
- Zdora, M.C.; Thibault, P.; Zhou, T.; Koch, F.J.; Romell, J.; Sala, S.; Last, A.; Rau, C.; Zanette, I. X-ray phase-contrast imaging and metrology through unified modulated pattern analysis. Phys. Rev. Lett. 2017, 118, 203903. [Google Scholar] [CrossRef]
- Berujon, S.; Ziegler, E. X-ray multimodal tomography using speckle-vector tracking. Phys. Rev. Appl. 2016, 5, 044014. [Google Scholar] [CrossRef]
- de La Rochefoucauld, O.; Bucourt, S.; Cocco, D.; Dovillaire, G.; Harms, F.; Idir, M.; Korn, D.; Levecq, X.; Piponnier, M.; Rungsawang, R.; et al. Hartmann wavefront sensor in the EUV and hard X-ray range for source metrology and beamline optimization (Conference Presentation). In Proceedings of the Relativistic Plasma Waves and Particle Beams as Coherent and Incoherent Radiation Sources III, Prague, Czech Republic, 3–4 April 2019; Volume 11036, p. 110360P. [Google Scholar]
- Vittoria, F.A.; Kallon, G.K.; Basta, D.; Diemoz, P.C.; Robinson, I.K.; Olivo, A.; Endrizzi, M. Beam tracking approach for single–shot retrieval of absorption, refraction, and dark–field signals with laboratory x–ray sources. Appl. Phys. Lett. 2015, 106, 224102. [Google Scholar] [CrossRef]
- Provinciali, G.B. X-ray Phase Imaging Based on Hartmann Wavefront Sensor for Applications on the Study of Neurodegenerative Diseases. Ph.D. Thesis, Institut Polytechnique de Paris, Palaiseau, France, 2022. [Google Scholar]
- Qiao, Z.; Shi, X.; Yao, Y.; Wojcik, M.J.; Rebuffi, L.; Cherukara, M.J.; Assoufid, L. Real-time X-ray phase-contrast imaging using SPINNet—A speckle-based phase-contrast imaging neural network. Optica 2022, 9, 391–398. [Google Scholar] [CrossRef]
- Mom, K.; Sixou, B.; Langer, M. Mixed scale dense convolutional networks for X-ray phase contrast imaging. Appl. Opt. 2022, 61, 2497–2505. [Google Scholar] [CrossRef] [PubMed]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quenot, L.; Bohic, S.; Brun, E. X-ray Phase Contrast Imaging from Synchrotron to Conventional Sources: A Review of the Existing Techniques for Biological Applications. Appl. Sci. 2022, 12, 9539. https://doi.org/10.3390/app12199539
Quenot L, Bohic S, Brun E. X-ray Phase Contrast Imaging from Synchrotron to Conventional Sources: A Review of the Existing Techniques for Biological Applications. Applied Sciences. 2022; 12(19):9539. https://doi.org/10.3390/app12199539
Chicago/Turabian StyleQuenot, Laurene, Sylvain Bohic, and Emmanuel Brun. 2022. "X-ray Phase Contrast Imaging from Synchrotron to Conventional Sources: A Review of the Existing Techniques for Biological Applications" Applied Sciences 12, no. 19: 9539. https://doi.org/10.3390/app12199539




