The Efficacy, Phytotoxicity, and Safety of Liquid Ethyl Formate Used to Control the Grape (Campbell Early) Quarantine Pest Pseudococcus comstocki
Abstract
:Featured Application
Abstract
1. Introduction
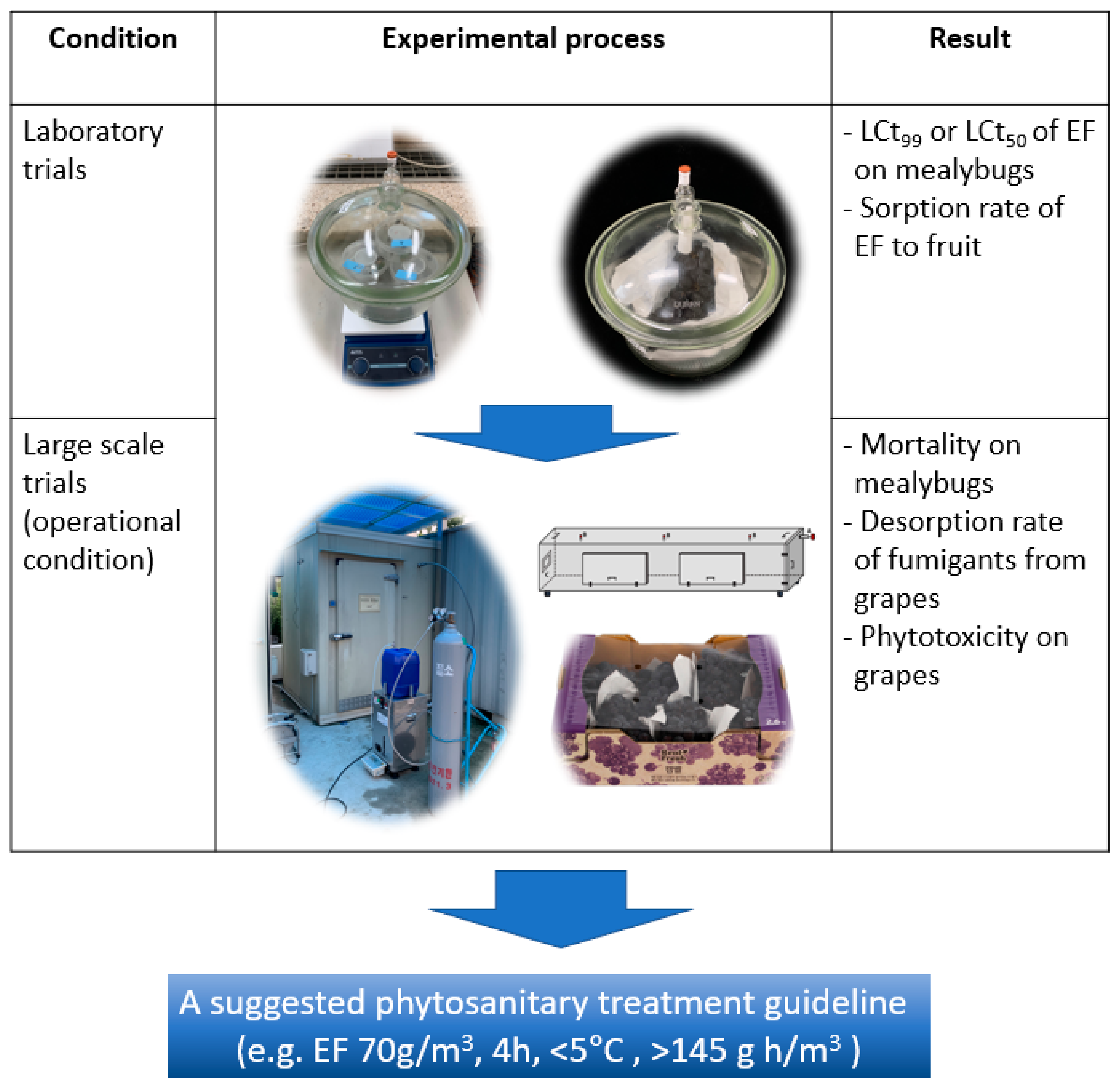
2. Materials and Methods
2.1. Fumigants
2.2. Insects
2.3. EF Concentration and Determination of the Ct (Concentration × Time) Product in Scaled-Up Fumigation
2.4. Efficacy of EF against P. comstocki in Laboratory Trials
2.5. EF Sorption under Different Loading Ratios of Grapes
2.6. Permeability of EF Gas through Packaging Film Used for Grapes
2.7. Large-Scale (10 m3) Fumigation Using Liquid EF on P. comstocki
2.8. Assessment of Phytotoxic Damage on Grapes Post-EF-Fumigation
2.9. Desorption of EF and MB from Fumigated Grapes
2.10. Statistical Analysis
3. Results
3.1. Efficacy of EF against P. comstocki in Laboratory Trials
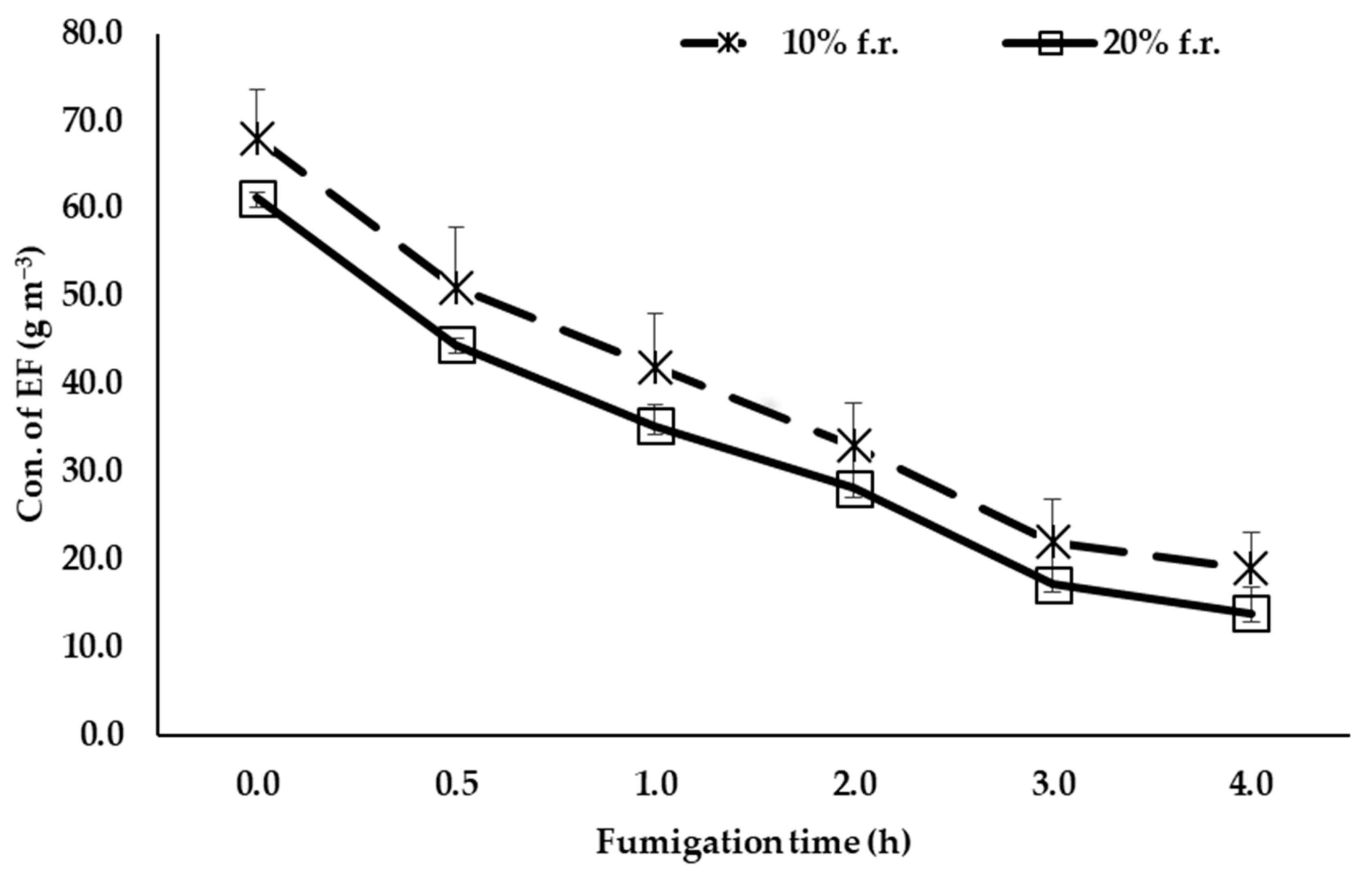
3.2. Sorption of EF with Respect to Different Loading Ratios of Grapes
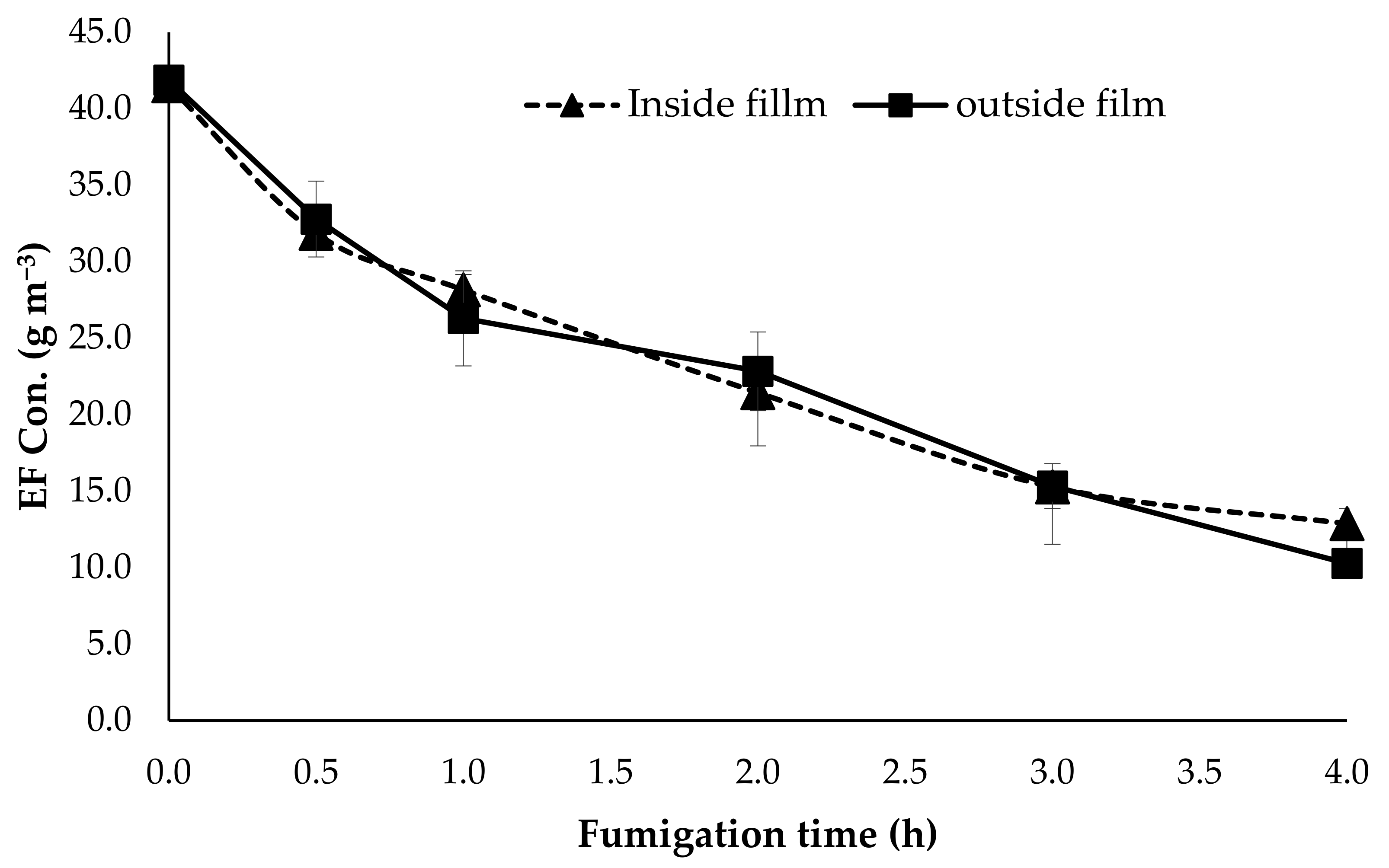
3.3. Permeability of EF Gas through the Grape Packaging Film
3.4. Large-Scale (10 m3) P. comstocki Fumigation with Liquid EF
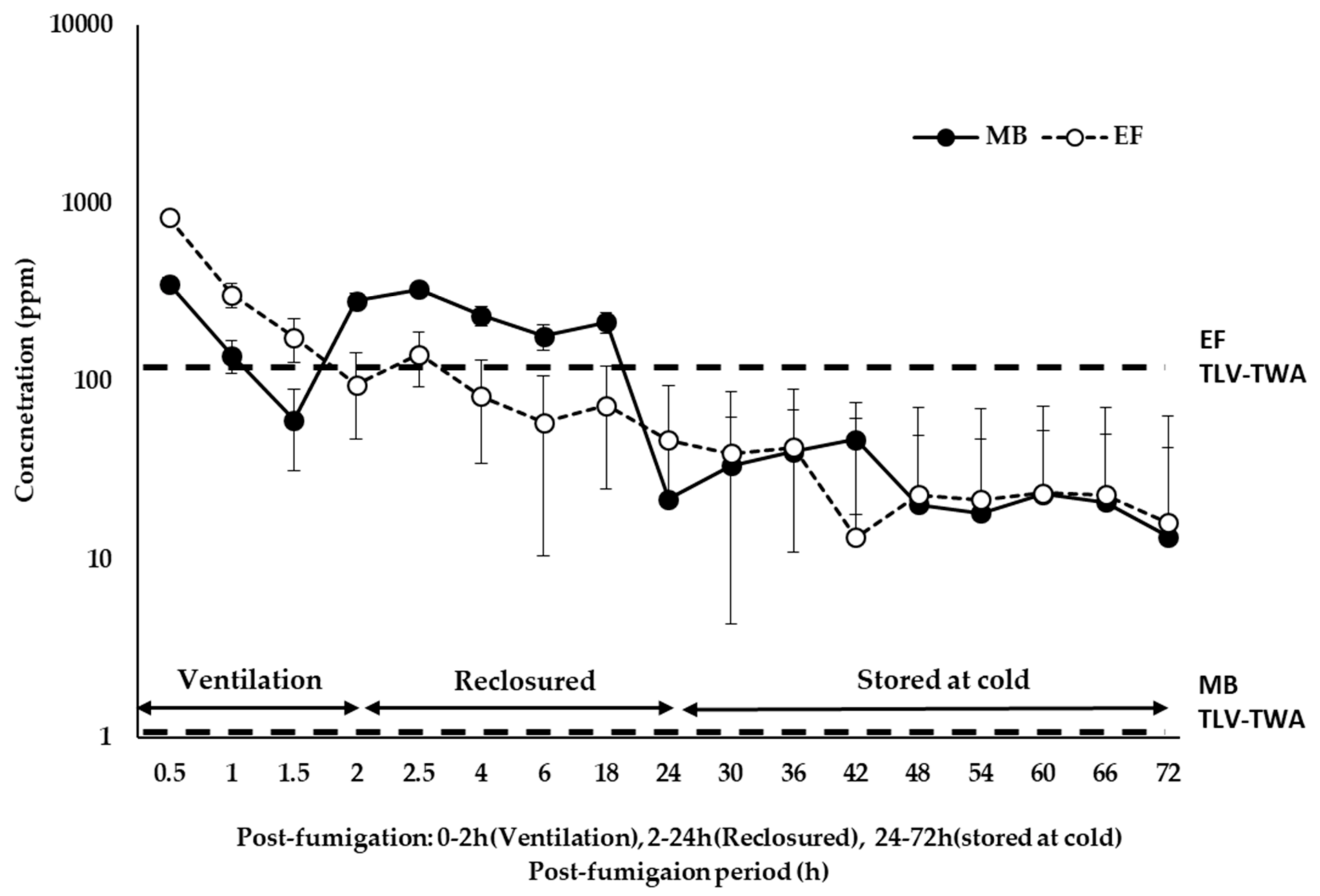
3.5. Evaluation of EF and MB Desorption from Fumigated Grapes
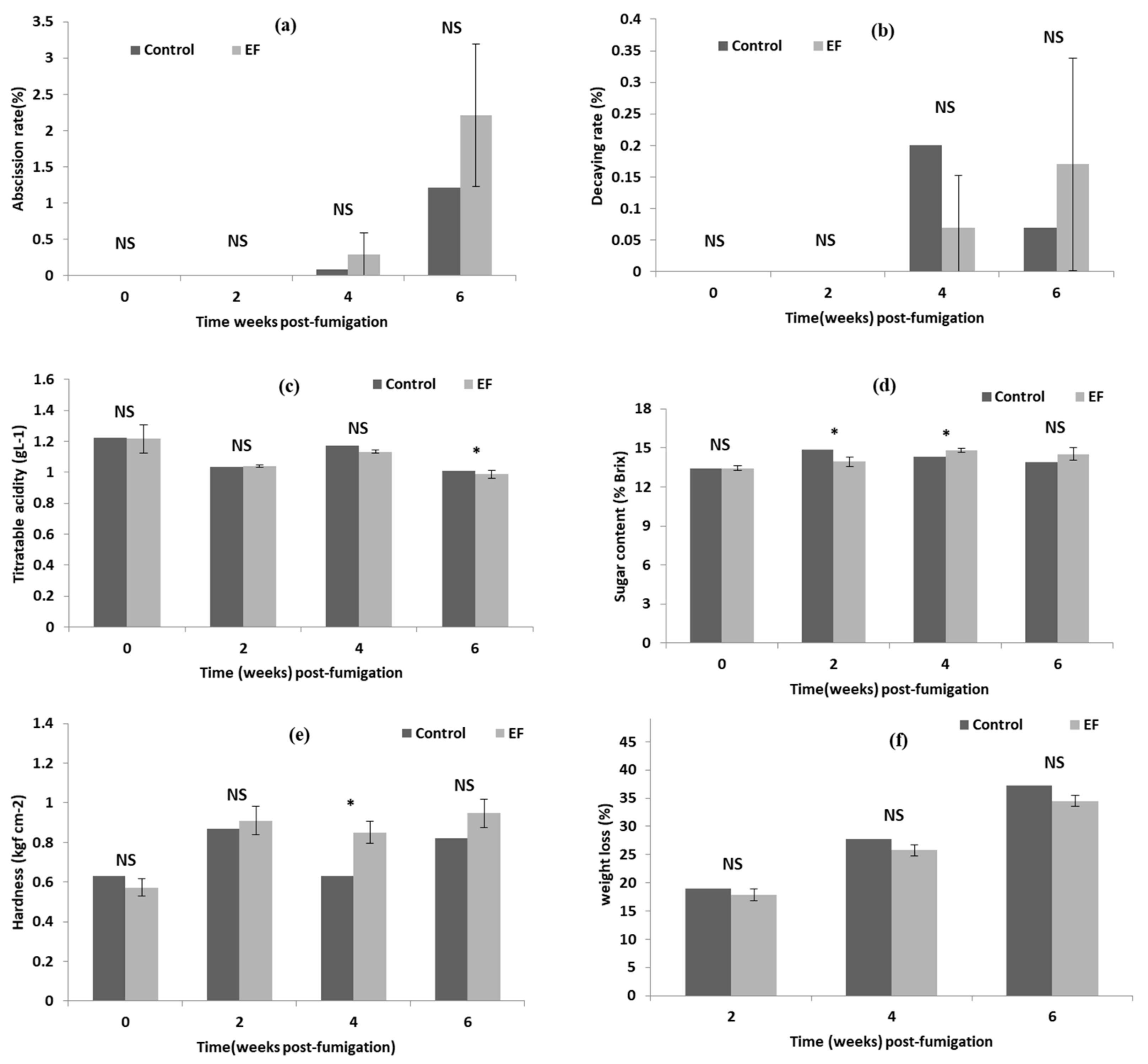
3.6. Assessment of Phytotoxic Damages on Grapes Post-EF Fumigation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OEC. Grapes. Available online: https://oec.world/en/profile/hs/grapes (accessed on 24 August 2022).
- KATI. Grape. Available online: https://m.kati.net/product/basisInfo.do?lcdCode=MD154 (accessed on 19 July 2022).
- APQA. Phytosanitary Disinfestation Guidelines. Available online: http://www.qia.go.kr/plant/disinpect/listXdclbzWebAction.do (accessed on 10 June 2022).
- UNEP. Handbook for the Montreal Protocol on Substances that Deplete the Ozone Layer, 12th ed.; United Nations Environment Programme: Nairobi, Kenya, 2018; p. 918. [Google Scholar]
- MBTOC. 2010 Report of the Methyl Bromide Technical Options Committee; 9789966200006; United Nations Environment Programme: Nairobi, Kenya, 2010; pp. 1–398. [Google Scholar]
- Suwanlaong, K.; Phanthumchinda, K. Neurological Manifestation of Methyl Bromide Intoxication. J. Med. Assoc. Thai. 2008, 91, 421–426. [Google Scholar] [PubMed]
- Baur, X.; Budnik, L.T.; Zhao, Z.; Bratveit, M.; Djurhuus, R.; Verschoor, L.; Rubino, F.M.; Colosio, C.; Jepsen, J.R. Health risks in international container and bulk cargo transport due to volatile toxic compounds. J. Occup. Med. Toxicol. 2015, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Preisser, A.M.; Budnik, L.T.; Baur, X. Health effects due to fumigated freight containers and goods: How to detect, how to act. Int. Marit. Health 2012, 63, 133–139. [Google Scholar] [PubMed]
- Deschamps, F.J.; Turpin, J.C. Methyl bromide intoxication during grain store fumigation. Occup. Med. 1996, 46, 89–90. [Google Scholar] [CrossRef]
- Park, M.-G.; Choi, J.; Hong, Y.-S.; Park, C.G.; Kim, B.-G.; Lee, S.-Y.; Lim, H.-J.; Mo, H.-h.; Lim, E.; Cha, W. Negative effect of methyl bromide fumigation work on the central nervous system. PLoS ONE 2020, 15, e0236694. [Google Scholar] [CrossRef]
- Park, M.-G.; Hong, Y.-S.; Park, C.G.; Gu, D.-C.; Mo, H.-H. Variations in methyl bromide concentration with distance and time during quarantine fumigation. Environ. Monit. Assess. 2021, 193, 397. [Google Scholar] [CrossRef]
- Choi, J.; Hong, Y.-S.; Cha, W.; Mo, H.-H.; Park, M.-G. Heart rate variability analysis in workers exposed to methyl bromide as a quarantine treatment. J. Occup. Environ. Med. 2021, 63, e32. [Google Scholar] [CrossRef]
- IPPC. Reccomendation on: Replacement or Reduction of the Use of Methyl bromide as a Phytosanitary Measure; FAO: Rome, Italy, 2008; pp. 1–8. [Google Scholar]
- Haritos, V.; Dojchinov, G. Cytochrome c oxidase inhibition in the rice weevil Sitophilus oryzae (L.) by formate, the toxic metabolite of volatile alkyl formates. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003, 136, 135–143. [Google Scholar] [CrossRef]
- American Conference of Governmental Industrial Hygienists. 2005 TLVs and BEIs; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 2005. [Google Scholar]
- Yang, J.O.; Kim, H.M.; Park, Y.J.; Park, M.G.; Ren, Y.L.; Lee, B.H. New Quarantine Trials for Using Liquid Ethyl Formate with Nitrogen Application on Imported Citrus Fruits_Cost Effectiveness and Worker Safety. In Proceedings of the International Symposium and Annual Meeting of the Korean Society of Pesticide Science (KSPS), Yeosu, Korea, 6–7 April 2017; p. 245. [Google Scholar]
- Apqa. Regulations for Phytosanitray Treatment of Import and Export Plant; Apqa: Gimcheon, Korea, 2019. [Google Scholar]
- Suh, S.-J.; Yu, H.M.; Hong, K.-J. List of Intercepted Scale Insects at Korean Ports of Entry and Potential Invasive Species of Scale Insects to Korea (Hemiptera: Coccoidea). Korean J. Appl. Entomol. 2013, 52, 141–160. [Google Scholar] [CrossRef]
- APQA. Available online: http://www.qia.go.kr/animal/prevent/listwebQiaCom.do?type=3_52jynb&clear=1 (accessed on 20 July 2022).
- APQA. Available online: http://www.qia.go.kr/bbs/lawAnn/listLawWebAction.do?type=0&key=%ED%8F%AC%EB%8F%84&firstkorkey=&nextkorkey=&lawQuery.jbbk_fl=all&pager.maxPageItems=10&pager.offset=0 (accessed on 20 July 2022).
- Park, M.-G.; Park, C.-G.; Yang, J.-O.; Kim, G.-H.; Ren, Y.; Lee, B.-H.; Cha, D.H. Ethyl Formate as a Methyl Bromide Alternative for Phytosanitary Disinfestation of Imported Banana in Korea with Logistical Considerations. J. Econ. Entomol. 2020, 113, 1711–1717. [Google Scholar] [CrossRef]
- Park, M.-G.; Lee, B.-H.; Yang, J.-O.; Kim, B.-S.; Roh, G.H.; Kendra, P.E.; Cha, D.H. Ethyl Formate as a Methyl Bromide Alternative for Fumigation of Citrus: Efficacy, Fruit Quality, and Workplace Safety. J. Econ. Entomol. 2021, 114, 2290–2296. [Google Scholar] [CrossRef]
- Bessi, H.; Bellagha, S.; Lebdi, K.G.; Bikoba, V.; Mitcham, E.J. Ethyl formate fumigation of dry and semidry date fruits: Experimental kinetics, modeling, and lethal effect on carob moth. J. Econ. Entomol. 2015, 108, 993–999. [Google Scholar] [CrossRef]
- Lee, B.-H.; Park, C.-G.; Park, M.-G.; Roh, G.-H.; Kim, D.; Riddick, E.W.; Chen, J.; Cha, D.H. Ethyl formate fumigation for the disinfestation of red imported fire ants Solenopsis invicta Buren. J. Asia-Pac. Entomol. 2019, 22, 838–840. [Google Scholar] [CrossRef]
- Kwon, T.H.; Park, C.G.; Lee, B.-H.; Zarders, D.R.; Roh, G.H.; Kendra, P.E.; Cha, D.H. Ethyl formate fumigation and ethyl formate plus cold treatment combination as potential phytosanitary quarantine treatments of Drosophila suzukii in blueberries. J. Asia-Pac. Entomol. 2021, 24, 129–135. [Google Scholar] [CrossRef]
- Simpson, T.; Bikoba, V.; Tipping, C.; Mitcham, E. Ethyl formate as a postharvest fumigant for selected pests of table grapes. J. Econ. Entomol. 2007, 100, 1084–1090. [Google Scholar] [CrossRef]
- Misumi, T.; Ogawa, N.; Yamada, K.; Shukuya, T. Susceptibilities of Five Species of Scales (Diaspididae and Coccidae) and Mealybugs (Pseudococcidae) to Fumigation with a Gas Mixture of Ethyl Formate and Carbon Dioxide under Normal Atmospheric Pressure or Vacuum. Res. Bullutine Plant Prot. Jpn. 2013, 49, 1–9. [Google Scholar]
- Pupin, F.; Bikoba, V.; Biasi, W.B.; Pedroso, G.M.; Ouyang, Y.; Grafton-Cardwell, E.E.; Mitcham, E.J. Postharvest Control of Western Flower Thrips (Thysanoptera: Thripidae) and California Red Scale (Hemiptera: Diaspididae) With Ethyl Formate and Its Impact on Citrus Fruit Quality. J. Econ. Entomol. 2014, 106, 2341–2348. [Google Scholar] [CrossRef]
- Jamieson, L.E.; Griffin, M.J.; Page-Weir, N.E.M.; Chhagan, A.; Redpath, S.P.; Connolly, P.G. Developing ethyl formate treatment for disinfesting pipfruit. N. Z. Plant Prot. 2014, 67, 96–102. [Google Scholar] [CrossRef]
- Yang, J.; Park, Y.; Hyun, I.-H.; Kim, G.-H.; Kim, B.-S.; Lee, B.-H.; Ren, Y. A combination treatment using ethyl formate and phosphine to control Planococcus citri (Hemiptera: Pseudococcidae) on pineapples. J. Econ. Entomol. 2016, 109, 2355–2363. [Google Scholar] [CrossRef]
- Ren, Y.L.; Lee, B.H.; Padovan, B. Penetration of methyl bromide, sulfuryl fluoride, ethanedinitrile and phosphine into timber blocks and the sorption rate of the fumigants. J. Stored Prod. Res. 2011, 47, 63–68. [Google Scholar] [CrossRef]
- Cho, M.-K.; Kwon, H.R.; Yu, Y.M.; Youn, Y.N. Development by Temperatures and Copulation Flight of Comstock mealybug, Pseudococcus comstocki. Korean J. Appl. Entomol. 2018, 57, 105–115. [Google Scholar]
- Lichter, A.; Zutahy, Y.; Kaplunov, T.; Lurie, S. Evaluation of table grape storage in boxes with sulfur dioxide-releasing pads with either an internal plastic liner or external wrap. HortTechnology 2008, 18, 206–214. [Google Scholar] [CrossRef] [Green Version]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Kim, K.; Park, M.-G.; Lee, Y.H.; Jeon, H.-J.; Kwon, T.H.; Kim, C.; Park, J.; Lee, B.-H.; Yang, J.O.; Lee, S.-E. Synergistic Effects and Toxic Mechanism of Phosphine with Ethyl Formate against Citrus Mealybug (Planococcus citri). Appl. Sci. 2021, 11, 9877. [Google Scholar] [CrossRef]
- Bikoba, V.; Pupin, F.; Biasi, W.; Rutaganira, F.; Mitcham, E. Use of Ethyl Formate Fumigation to Control Adult Bean Thrips in Navel Oranges. J. Econ. Entomol. 2019, 112, 591–596. [Google Scholar] [CrossRef]
- Lee, B.H.; Kim, H.M.; Kim, B.S.; Yang, J.O.; Moon, Y.M.; Ren, Y. Evaluation of the synergistic effect between ethyl formate and phospine for control of Aphis gossypii (Homoptera: Aphididae). J. Econ. Entomol. 2016, 109, 143–147. [Google Scholar] [CrossRef]
- Kim, K.; Lee, Y.H.; Kim, G.; Lee, B.-H.; Yang, J.-O.; Lee, S.-E. Ethyl formate and phosphine fumigations on the two-spotted spider mite, Tetranychus urticae and their biochemical responses. Appl. Biol. Chem. 2019, 62, 50. [Google Scholar] [CrossRef]
- Kwon, T.H.; Kim, D.B.; Kim, K.W.; Park, M.G.; Roh, G.H.; Lee, B.H. Scaled-up ethyl formate fumigation to replace methyl bromide on traded mushroom to disinfest mushroom fly (Lycoriella mali). Appl. Biol. Chem. 2021, 64, 64. [Google Scholar] [CrossRef]
- IPPC. Phytosanitary Treatments for Regulated Pests; ISPM 28; FAO/IPPC: Rome, Italy, 2007; pp. 1–11. [Google Scholar]
- Bond, E.J. Manual of Fumigation for Insect Control, 2nd ed.; FAO: Rome, Italy, 1984; pp. 134–135. [Google Scholar]
- Park, M.G.; Ren, Y.; Lee, B.H. Preliminary study to evaluate ethanedinitrile (C2 N2) for quarantine treatment of four wood destroying pests. Pest Manag. Sci. 2021, 77, 5213–5219. [Google Scholar] [CrossRef]
- Kyung, Y.; Kim, H.K.; Cho, S.W.; Kim, B.-S.; Yang, J.-O.; Koo, H.-N.; Kim, G.-H. Comparison of the efficacy and phytotoxicity of phosphine and ethyl formate for controlling Pseudococcus longispinus (Hemiptera: Pseudococcidae) and Pseudococcus orchidicola on imported foliage nursery plants. J. Econ. Entomol. 2019, 112, 2149–2156. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, H.K.; Kyung, Y.; Park, G.-H.; Lee, B.-H.; Yang, J.-O.; Koo, H.-N.; Kim, G.-H. Fumigation activity of ethyl formate and phosphine against Tetranychus urticae (Acari: Tetranychidae) on imported sweet pumpkin. J. Econ. Entomol. 2018, 111, 1625–1632. [Google Scholar] [CrossRef]
- Lee, B.-H.; Park, C.-G.; Yang, J.-O.; Kim, K.; Lee, S.-E. Concurrent application of ethyl formate and 1-methylcyclopropene to control Tetranychus urticae on exported sweet persimmons (Diospyros kaki Thunb. ‘Fuyu’). Entomol. Res. 2018, 48, 198–203. [Google Scholar] [CrossRef]

| Insect | Stage | Temp (°C) | LCt50% (95% CL, g h m−3) | LCt99% (95% CL, g h m−3) | Slope ± SE | df | X2 |
|---|---|---|---|---|---|---|---|
| Pseudococcus comstocki | Adult | 5 | 29.41 (28.19–30.60) | 47.36 (43.88–52.70) | 11.24 ± 1.05 | 6 | 24.46 |
| Egg | 52.00 (48.72–55.33) | 145.85 (127.96–172.73) | 5.19 ± 0.37 | 10 | 22.43 |
| Loading Ratio (w/v %) | 70 g/m3 (g h m−3) | 80 g/m3 (g h m−3) | 90 g/m3 (g h m−3) |
|---|---|---|---|
| 10 | 148.33 ± 1.8 a 1 | 167.91 ± 1.3 a | 188.63 ± 1.0 a |
| 15 | 125.65 ± 1.2 b | 149.48 ± 2.1 b | 161.66 ± 1.4 b |
| 20 | 115.69 ± 1.1 c | 133.08 ± 1.2 c | 149.86 ± 1.2 c |
| CT Product (g h/m3) | Loading Ratio of Grapes (w/v) | No. of Eggs Used | No. of Emerging Nymphs | Corrected Mortality * (%) |
|---|---|---|---|---|
| 114.8 ± 12.36 | 20 | 1870 | 60 | 96.12 ± 0.28 |
| 148.2 ± 12.36 | 10 | 1800 | 0 | 100.00 ± 0.00 |
| Untreated | - | 1863 | 1822 | 2.50 ± 0.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.-H.; Hong, K.-J.; Park, M.-G. The Efficacy, Phytotoxicity, and Safety of Liquid Ethyl Formate Used to Control the Grape (Campbell Early) Quarantine Pest Pseudococcus comstocki. Appl. Sci. 2022, 12, 9769. https://doi.org/10.3390/app12199769
Lee B-H, Hong K-J, Park M-G. The Efficacy, Phytotoxicity, and Safety of Liquid Ethyl Formate Used to Control the Grape (Campbell Early) Quarantine Pest Pseudococcus comstocki. Applied Sciences. 2022; 12(19):9769. https://doi.org/10.3390/app12199769
Chicago/Turabian StyleLee, Byung-Ho, Ki-Jeong Hong, and Min-Goo Park. 2022. "The Efficacy, Phytotoxicity, and Safety of Liquid Ethyl Formate Used to Control the Grape (Campbell Early) Quarantine Pest Pseudococcus comstocki" Applied Sciences 12, no. 19: 9769. https://doi.org/10.3390/app12199769





