Abstract
Our major goal was the physicochemical, biochemical and microbiological characterization of Cobrançosa table olives, as support for the eventual granting of a PDO status. Seven producers were accordingly sampled throughout eleven months. Brines were analyzed for pH, salinity, acidity, and organic and phenolic compounds. Yeasts and Latic Acid Bacteria (LAB) were enumerated, and the dominant strains duly identified. Despite process variabilities, two stages appear to be shared by all manufacturers: sweetening—the renewal of water to remove bitter compounds; and salting—gradual addition of salt to brine for preservation. Yeasts dominated during sweetening, but LAB tended to be similar in viable counts (7 log CFU/mL) by the end of salting. Lactiplantibacillus (Lpb.) pentosus, Lpb. paraplantarum, Pediococcus parvulus, and Oenococcus kitaharae were the most abundant LAB found, together with an average pH of 4.1 and 6–9% for salt content. All organic acids exhibited an inverted parabolic evolution, with maxima of 3450 mg/L for lactic and 4000 mg/L for succinic by 3 months, and 2750 mg/L for acetic and 2950 mg/L for citric by 4 months. Oleuropein levels were affected by the frequency of brine renewal but decreased from 1350 to 700 mg/L, with hydroxityrosol and tyrosol increasing from 10 to 2000 mg/L and 2 to 550 mg/L, respectively, within 11 months.
1. Introduction
Table olives is the label utilized for the product prepared from the fruits of Olea europaea, processed to remove their bitterness and somehow preserved so as to ensure stability and prevent deterioration thereof under regular storage conditions.
There are three main types of table olives depending on the degree of maturation of the fruits at the time of harvest—green, mixed or black. There are more than 139 varieties from 23 olive-growing countries, which account for almost 85% of the whole olive crop area [1].
Fermentation processes are strongly influenced by such processing factors as temperature and salt concentration in the brine. Hence, the physicochemical conditions and availability of fermentable substrates as modulated by salt content will eventually contribute to the formation of products with distinct properties and, as such, different levels of quality and consumer acceptance, or even resistance to spoilage; in particular, abnormalities in fermentation may lead to a defective final product. The application of starter cultures would accordingly be recommended in order to gain greater control over fermentation, and thus reduce the risk of growth of undesirable strains; such an approach has been expanding in recent times [2]. These starter cultures reduce the risk of spoilage by accelerating acidification of the brine, while contributing to a more efficient fermentation and concomitant reduction in metabolic energy required [3].
The existing processes at commercial scale are: (i) treated green olives (Spanish style), (ii) olives darkened by oxidation (ripe olives), (iii) dehydrated and/or shriveled olives, and (iv) natural black olives (Greek style) [4]. Fermentative methods have been gaining importance over strictly chemical methods, since they minimize loss of nutrients and polyphenols (as components of color and flavor) with anticarcinogenic features. However, these methods are more expensive for requiring longer processing times, extra work and energy, and considerably larger loss of mass [5]. During olive fermentation, the final product submerged in the brine interacts with the microorganisms therein; hence, their metabolism plays a crucial role upon transformation of native substrates in the olive drupe—which improves their nutritional value, appearance and flavor, while favoring the degradation of undesirable traits and thus contributing to a safer product.
Of the diverse microbial populations involved in olive fermentation, LAB are, alongside yeasts, the most relevant [2]. If present as adventitious or added microorganisms, LAB will grow spontaneously in treated olives, whereas they may be essentially replaced by yeasts in native olives. LAB are known to improve preservation, as they bring about gradual acidification of brine—further to releasing bacteriocins that prevent growth of contaminating microorganisms. Some strains also aid in the removal of bitterness via hydrolysis of oleuropein, thus improving the final flavor [6]. Portilha-Cunha et al. [7] described the large variety of adventitious lactic acid bacteria found in table olives—with more than 40 species belonging to nine genera of LAB being reported; the genus most frequently cited is Lactobacillus, under such species as Lpb. plantarum and Lpb. pentosus. Other less common species have been mentioned to illustrate biodiversity across major producers in Europe: Paulilactobacillus vaccinostercus and Paulilactobacillus suebicus in Spanish Aloreña [5], Loigolactobacillus coryniformis in Greek Conservolea [8], Secundilactobacillus collinoides in Italian Tonda di Cagliari [9], and Pediococcus pentosaceus in Portuguese Galega [10].
Yeasts also play an important role in table olive processing, especially when spontaneous fermentation is at stake [11]. This includes effects upon taste and aroma, via: (i) esterase and lipase activities, associated with production of volatile compounds, e.g., alcohols, glycerol, higher alcohols, esters, and free fatty acids [12]; (ii) β-glucosidase activity, able to degrade polyphenols that aid in debittering [11]; and (iii) capacity to (under aerobic conditions) take up lactic and acetic acids, produced during fermentation or added for final storage [13]. They can also enhance the growth of LAB due to their ability to synthesize such compounds as vitamins, amino acids, and purines; as well as to break down complex carbohydrates to smaller sugars, essential for the growth of (nutritionally fastidious) Lactobacillus [14].
The production of table olives worldwide is regulated by the International Oil Council—the only intergovernmental organization in the field [15]. Among the 2.96 million tons of table olives produced in 2019/20, the largest world producers were: European Union (26.0%), Egypt (22.0%), Turkey (14.0%), Algeria (11.0%), and Morocco (4.6%). Within the European Union (771.0 thousand tons), Spain (59.4%), Greece (28.8%), Italy (7.8%), and Portugal (3.2%) account for most production—with Cyprus, France, and Croatia contributing residuals amounts. In addition to belonging to the top-five group of producers of table olives in Europe, Portugal has been steadily expanding; for instance, its production in 2017 was four-fold that in 2000. In addition, structural changes have taken place over the latest decade; from a traditional and hardly competitive activity, Trás-os-Montes (Northeast of Portugal) has stepped up and is now second only to Alentejo [16] in table olive production.
Cobrançosa is native to Trás-os-Montes and known for the consistency of its pulp—a textural requirement for effective preservation and consumption as table olives. The demand for table olives has meanwhile doubled, probably owing to an ever-increasing proportion of health-aware consumers [15]. This has come along with a massive investment in the planting of new olive trees, with ca. EUR 675 million funded within the 2014–2020 period by the Rural Development Program (PDR2020, Portugal). Only Negrinha do Freixo has to date earned a PDO status—but the textural and sensory advantages of Cobrançosa fully justify their receiving a similar status [17].
When searching for terms (“Portugal” OR “Portuguese”) AND “table olives” in the published literature (Title, Abstract, and Keywords) listed in databases such as SCOPUS, Science Direct, and PubMed, a total of 50 documents can be found; however, only 7 are research articles related to Northeast Cobrançosa table olives, concerning physicochemical and nutritional [18], sensory [18,19,20], volatile and phenolic profiles, and antioxidant activity [19,21,22,23,24]. However, none of these studies describe technological recipes for processing of this cultivar, and thus putative implications upon microbiological, physicochemical, and biochemical profiles throughout the fermentation period.
Therefore, the present study is the first consistent research work dealing with the characterization of the processing method of and native LAB strains from Cobrançosa table olives. It is hoped that our results provide a useful scientific and technical contribution—not only to the fundamental understanding of the basic dynamics prevailing during fermentation but also to the design of a dedicated starter culture that may eventually support the establishment of a Cahier d’Écharges for the granting of a PDO label.
2. Materials and Methods
2.1. Table Olives Sampling
Sampling of table olives from cultivar Cobrançosa was performed by seven independent producers in Trás-os-Montes—all of them following traditional protocols of spontaneous fermentation, under the auspices and the logistic cooperation of the Regional Directorate for Northern Agriculture and Fisheries (DRAPN). A timetable for the collection of brine and olive samples was duly agreed upon. Sampling was carried out by a certified DRAPN technician, who collected samples as deep as possible from two fermentation drums per producer and immediately placed them in previously labeled, airtight, and sterile boxes; these were transported under refrigeration to our laboratory and kept as such for no longer than 18 h prior to chemical and microbiological analyses. Sampling started on 19 November 2018 (right after harvest) and was concluded on 10 October 2019 (right after the product was ready for selling). Further to the samples at those times, an extra five samples were collected on 12 December, 21 January, 17 March, 2 May, and 11 July.
2.2. Processing Method Questionnaire
Recall that Cobrançosa table olives are processed following traditional methods, yet each producer follows their own, unique protocol; hence, each producer was requested to fill a questionnaire on all procedures taken between sampling times. Most questions dealt with calibration/cleaning of drupes, proportion of olive:water:salt (w/v/w) added, washing frequency, agitation frequency, type of aeration, and package characteristics.
2.3. Chemicals, Reagents, and Microbiological Media
Phenolic standards and organic acids, as well as HPLC reagents, were purchased from SIGMA Aldrich (Darmstadt, Germany). Other chemicals and reagents were obtained from VRW (Milano, Italy), and microbiological media were obtained from VRW (Leuven, Belgium).
2.4. pH, Titratable Acidity, and Salt Measurements
The pH was determined by potentiometry (MU-6100L pHenomenal® pH meter, VWR). To ascertain salinity (expressed as percentage, w/v), a portable refractometer (YIERYI, China) was utilized. Determination of the total titratable acidity (TTA) followed Pino et al. [25] and was expressed as percentage of lactic acid (w/v). All analyses were performed in duplicate.
2.5. Quantification of Yeasts and Lactic Acid Bacteria
Microbiological analyses were carried out on samples of both olive drupes and brine at a 1:1 (w/v) ratio. Samples consisted of 100 g of whole olives together with 100 mL of extra brine and were crushed in a Stomacher Star Blender LB400 (VWR) for 60 s—the blend is hereafter termed “enriched brine”. These samples were serially diluted in 0.85% (w/v) sterile saline solution and spread plated in triplicate on the following media: Rose Bengal agar supplemented with 0.1 g/L of chloramphenicol for yeasts and de Man, Rogosa, and Sharpe (MRS) agar supplemented with 0.4 g/L of sodium azide for LAB. Counts were obtained by 48 h of incubation at 30 °C for LAB and 5 days at 25 °C for yeasts—using a colony counter (Scan® 100, VWR). Results were calculated as means of three determinations.
2.6. Organic Acids Analysis
Samples were prepared using Oasis PRiME HLB (Waters, Milford, MA, USA) for clean-up, followed by acidification with 0.01% TFA. The concentrations of organic acids in brine samples were determined in duplicate by HPLC, following the method of Ghabbour et al. [26] with a few modifications. A Waters Alliance HPLC system (model 2695), equipped with photodiode array detector (model 2998) set at 210 nm, and an Atlantis Premier BEH C18 Ax column (4.6 × 150 mm, 5 μm bondapack) (Waters) were used. Separation was carried out at 30 °C for the column oven under a flow rate 1 mL/min using ultrapure water with 0.05% TFA (A) and acetonitrile (B) under linear gradients between: 0 min, 98% A; 3 min, 98% A; 12 min, 40% A; 14 min, 40% A; and 20 min, 98% A. Compound concentration was expressed in mg/L.
2.7. Phenolic Compounds Analysis
The concentrations of phenolic compounds in brine samples were ascertained by HPLC, following the method of Cosmai et al. [27] with modifications. Samples were preserved at 4 °C. Separations were performed using a C18 Cortecs column (4.6 mm × 150 mm, 2.7 μm) (Waters) coupled to a C18 Cortecs VanGuard (3.9 × 5 mm, 2.7 μm bondapack) maintained at 35 °C and using a photodiode array detector (model 2998) set at 280 nm. Ultrapure water with 0.02% TFA (A) and acetonitrile (B) were used as mobile phases under a flow rate 1 mL/min and linear gradients between: 0 min, 5% B; 3 min, 20% B; 4 min, 40% B; 10 min, 80% B; and 111 min, 80% B. Concentrations of compounds were expressed in mg/L, as means of two determinations.
2.8. Isolation and Identification of Lactic Acid Bacteria
To characterize the predominant LAB during spontaneous fermentation of Cobrançosa olives, a collection of 316 isolates were obtained from MRS agar plates seeded with the highest dilutions. Colonies (10%) were picked up and purified via plating on the same medium. After 48 h of incubation at 30 °C, the purity of the isolates was checked microscopically using Gram staining, complemented by assessment of catalase and oxidase activities. Pure cultures were stored at –80 °C in MRS broth with 15% (v/v) glycerol.
Genomic DNA was extracted using the GenElute™ Bacterial Genomic DNA kit (Sigma-Aldrich, St. Louis, MO, USA) according to manufacturer’s instructions. LAB isolates underwent preliminary genotyping with RAPD-PCR (primer OPL5), as reported by Maldonado-Barragan et al. [28]. Amplifications were performed on an Uno Cycler (VWR) thermocycler. The NZYDNA Ladder VIII (NZYTech, Lisboa, Portugal) was run as molecular size marker and as reference lanes for band matching and inter-gel comparisons. Gels were visualized under UV light and digitally captured using a gel documentation system (Cleaver Scientific, Rugby, UK). The RAPD profiles were analyzed visually and further translated into binary matrices. Only reproducible bands representing amplicons between 200–5000 bp in size were considered. The similarity of band patterns was calculated using the band-based Jaccard similarity coefficient, and the clustering analysis was based on the UPGMA approach. At least one representative isolate of each profile was identified through amplification and sequencing of the 16S rRNA gene. The nucleotide sequences obtained were used to query the EzBioCloud database [29], and thus retrieve the closest type strain—as per identification of isolates at species level.
2.9. Statistical Analysis
Descriptive and regression analyses were performed with the aid of Excel Microsoft Office 2019. The tendency lines were obtained by regression options of the software (2nd-order polynomial, logarithmic, and linear), except for TTA and individual phenolic compounds, for which the model used stemmed from a Poisson distribution curve. To visualize dissimilarities among olive drums according to LAB strain diversity, hierarchical clustering analysis was applied to the abundance dataset—using Euclidean distance and aggregation criterion average linkage between groups. The results are reported in the form of Heatmap, generated under RStudio software.
3. Results and Discussion
The most important parameters of the process are summarized in Table 1, encompassing all seven producers tested.

Table 1.
Characterization of Cobrançosa table olive processing.
The differences found between producers’ protocols justify our efforts to define a more standardized protocol—required to effectively support the ultimate goal of PDO definition.
3.1. Processing Method Characterization Based on Producer Information
First of all, one realized that harvest time does not coincide among producers— this is reflected upon differences in drupe color and ripeness stage (e.g., A and F in Table 1, separated by one month). It has been reported that the stage of ripeness of Arbequina table olives influences both microbiota and the final sensory characteristics thereof [30]. Therefore, predefinition of the stage of ripeness of Cobrançosa is in order, probably by some easily assessed reference parameter, e.g., the maturity index (MI) elected by Bengana et al. [31]; note that this is a simple method, based on assessment of the color of olive skin and pulp (MI values range from 0—very green skin up to 7—purple flesh and black skin). However, looking at Table 1, it appears that most producers harvest the olives in the turning color ripening time.
According to the information conveyed by the producers—and after careful removal of rotten drupes, stalks, and leaves—the olives are washed and added with spring water to different proportions and kept thereafter in water for 4–6 months. During this period, the table olives are washed periodically, and fresh water is added; the period between renewals of brine ranges, however, from 1 week up to two months (Table 1). This first stage of the processing of table olives is unique, and termed sweetening stage by the producers because its major goal is to remove bitterness. Considering that oleuropein is the dominant bitter compound present in olives, it became important to follow the profile of that (and other) phenolic compounds throughout the process. Meanwhile, the drums are covered or closed, depending on the modus operandi of each producer. After the sweetening stage, the salting stage (as so named by the producers) is in order. During this period, the water is no longer changed until the product is ready for the market; however, salt is gradually added to the brine—up to 7–10% salt content by the moment of selling.
Temperature is a quite important technological parameter, in that it influences the microbiological and chemical profiles throughout fermentation and, consequently, the sensory properties of the final product [32]. Despite the beneficial effects of controlled temperature upon table olive fermentation, such control is too expensive, and is thus not generally applied in current artisanal manufacture. Although the room temperature in Trás-os-Montes is normally rather low in winter and rather high in summer, a noteworthy temperature amplitude is also observed within each day (Table 1)—which is expected to somehow affect the fermentation process of Cobrançosa table olives.
After eleven months, table olives are packed in 5–10 kg plastic buckets, after renewing the brine one final time with exchange by another bearing a similar percentage of salt (and devoid of any extra additives or preservatives). Finally, the buckets are sealed and duly labeled for selling.
3.2. Processing Physicochemical Profile in Brine
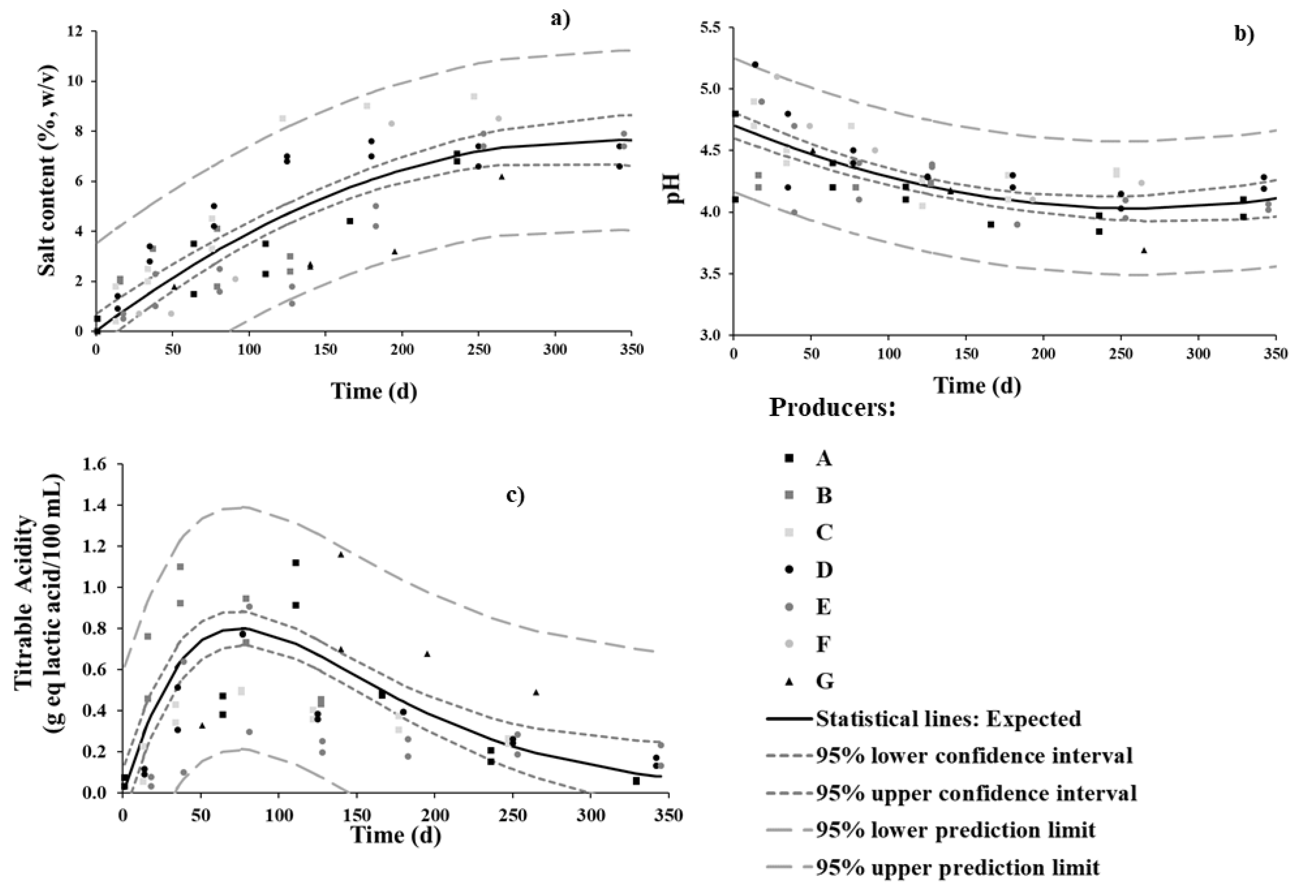
Directly brined olives are among the most popular and established commercial styles for table olive preparation [7,33]. However, the Cobrançosa brining process makes it quite different from most other table olive treatments. The evolution in salt content throughout the fermentation process of Cobrançosa olives is presented in Figure 1a. Contrary to what was stated by the producers in the questionnaire—that salt is not added during the sweetening stage—our results show that said statement was not completely true for all producers. When looking for a general pattern in Figure 1a, it can be said that salt is added during the sweetening stage in a steady fashion, from 0 up to 5%, and afterward at a slower pace, from 5 up to 7% or 10%, within the following 5 and 6 months, respectively. A study focused on reducing sodium content of Picual table olives from Cyprus supported the hypothesis that reduced NaCl levels are feasible; 7% salt-containing table olives were even appreciated by the sensory panel as better than their 10% counterparts [6]. Owing to market demand for low-salt and sodium-reduced food products, the value of 7% (w/v) appears a good choice—besides lying below current values for competing products from other countries [34]. Therefore, future standardization of the Cobrançosa process should seriously consider reducing the salt content in the second stage of the process.
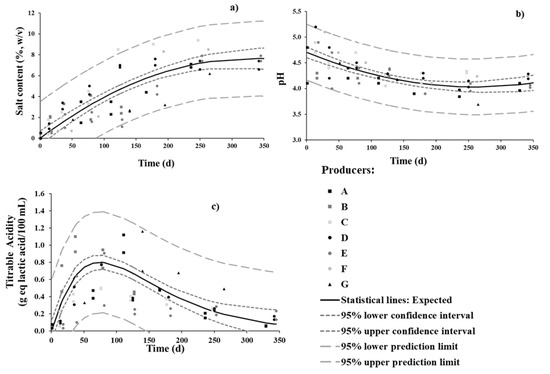
Figure 1.
Changes in (a) salt, (b) pH, and (c) total titratable acidity in brine during spontaneous fermentation of Cobrançosa table olives.
The initial pH in brines (up to 15 d) ranged from 4.1 to 5.2, depending on the producer; said discrepancy can be explained by the pH of the water source (typically natural mines and holes). Nevertheless, a similar acidification profile was observed for all producers. The pH values decreased gradually until 7 months of fermentation (Figure 1b)and stabilized thereafter at 4.0–4.3 toward the end of fermentation; this agrees with reports elsewhere [6,33,35,36,37]. Based on these results, a different salt content (as previously discussed) appears not to have a significant impact upon acidification; note that the safety issue is conveniently addressed via the addition of salt, so as to allows preferential growth of yeasts and LAB and subsequent decrease in pH. A study confirmed that table olives consubstantiate adverse habitats for foodborne pathogenic microorganisms, e.g., Escherichia coli, Staphylococcus aureus, Listeria monocytogenes, and Salmonella enterica) in Aloreña de Malaga table olive brine [38].
Regarding TTA, an increase of up to 0.8 g eq lactic acid/100 mL of brine was observed during the first 3 months; a slower decrease was recorded thereafter, down to 0.2 by 7 months—when a stable plateau was eventually reached (Figure 1c). Remember that there is no fundamental correlation between TTA and pH profiles, since the acids produced are weak, and therefore do not undergo full ionization. The increase in TTA is related to the release of organic acids [39], such as lactic, acetic, succinic, and citric, by LAB and yeasts as they take up and metabolize sugars (e.g., glucose and fructose) contributed by olives [6,32]. Said acids are ubiquitous in the fermentation brines of green and black olives [6,32]. The profiles of organic acids were duly monitored and are discussed in due course.
3.3. Microbiological Profile of Table Olive and Brine Together
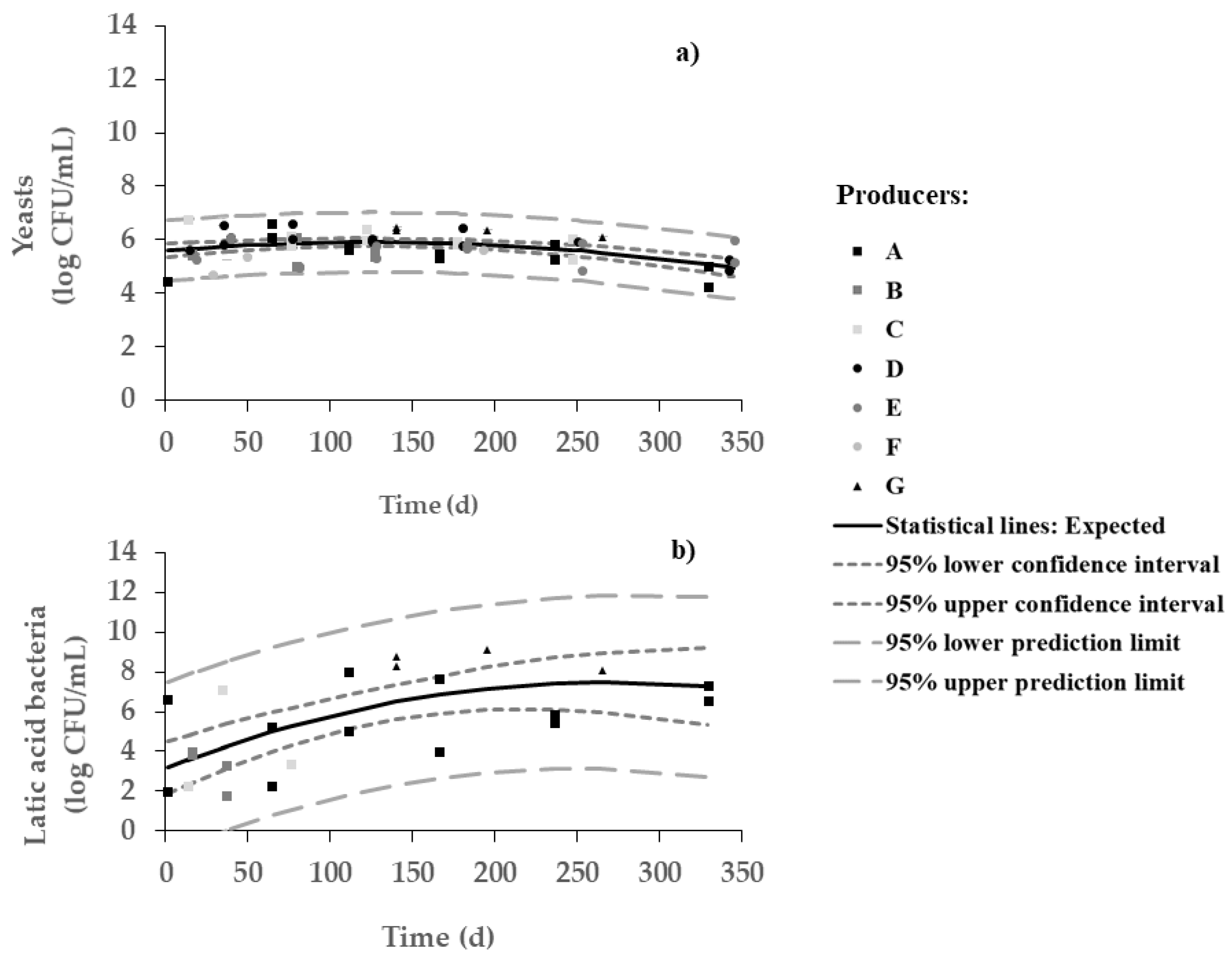
Yeasts were dominant over lactic acid bacteria during most of the fermentation period (Figure 2). Changes in viable numbers of yeasts (between 6 and 7 log CFU/mL) were indeed not very significant throughout the process. On the other hand, a significant increase in the number of LAB (from 3 to 7 log CFU/mL) throughout 7 months was observed, with a plateau thereafter. As expected, such increase in viable LAB runs along with pH decrease (Pearson, r = 0.999 and p = 0.000). In other words, brine starts with a richer population of yeasts but ends with a microbial population of yeasts and LAB similar in numbers—with pH and salt playing a major role in facilitating the survival of pH- and salt-resistant strains of LAB. The microbial framework depends on olive cultivar, crop management, fermentation style, and technology processing type [4]; the complex olive/brine matrix can, in turn, affect the formation of the microbial consortium responsible for fermentation, and thus influence the characteristics of the final product. Therefore, it is hard to compare microbial evolution in different types of table olives. For instance, yeasts are the main microorganisms involved in the fermentation of Arbequina table olives, but lactic acid bacteria become important when such olives undergo maturation prior to packaging—thus unfolding a trend quite similar to our results; on the other hand, the dominant microflora from Picual table olives are LAB, although yeasts can also be detected to significant numbers [6].
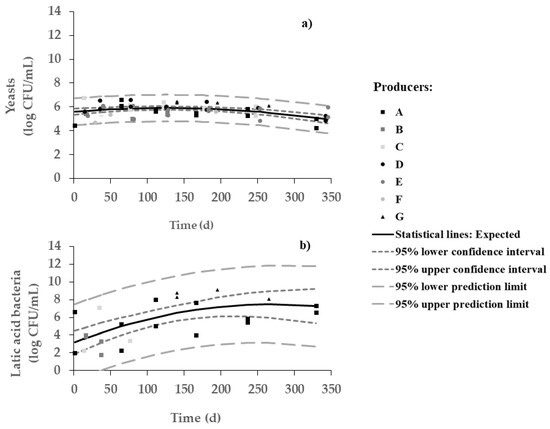
Figure 2.
Changes in viable numbers of (a) yeasts and (b) lactic acid bacteria in olive samples during spontaneous fermentation of Cobrançosa table olives.
The large variability in LAB counts was also anticipated in view of the differences among protocols followed by the various producers. For instance, no counts were obtained for producers D, E, and F—this fact is certainly related to the too frequent changes in water (Table 1). For producers B and C, the counts of LAB were rather unstable and could not be perceived any more beyond 3 months.
Additionally, the disappearance of food-borne pathogens is somewhat guaranteed by the antimicrobial activity of some species of yeasts and LAB, further to the previously discussed harsh environmental conditions derived from salt concentration and pH [40]. The antimicrobial action of yeast strains is related to mycocins, or yeast killer toxins; the latter are toxins active against members of the same species, or closely related species—with activities analogous to those of bacteriocins in bacterial species [41].
3.4. Identification and Quantification of Organic Acids Compounds in Brine
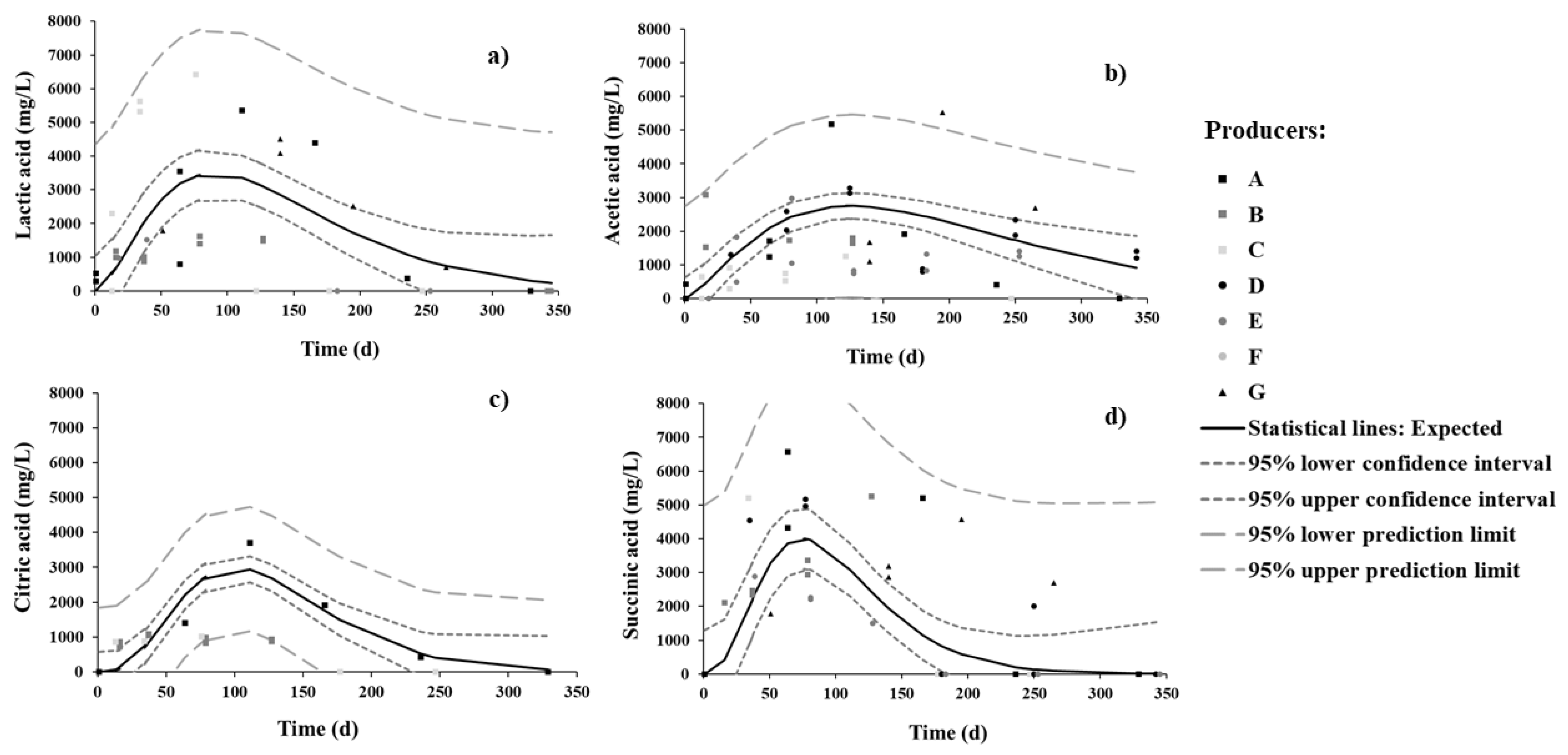
The evolution of organic acids was in general similar to that of TTA (and a parabolic pattern was expected) (Figure 3). During sweetening, succinic is the dominant organic acid, followed by lactic, and then by acetic and citric (to similar concentrations).
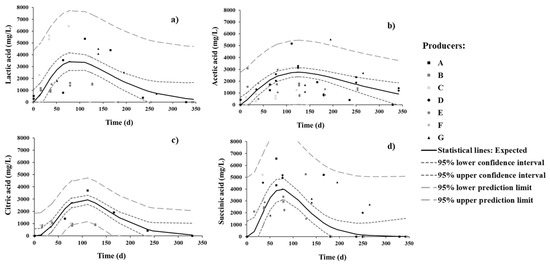
Figure 3.
Changes in (a) lactic, (b) acetic, (c) citric, and (d) succinic organic acids in brine during spontaneous fermentation of Cobrançosa table olives.
Citric acid is inherent in olive flesh, while lactic and acetic acids arise from microbial activity [42]. The presence of succinic acid is related to microbial conversion of citric to succinic acid via a potential shift from hetero- to homo-fermentative metabolism of LAB [43]; this appears to be the case in our study, as discussed below in further detail. The peaks of concentrations of succinic and lactic acids were attained by 3 months, whereas citric and acetic acids peaked at 4 months. By the end of the process, acetic acid became dominant, seconded by lactic acid. However, succinic acid decreased faster than the others by 7 months, followed by lactic and citric—and acetic acid, at a much lower rate.
Based on previous studies, a higher salt content and mild room temperature favor the enzymatic activity of LAB over yeasts, thus causing lactic acid concentration to increase as the numbers of LAB increase and to decrease more slowly than others do thereafter; yeasts resistant to salt could, however, also be responsible for the slower decrease in acetic acid at the final stage of fermentation [6,36].
It is noteworthy that the presence of acetic acid can be attributed to the activity of yeasts or heterofermentative LAB, able to generate acetic acid from fermentable material under particular conditions of environmental stress, as well as to metabolize citric acid [37]. Nevertheless, acetic acid did not vanish by 11 months and even became the most concentrated organic acid in brine; this is so because some producers deliberately add vinegar during the process in attempts to empirically control pH—information not provided in the questionnaire (as requested) but confirmed a posteriori after having visited the producers.
Inspection of Figure 3 unfolds a large probability that citric and succinic acids were depleted in full; this agrees with the findings by Tassou, Panagou, and Katsaboxakis [32], who reported similar observations at the final stage for other varieties of table olives.
3.5. Identification and Quantification of Phenolic Compounds in Brine
Oleuropein and other β-glucosides are the major fermentative substrates from the olive drupe; they are enzymatically hydrolyzed by β-glucosidase, which releases glucose and aglycones. The latter are then quantitatively degraded by esterase to simple and non-bitter phenolics, such as hydroxytyrosol and tyrosol. Some strains of LAB can accelerate the process, by hydrolyzing such glycosides as oleuropein and ligstroside—mainly responsible for the bitter taste of olives. The breakdown of oleuropein and ligstroside takes place in two phases: (i) hydrolysis of the glycosidic bond by β-glucosidase, with formation of oleuropein or ligstroside-aglycons and (ii) hydrolysis of the aglycons by esterase, with formation of elenolic acid and hydroxytyrosol (from oleuropein-aglycon) or tyrosol (from ligstroside-aglycon) [33,44,45]. The hydrolysis of oleuropein during fermentation is also attributed to the acid pH, coupled with the activity of β-glucosidase produced by oleuropeinolytic yeasts [40].
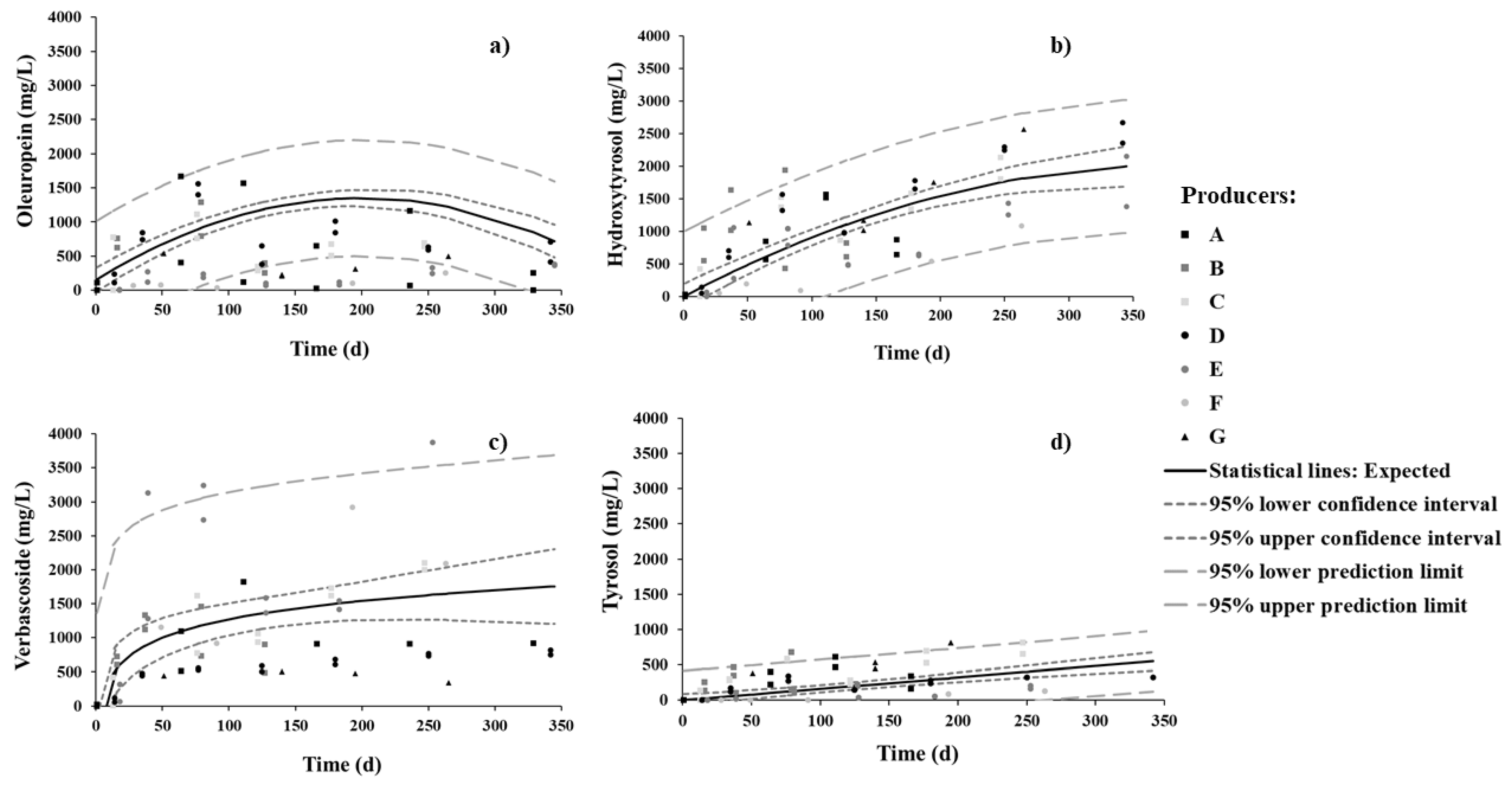
The concentrations of oleuropein, hydroxytyrosol, verbascoside, and tyrosol were monitored throughout the fermentation process (Figure 4). Oleuropein attained its maximum value, ca. 1200 mg/L, by 7 months. Within the same period, hydroxytyrosol increased steadily up to 1700 mg/L and went on increasing up to 2000 mg/L by the end of the process, although at a much lower rate. Verbascoside reached 1500 mg/L within just 2 months, and then increased slowly up to 1700 mg/L by the end of the process. Tyrosol was found to much lower levels (about one third) than the other phenolic compounds and exhibited a linear pattern throughout the process.
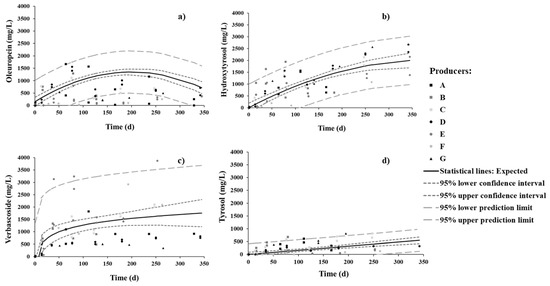
Figure 4.
Changes in (a) oleuropein, (b) hydroxytyrosol, (c) verboscoside, and (d) tyrosol phenolic compounds in brine during spontaneous fermentation of Cobrançosa table olives.
As for oleuropein, the importance of the sweetening stage (with brine changes) in removing this compound is apparent, as it leaches off the pulp into the surrounding water. In the salting stage, LAB and yeasts able to degrade oleuropein (associated to a bitter taste) into hydroxytyrosol (associated to a non-bitter taste) accordingly attain a great importance. Hydroxytyrosol is considered the main marker to estimate oleuropein degradation, as well as the diffusion of phenols from fruit to brine [6,13,33]. Our results corroborate the continuous increase in hydroxytyrosol throughout fermentation versus the oleuropein profile, which attains a maximum at 5–6 months (or end of sweetening stage); oleuropein reaches a maximum because it is released into the brine but simultaneously undergoes conversion to hydroxytyrosol by LAB and yeasts present therein, while hydroxytyrosol grows monotonically for being a final product. This growth is accompanied by significant growth of LAB—their population by ca. 6 months became slightly higher in number than the yeast population (ca. 1 log CFU/mL).
Another explanation for the higher rate of production of hydroxytyrosol in the first 3 months (typically November to January) than in the later 3 months may be temperature (typically July to August, Table 1). Medina et al. [46] indeed reported that inactivation of β-glucosidase during olive brining increases with increasing room temperature. Considering that the drums are kept inside warehouses not designed to maintain a constant temperature, the hydroxytyrosol profile developed during fermentation might also be influenced by environmental temperature.
The evolution of the concentration of verbascoside agrees with Pereira et al. [47], who found verbascoside in all debittering methods of fermentation. Concerning table olive cultivars, it was different—as expected from the results reported by Ait Chabane et al. [48] and Salis et al. [49]. Note that the latter study claims that high-performance liquid chromatography diode array detector (HPLC-DAD) and ultrahigh-performance liquid chromatography tandem mass spectrometry (LC-(ESI)-MS/MS) produce different results when selected for analytical methods.
Tyrosol exhibited the lowest concentration of all four phenolic compounds assayed for; its profile throughout the process looks similar to that reported for spontaneous fermentation of other cultivars [13,33,47]. This realization is probably a consequence of hydrolysis of tyrosol glucoside, as well as the diffusion of tyrosol from olive pulp to brine.
3.6. Molecular Identification of Lactic Acid Bacteria
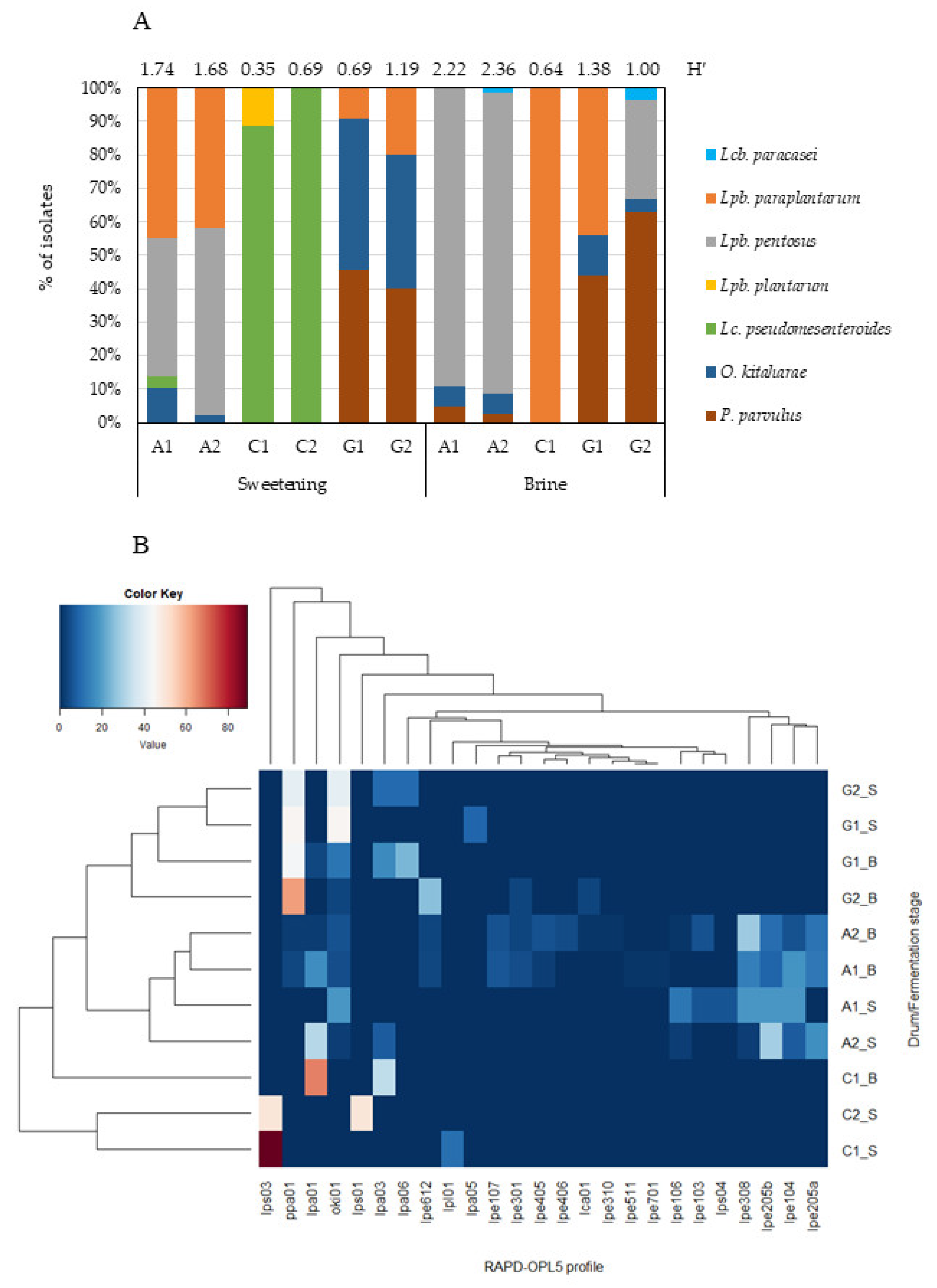
Results from RAPD-PCR and sequence analysis of a representative number of isolates reveal seven different LAB species belonging to five different bacterial genera—Lacticaseibacillus (Lcb.), Lactiplantibacillus (Lpb.), Leuconostoc (Lc.), Oenococcus (O.), and Pediococcus (P.). Their relative abundance, in each sampled drum and at each fermentation stage, is depicted in Figure 5A.
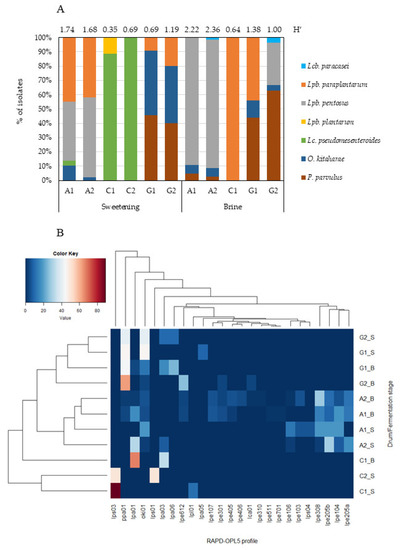
Figure 5.
(A) Relative abundance (%) of the LAB species identified in Cobrançosa table olive drums at each fermentation stage. Shannon–Weaver’s index of diversity (H′), based on the RAPD-OPL5 profiles, is reported for each drum at each fermentation stage; (B) heatmap depicting the relative percentage of each RAPD-OPL5 profile within each Cobrançosa table olive drum (A, C, and G) at sweetening (_S) and brining (_B) stages. Hierarchical clustering was performed using Euclidean distance and average linkage clustering.
The species Lpb. pentosus, Lpb. paraplantarum, Lc. pseudomesenteroides, O. kitaharae, and P. parvulus were the most frequently found, in contrast to Lpb. plantarum and Lcb. paracasei. These findings partially agree with Portilha-Cunha, Macedo, and Malcata [7], who reported that the most cited species worldwide during fermentation of (green or black) table olives are Lpb. pentosus and Lpb. plantarum, although pertaining to different processing styles (natural versus treated), as well as Lpb. paraplantarum and Lc. mesenteroides to a lesser extent.
Lpb. pentosus and O. kitaharae were frequently found in both stages, whereas Lpb. paraplantarum and Lc. pseudomesenteroides appeared to be better adapted to the sweetening stage. The highest LAB diversity was found for producer A—with all identified species detected, except Lpb. Plantarum, followed by producer G, for whom only the species Lpb. plantarum and Lc. pseudomesenteroides could not be found (Figure 5A). When cluster analysis was applied (Figure 5B), producers A and G indeed gathered as a cluster, clearly separated from producer C. Producer A was characterized by the occurrence of Lpb. pentosus, regardless of fermentation stage. This species displayed the greatest genotypic variability, with 14 distinct fingerprints—again all found in producer A; they justified the highest diversity of LAB recorded during the whole process. Only 2 genotypes (lpe301, lpe612) out of 14 were recovered from more than one producer along fermentation.
Olives from producer G constituted a separate cluster on the basis of abundance of P. parvulus and O. kitaharae. These species were recovered at higher frequency in producer G and accounted for 86% and 62% in sweetening and brining stages, respectively. No genetic diversity was found among isolates belonging to species O. kitaharae and P. parvulus.
Producer C showed the highest level of dissimilarity among samples at the sweetening stage because of the high incidence of Lc. pseudomesenteroides—with two distinct RAPD profiles (lps01 and lps03). However, he clustered close to producers A and G at the brining stage, thus underlining the presence of genetic profiles that colonize those ecosystems (P. parvulus and Lcb. paracasei). In fact, Lpb. paraplantarum—the single species found in the brine stage of producer C—was also present in the sweetening and salting stages of producers A (28% and 9%, respectively) and G (14% and 21%, respectively). Of the four different fingerprints found throughout fermentation, two (lpa01 and lpa03) were recovered from more than one producer (A, C, and G), and the others were found in producer G, during either the sweetening stage (lpa05) or both stages (lpa06). Such LAB species as Lpb. plantarum (1 fingerprint) and Lcb. paracasei (1 fingerprint) accounted for ca. 2% (from producer C, sweetening stage) and 1% (from producers A and G, salting stage) of the isolates.
Except for producer C, no specific separation could be observed between producers A and G based on the stage of fermentation. Olives from such producers underwent slight changes in their LAB profiles as fermentation proceeded. The LAB diversity along the fermentation of Cobrançosa olives ranged from ca. 0.52 (producer C, sweetening) to 2.29 (producer A, brine), as indicated by the Shannon diversity index (Figure 5A). As mentioned before, LAB did not dominate in the batches by producers B, D, E, and F—thus unfolding the role that yeasts may play in the fermentation of Cobrançosa olives by such producers.
3.7. Standardization of the Process
As far as our knowledge goes, French Nyons and Spanish Empeltre table olives resemble Cobrançosa from a technological perspective—considering harvest period, their undergoing spontaneous fermentation without starters, and duration of fermentation of up to (at least) 1 year [13,46]. On the other hand, Nyons olives are directly submerged in brine (10% salt) and then sealed with a heavy lid to halt aeration, whereas Empeltre olives are treated under aerobic conditions during fermentation by fitting an aeration column to the tank. They exhibit, however, quite different microbial dynamics—with yeasts clearly overriding and a poor contribution of LAB to fermentation. This explains why their profiles of organic acids are rather different from ours, with those differences expanding as fermentation time elapses. Such facts reinforce that the existence of sweetening and brining stages in fermentation (involving microaeration) is crucial for the uniqueness of Cobrançosa olives—arising from specific population dynamics between yeasts and LAB and consequent biochemical profiles.
Pino et al. [25] additionally suggest a stronger oleuropeinolytic activity of LAB starters under 5% than 8% salt concentration—prone to reducing the bitterness of the final product. These authors claimed that the reduction in salt would be not a risk factor toward an increase in pathogen numbers, provided that pH remained the same throughout fermentation. Furthermore, the best sensory scores received by Nocellara Etnea table olives were recorded for 5% salt when compared with 4, 6, and 8% salt [25], while said low-salt concentration did not compromise microbiological safety of the product. Finally, note that lactic acid fermentation is favored when LAB outnumber yeasts, thus rendering a more acidic product with a lower pH. The opposite occurs with un-debittered table olives, for which LAB growth is hampered by the high concentration of polyphenols—and oleuropein in particular. As a consequence, yeasts become the prevailing group, responsible for a fruity flavor coupled with a slightly bitter taste of the final product [44].
4. Conclusions
The production of Northeast Cobrançosa table olives follows traditional manufacturing processes, typically uncontrolled and hardly standardized to date. Our pioneer study revealed that considerable variability exists in the physicochemical, biochemical, and microbiological features throughout fermentation. Nevertheless, a few trends could be ascertained—which, as a whole, will likely support the definition of a standard protocol for Cobrançosa table olive processing technology: harvest at intermediate stage of maturation (turning color), ratio of 2:1 for table olives:water, and drums covered with tight lids. The sweetening stage should last 2 months in water, renewed every two weeks, and the salting stage should encompass 3% salt for 2 months, 5% salt for 4 more months, and 7% salt for the remaining 5 months. This improved protocol—necessary for the establishment of the Cahier d’Écharges required for granting of a PDO status to said table olives—will necessarily include a specific starter culture to be designed based on the microbiological profile described. The starter strain selection should then be based on their in vitro characteristics, especially ability to survive under higher salt concentrations, tolerance to a wider pH range, ability to grow at low temperature, and presence of β-glucosidase activity. Our groundbreaking research effort accordingly laid the foundations for the technological development of a starter expected to bear probiotic traits. Toward this specific goal, three major steps are envisaged: (i) screen the identified LAB and select those exhibiting the best probiotic performance in vitro; (ii) optimize biomass production thereof; and (iii) validate said performance in loco. It is expected that the utilization of said starter for olive fermentation will allow the standardization of the final product, namely in sensory characteristics, while the associated probiotic potential will bring about an extra added value to the final product.
Author Contributions
Conceptualization, P.J.M.R., F.X.M. and A.C.M.; methodology, P.J.M.R. and T.G.T.; validation, P.J.M.R. and F.X.M.; formal analysis, P.J.M.R. and A.C.M.; investigation, P.J.M.R., T.G.T. and J.M.R.; data curation, P.J.M.R., T.G.T. and J.M.R.; writing—review and editing, P.J.M.R., F.X.M. and A.C.M.; supervision, P.J.M.R., F.X.M. and A.C.M.; project administration, F.X.M. and A.C.M.; funding acquisition, F.X.M. and A.C.M.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by: Project PROMETHEUS-POCI-01-0145-FEDER-029284, funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES; and LA/P/0045/2020 (ALiCE), UIDB/00511/2020 and UIDP/00511/2020 (LEPABE), funded by national funds through FCT/MCTES (PIDDAC).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Rocha Fernandes, c/o Direção Regional de Agricultura e Pescas do Norte, for support in selecting olive producers for screening and in transporting raw materials to our laboratory premises.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anonymous. World Catalogue of Olive Varieties. Available online: https://theolivecentre.com/product/World-Catalogue-of-Olive-Varieties (accessed on 12 September 2022).
- Heperkan, D. Microbiota of table olive fermentations and criteria of selection for their use as starters. Front. Microbiol. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Bonatsou, S.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.E. Table olive fermentation using starter cultures with multifunctional potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Khairul Alam, M.; Corsetti, A. Table olives more than a fermented food. Foods 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Rejano, L.; Montaño, A.; Casado, F.J.; Sánchez, A.H.; Castro, A.D. Table Olives: Varieties and Variations. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 5–15. [Google Scholar]
- Anagnostopoulos, D.A.; Kamilari, E.; Tsaltas, D. Evolution of bacterial communities, physicochemical changes and sensorial attributes of natural whole and cracked Picual table olives during spontaneous and inoculated fermentation. Front. Microbiol. 2020, 11, 1128. [Google Scholar] [CrossRef]
- Portilha-Cunha, M.F.; Macedo, A.C.; Malcata, F.X. A review on adventitious lactic acid bacteria from table olives. Foods 2020, 9, 948. [Google Scholar] [CrossRef]
- Doulgeraki, A.I.; Hondrodimou, O.; Iliopoulos, V.; Panagou, E.Z. Lactic acid bacteria and yeast heterogeneity during aerobic and modified atmosphere packaging storage of natural black Conservolea olives in polyethylene pouches. Food Control 2012, 26, 49–57. [Google Scholar] [CrossRef]
- Comunian, R.; Ferrocino, I.; Paba, A.; Daga, E.; Campus, M.; Di Salvo, R.; Cauli, E.; Piras, F.; Zurru, R.; Cocolin, L. Evolution of microbiota during spontaneous and inoculated Tonda di Cagliari table olives fermentation and impact on sensory characteristics. LWT-Food Sci. Technol. 2017, 84, 64–72. [Google Scholar] [CrossRef]
- Oliveira, M.; Brito, D.; Catulo, L.; Leitão, F.; Gomes, L.; Silva, S.; Vilas Boas, L.; Peito, A.; Fernandes, I.; Gordo, F.; et al. Biotechnology of olive fermentation of Galega Portuguese variety. Grasas Y Aceites 2004, 55, 219–226. [Google Scholar] [CrossRef]
- Arroyo-Lopez, F.N.; Medina, E.; Ruiz-Bellido, M.A.; Romero-Gil, V.; Montes-Borrego, M.; Landa, B.B. Enhancement of the Knowledge on Fungal Communities in Directly Brined Alorena de Malaga Green Olive Fermentations by Metabarcoding Analysis. PLoS ONE 2016, 11, e0163135. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Rodriguez-Gomez, F.; Barrio, E.; Querol, A.; Garrido-Fernandez, A.; Arroyo-Lopez, F.N. Exploring the yeast biodiversity of green table olive industrial fermentations for technological applications. Int. J. Food Microbiol. 2011, 147, 89–96. [Google Scholar] [CrossRef]
- Penland, M.; Deutsch, S.M.; Falentin, H.; Pawtowski, A.; Poirier, E.; Visenti, G.; Le Meur, C.; Maillard, M.B.; Thierry, A.; Mounier, J.; et al. Deciphering microbial community dynamics and biochemical changes during Nyons black olive natural fermentations. Front. Microbiol. 2020, 11, 586614. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, B.C. Yeast Ecological Interactions. Yeast-Yeast, Yeast-Bacteria, Yeast-Fungi Interactions and Yeasts as Biocontrol Agents. In Yeasts in Food and Beverages; Querol, A., Fleet, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 83–110. [Google Scholar]
- Anonymous. World Table Olive Figures. Available online: https://www.internationaloliveoil.org/what-we-do/economic-affairs-promotion-unit/#figures (accessed on 9 June 2022).
- Anonymous. Produção das Principais Culturas Agrícolas por Região Agrária. Agricultura, Floresta e Pescas. Available online: https://www.ine.pt/xportal/xmain?xpid=INE&xpgid=ine_indicadores&indOcorrCod=0000705&xlang=pt&contexto=bd&selTab=tab2 (accessed on 9 June 2022).
- Anonymous. Azeitona de Conserva Negrinha de Freixo PDO. Available online: https://tradicional.dgadr.gov.pt/en/categories/olive-oils-and-olives/353-azeitona-de-conserva-negrinha-de-freixo-dop-en (accessed on 9 June 2022).
- Malheiro, R.; Casal, S.; Sousa, A.; de Pinho, P.G.; Peres, A.M.; Dias, L.G.; Bento, A.; Pereira, J.A. Effect of Cultivar on Sensory Characteristics, Chemical Composition, and Nutritional Value of Stoned Green Table Olives. Food Bioprocess Technol. 2012, 5, 1733–1742. [Google Scholar] [CrossRef]
- Rodrigues, N.; Marx, Í.M.G.; Dias, L.G.; Veloso, A.C.A.; Pereira, J.A.; Peres, A.M. Monitoring the debittering of traditional stoned green table olives during the aqueous washing process using an electronic tongue. LWT-Food Sci. Technol. 2019, 109, 327–335. [Google Scholar] [CrossRef]
- Peres, A.M.; Baptista, P.; Malheiro, R.; Dias, L.G.; Bento, A.; Pereira, J.A. Chemometric classification of several olive cultivars from Trás-os-Montes region (Northeast of Portugal) using artificial neural networks. Chemom. Intell. Lab. Syst. 2011, 105, 65–73. [Google Scholar] [CrossRef]
- Malheiro, R.; Mendes, P.; Fernandes, F.; Rodrigues, N.; Bento, A.; Pereira, J.A. Bioactivity and phenolic composition from natural fermented table olives. Food Funct. 2014, 5, 3132–3142. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, R.; Sousa, A.; Casal, S.; Bento, A.; Pereira, J.A. Cultivar effect on the phenolic composition and antioxidant potential of stoned table olives. Food Chem. Toxicol. 2011, 49, 450–457. [Google Scholar] [CrossRef]
- Malheiro, R.; de Pinho, P.G.; Casal, S.; Bento, A.; Pereira, J.A. Determination of the volatile profile of stoned table olives from different varieties by using HS-SPME and GC/IT-MS. J. Sci. Food Agric. 2011, 91, 1693–1701. [Google Scholar] [CrossRef]
- Seabra, R.M.; Andrade, P.B.; Valentão, P.; Faria, M.; Paice, A.; Oliveira, M.B.P.P. Phenolic Profiles of Portuguese Olives: Cultivar and Geographics. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2010; pp. 177–186. [Google Scholar]
- Pino, A.; Vaccalluzzo, A.; Solieri, L.; Romeo, F.V.; Todaro, A.; Caggia, C.; Arroyo-López, F.N.; Bautista-Gallego, J.; Randazzo, C.L. Effect of sequential inoculum of beta-Glucosidase positive and probiotic strains on brine fermentation to obtain low salt sicilian table olives. Front. Microbiol. 2019, 10, 174. [Google Scholar] [CrossRef]
- Ghabbour, N.; Lamzira, Z.; Thonart, P.; Peres, C.; Markaoui, M.; Asehraou, A. Selection of oleuropein-degrading lactic acid bacteria strains isolated from fermenting Moroccan green olives. Grasas Y Aceites 2011, 62, 84–89. [Google Scholar] [CrossRef]
- Cosmai, L.; Campanella, D.; De Angelis, M.; Summo, C.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Use of starter cultures for table olives fermentation as possibility to improve the quality of thermally stabilized olive-based paste. LWT-Food Sci. Technol. 2018, 90, 381–388. [Google Scholar] [CrossRef]
- Maldonado-Barragan, A.; Caballero-Guerrero, B.; Lucena-Padros, H.; Ruiz-Barba, J.L. Induction of bacteriocin production by coculture is widespread among plantaricin-producing Lactobacillus plantarum strains with different regulatory operons. Food Microbiol. 2013, 33, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozes, N. Influence of fruit ripeness and salt concentration on the microbial processing of Arbequina table olives. Food Microbiol. 2009, 26, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Bengana, M.; Bakhouche, A.; Lozano-Sánchez, J.; Amir, Y.; Youyou, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Influence of olive ripeness on chemical properties and phenolic composition of Chemlal extra-virgin olive oil. Food Res. Int. 2013, 54, 1868–1875. [Google Scholar] [CrossRef]
- Tassou, C.C.; Panagou, E.Z.; Katsaboxakis, K.Z. Microbiological and physicochemical changes of naturally black olives fermented at different temperatures and NaCl levels in the brines. Food Microbiol. 2002, 19, 605–615. [Google Scholar] [CrossRef]
- Kiai, H.; Raiti, J.; El Abbassi, A.; Hafidi, A. Chemical profiles of Moroccan Picholine olives and its brines during spontaneous fermentation. Int. J. Fruit Sci. 2020, 20, S1297–S1312. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Hondrodimou, O.; Mallouchos, A.; Nychas, G.J. A study on the implications of NaCl reduction in the fermentation profile of Conservolea natural black olives. Food Microbiol. 2011, 28, 1301–1307. [Google Scholar] [CrossRef]
- Abriouel, H.; Benomar, N.; Cobo, A.; Caballero, N.; Fernandez Fuentes, M.A.; Perez-Pulido, R.; Galvez, A. Characterization of lactic acid bacteria from naturally-fermented Manzanilla Aloreña green table olives. Food Microbiol. 2012, 32, 308–316. [Google Scholar] [CrossRef]
- Aponte, M.; Ventorino, V.; Blaiotta, G.; Volpe, G.; Farina, V.; Avellone, G.; Lanza, C.M.; Moschetti, G. Study of green Sicilian table olive fermentations through microbiological, chemical and sensory analyses. Food Microbiol. 2010, 27, 162–170. [Google Scholar] [CrossRef]
- Tzamourani, A.P.; Di Napoli, E.; Paramithiotis, S.; Economou-Petrovits, G.; Panagiotidis, S.; Panagou, E.Z. Microbiological and physicochemical characterisation of green table olives of Halkidiki and Conservolea varieties processed by the spanish method on industrial scale. Int. J. Food Sci. Technol. 2021, 56, 3845–3857. [Google Scholar] [CrossRef]
- Medina, E.; Romero-Gil, V.; Garrido-Fernandez, A.; Arroyo-Lopez, F.N. Survival of foodborne pathogens in natural cracked olive brines. Food Microbiol. 2016, 59, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Hutkins, R.W. Microbiology and Technology of Fermented Foods; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Silva, T.; Reto, M.; Sol, M.; Peito, A.; Peres, C.M.; Peres, C.; Malcata, F.X. Characterization of yeasts from Portuguese brined olives, with a focus on their potentially probiotic behavior. LWT-Food Sci. Technol. 2011, 44, 1349–1354. [Google Scholar] [CrossRef]
- Psani, M.; Kotzekidou, P. Technological characteristics of yeast strains and their potential as starter adjuncts in Greek-style black olive fermentation. World J. Microbiol. Biotechnol. 2006, 22, 1329–1336. [Google Scholar] [CrossRef]
- Bleve, G.; Tufariello, M.; Durante, M.; Perbellini, E.; Ramires, F.A.; Grieco, F.; Cappello, M.S.; De Domenico, S.; Mita, G.; Tasioula-Margari, M.; et al. Physico-chemical and microbiological characterization of spontaneous fermentation of Cellina di Nardo and Leccino table olives. Front. Microbiol. 2014, 5, 570. [Google Scholar] [CrossRef]
- Papadelli, M.; Zoumpopoulou, G.; Georgalaki, M.; Anastasiou, R.; Manolopoulou, E.; Lytra, I.; Papadimitriou, K.; Tsakalidou, E. Evaluation of two lactic acid bacteria starter cultures for the fermentation of natural black table olives (Olea europaea L. cv Kalamon). Pol. J. Microbiol. 2015, 64, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B. Abnormal fermentations in table-olive processing: Microbial origin and sensory evaluation. Front. Microbiol. 2013, 4, 91. [Google Scholar] [CrossRef]
- Lanza, B.; Zago, M.; Di Marco, S.; Di Loreto, G.; Cellini, M.; Tidona, F.; Bonvini, B.; Bacceli, M.; Simone, N. Single and multiple inoculum of Lactiplantibacillus plantarum strains in table olive lab-scale fermentations. Fermentation 2020, 6, 126. [Google Scholar] [CrossRef]
- Medina, E.; García-García, P.; Romero, C.; Castro, A.; Brenes, M. Aerobic industrial processing of Empeltre cv. natural black olives and product characterisation. Int. J. Food Sci. Technol. 2019, 55, 534–541. [Google Scholar] [CrossRef]
- Pereira, J.A.; Pereira, A.P.G.; Ferreira, I.C.F.R.; Valentão, P.; Andrade, P.B.; Seabra, R.; Estevinho, L.; Bento, A. Table olives from Portugal: Phenolic compounds, antioxidant potential, and antimicrobial activity. J. Agric. Food Chem. 2006, 54, 8425–8431. [Google Scholar] [CrossRef]
- Ait Chabane, F.; Tamendjari, A.; Rovellini, P.; Romero, C.; Medina, E. Chemical parameters and antioxidant activity of turning color natural-style table olives of the Sigoise cultivar. Grasas Y Aceites 2021, 72, e419. [Google Scholar] [CrossRef]
- Salis, C.; Papadakis, I.E.; Hagidimitriou, M. Identification and quantification of phenolic compounds in fresh and processed table olives of cv. ‘Kalamata’. Not. Bot. Horti. Agrobo. 2021, 49, 12394. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).