Double-Facet Effect of Artificial Mechanical Stress on Red Blood Cell Deformability: Implications for Blood Salvage
Abstract
:1. Introduction
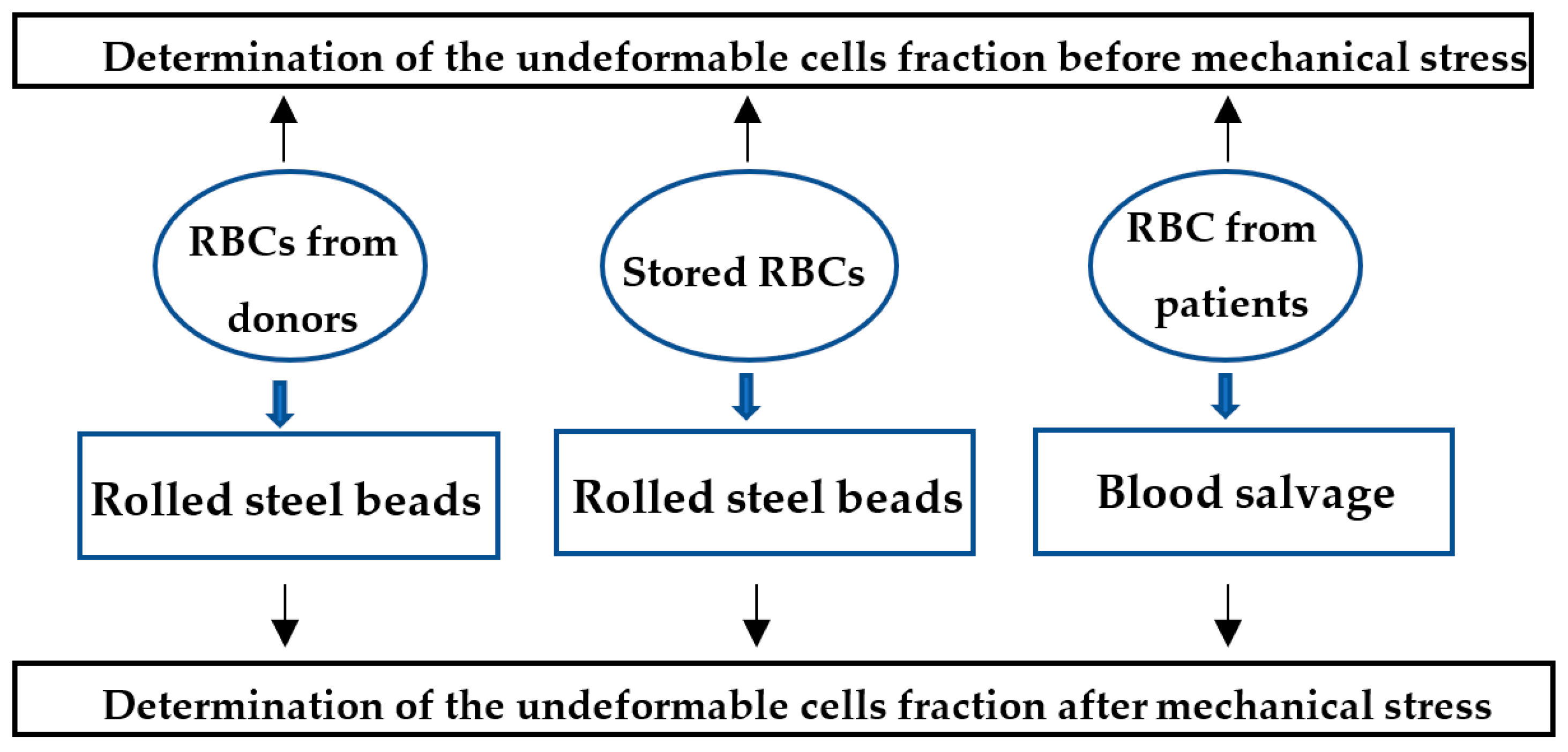
2. Materials and Methods
- RBCs suspended in PBS buffer were rolled with steel beads [61].
- Blood salvage procedure, in which the whole blood of a patient undergoing orthopedic surgery was collected and processed for re-administration to the patient, using the OrthoPAT (Haemodynamics, Boston, MA, USA). RBCs were collected before and after the mechanical stress exposure, and cell deformability was assessed as described below.
2.1. Materials
2.2. Freshly Donated Blood
2.3. RBC from Packed RBC Units (PRBC)
2.4. Preparation of RBC from Freshly Collected Blood
2.5. Application of Mechanical Stress
2.6. Surgery Patients
2.7. RBC from Salvage Procedure
2.8. Preparation of RBC from the Operating Room
2.9. RBC Deformability
2.10. Statistical Analysis
3. Results
3.1. Effect of Moderate Mechanical Stress on RBC Deformability
3.2. Effect of Blood Salvage on RBC Deformability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, J.; Kwon, J.H.; Lee, S.H.; Lee, J.H.; Min, J.J.; Kim, J.; Oh, A.R.; Seo, W.; Hyeon, C.W.; Yang, K.; et al. Intraoperative blood loss may be associated with myocardial injury after non-cardiac surgery. PLoS ONE 2021, 16, e0241114. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Palmer, A.J.R.; Klein, A.A. Strategies to minimize intraoperative blood loss during major surgery. Br. J. Surg. 2020, 107, e26–e38. [Google Scholar] [CrossRef] [Green Version]
- Fulop, A.; Turoczi, Z.; Garbaisz, D.; Harsanyi, L.; Szijarto, A. Experimental models of hemorrhagic shock: A review. Eur. Surg. Res. 2013, 50, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacagnella, R.C.; Souza, J.P.; Durocher, J.; Perel, P.; Blum, J.; Winikoff, B.; Gulmezoglu, A.M. A systematic review of the relationship between blood loss and clinical signs. PLoS ONE 2013, 8, e57594. [Google Scholar] [CrossRef]
- Charalambides, M.; Mavrou, A.; Jennings, T.; Powar, M.P.; Wheeler, J.; Davies, R.J.; Fearnhead, N.S.; Simillis, C. A systematic review of the literature assessing operative blood loss and postoperative outcomes after colorectal surgery. Int. J. Colorectal Dis. 2022, 37, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Guo, J.; Hou, Z. Risk Factors for Perioperative Hidden Blood Loss After Intertrochanteric Fracture Surgery in Chinese Patients: A Meta-Analysis. Geriatr. Orthop. Surg. Rehabil. 2022, 13, 21514593221083816. [Google Scholar] [CrossRef]
- Carson, J.L.; Stanworth, S.J.; Dennis, J.A.; Trivella, M.; Roubinian, N.; Fergusson, D.A.; Triulzi, D.; Doree, C.; Hebert, P.C. Transfusion thresholds for guiding red blood cell transfusion. Cochrane Database Syst Rev. 2021, 12, CD002042. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Ghidini, A.; Sgroi, G.; Vavassori, I.; Petro, D.; Cabiddu, M.; Aiolfi, A.; Bonitta, G.; Zaniboni, A.; et al. Red blood cell transfusions and the survival in patients with cancer undergoing curative surgery: A systematic review and meta-analysis. Surg. Today 2021, 51, 1535–1557. [Google Scholar] [CrossRef]
- Frank, S.M.; Sikorski, R.A.; Konig, G.; Tsilimigras, D.I.; Hartmann, J.; Popovsky, M.A.; Pawlik, T.M.; Waters, J.H. Clinical Utility of Autologous Salvaged Blood: A Review. J. Gastrointest. Surg. 2020, 24, 464–472. [Google Scholar] [CrossRef]
- Kameneva, M.V.; Antaki, J.F.; Borovetz, H.S.; Griffith, B.P.; Butler, K.C.; Yeleswarapu, K.K.; Watach, M.J.; Kormos, R.L. Mechanisms of red blood cell trauma in assisted circulation. Rheologic similarities of red blood cell transformations due to natural aging and mechanical stress. ASAIO J. 1995, 41, M457–M460. [Google Scholar] [CrossRef]
- Kameneva, M.V.; Undar, A.; Antaki, J.F.; Watach, M.J.; Calhoon, J.H.; Borovetz, H.S. Decrease in red blood cell deformability caused by hypothermia, hemodilution, and mechanical stress: Factors related to cardiopulmonary bypass. ASAIO J. 1999, 45, 307–310. [Google Scholar] [CrossRef] [PubMed]
- McNamee, A.P.; Simmonds, M.J.; Inoue, M.; Horobin, J.T.; Hakozaki, M.; Fraser, J.F.; Watanabe, N. Erythrocyte morphological symmetry analysis to detect sublethal trauma in shear flow. Sci. Rep. 2021, 11, 23566. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Apel, J.; Klaus, S.; Schugner, F.; Schwindke, P.; Reul, H. Shear stress related blood damage in laminar couette flow. Artif. Organs 2003, 27, 517–529. [Google Scholar] [CrossRef]
- Watanabe, N.; Shimada, T.; Hakozaki, M.; Hara, R. Visualization of erythrocyte deformation induced by supraphysiological shear stress. Int. J. Artif. Organs 2018, 41, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Faghih, M.M.; Sharp, M.K. Modeling and prediction of flow-induced hemolysis: A review. Biomech. Model. Mechanobiol. 2019, 18, 845–881. [Google Scholar] [CrossRef]
- Horobin, J.T.; Sabapathy, S.; Simmonds, M.J. Repetitive Supra-Physiological Shear Stress Impairs Red Blood Cell Deformability and Induces Hemolysis. Artif. Organs 2017, 41, 1017–1025. [Google Scholar] [CrossRef]
- Ricci, G.; Martinelli, L.; Vigano, M.; Strozzi, C. Reduced membrane sialic acid contents of the erythrocytes after heart valve replacement with prosthetic devices. Biomed. Pharmacother. 1986, 40, 25–27. [Google Scholar]
- Gu, Y.J.; Vermeijden, W.J.; de Vries, A.J.; Hagenaars, J.A.; Graaff, R.; van Oeveren, W. Influence of mechanical cell salvage on red blood cell aggregation, deformability, and 2,3-diphosphoglycerate in patients undergoing cardiac surgery with cardiopulmonary bypass. Ann. Thorac. Surg. 2008, 86, 1570–1575. [Google Scholar] [CrossRef]
- Pstras, L.; Debowska, M.; Wojcik-Zaluska, A.; Zaluska, W.; Waniewski, J. Hemodialysis-induced changes in hematocrit, hemoglobin and total protein: Implications for relative blood volume monitoring. PLoS ONE 2019, 14, e0220764. [Google Scholar] [CrossRef] [Green Version]
- Reinhart, W.H.; Cagienard, F.; Schulzki, T.; Venzin, R.M. The passage of a hemodialysis filter affects hemorheology, red cell shape, and platelet aggregation. Clin. Hemorheol. Microcirc. 2014, 57, 49–62. [Google Scholar] [CrossRef]
- Krisher, J.A.; Malinauskas, R.A.; Day, S.W. The effect of blood viscosity on shear-induced hemolysis using a magnetically levitated shearing device. Artif. Organs 2022, 46, 1027–1039. [Google Scholar] [CrossRef] [PubMed]
- McNamee, A.P.; Tansley, G.D.; Sabapathy, S.; Simmonds, M.J. Biphasic impairment of erythrocyte deformability in response to repeated, short duration exposures of supraphysiological, subhaemolytic shear stress. Biorheology 2016, 53, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.D.; da Cunha Vieira Perini, F.; de Sousa, L.C.B.; Buffolo, E.; Chaccur, P.; Arrais, M.; Jatene, F.B. Autologous blood salvage in cardiac surgery: Clinical evaluation, efficacy and levels of residual heparin. Hematol. Transfus. Cell Ther. 2021, 43, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.H. Cell salvage in trauma. Curr. Opin. Anaesthesiol. 2021, 34, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Gueye, P.M.; Bertrand, F.; Duportail, G.; Lessinger, J.M. Extracellular haemoglobin, oxidative stress and quality of red blood cells relative to perioperative blood salvage. Clin. Chem. Lab. Med. 2010, 48, 677–683. [Google Scholar] [CrossRef]
- Jin, D.; Shen, L.; Huang, Y. Intraoperative Cell-Saver Caused More Autologous Salvage Hemolysis in a Hereditary Spherocytosis Patient Than in a Normal Erythrocyte Patient. Front. Physiol. 2022, 13, 926398. [Google Scholar] [CrossRef]
- Liang, H.; Zhao, Y.; Wang, D.; Wang, B. Evaluation of the quality of processed blood salvaged during craniotomy. Surg. Neurol. 2009, 71, 74–80. [Google Scholar] [CrossRef]
- Klein, A.A.; Nashef, S.A.; Sharples, L.; Bottrill, F.; Dyer, M.; Armstrong, J.; Vuylsteke, A. A randomized controlled trial of cell salvage in routine cardiac surgery. Anesth. Analg. 2008, 107, 1487–1495. [Google Scholar] [CrossRef]
- Liao, X.; Du, K.; Zhang, J.; Meng, W.; Zuo, S.; Huang, Q.; Wang, H.; Gou, D. Red blood cells are damaged by intraoperative blood salvage via Ca(2+)-dependent and -independent mechanisms. Life Sci. 2019, 227, 114–121. [Google Scholar] [CrossRef]
- Liao, X.Y.; Zuo, S.S.; Meng, W.T.; Zhang, J.; Huang, Q.; Gou, D.M. Intraoperative blood salvage may shorten the lifespan of red blood cells within 3 days postoperatively: A pilot study. Medicine 2017, 96, e8143. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Red blood cell mechanical stability test. Clin. Hemorheol. Microcirc. 2013, 55, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sakota, D.; Sakamoto, R.; Sobajima, H.; Yokoyama, N.; Waguri, S.; Ohuchi, K.; Takatani, S. Mechanical Damage of Red Blood Cells by Rotary Blood Pumps: Selective Destruction of Aged Red Blood Cells and Subhemolytic Trauma. Artif. Organs 2008, 32, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Kameneva, M.V.; Repko, B.M.; Krasik, E.F.; Perricelli, B.C.; Borovetz, H.S. Polyethylene glycol additives reduce hemolysis in red blood cell suspensions exposed to mechanical stress. ASAIO J. 2003, 49, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Olia, S.E.; Maul, T.M.; Antaki, J.F.; Kameneva, M.V. Mechanical blood trauma in assisted circulation: Sublethal RBC damage preceding hemolysis. Int. J. Artif. Organs 2016, 39, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Engel, S.; Janiga, G.; Thevenin, D. A Review of Hemolysis Prediction Models for Computational Fluid Dynamics. Artif. Organs 2017, 41, 603–621. [Google Scholar] [CrossRef] [Green Version]
- Simmonds, M.J.; Meiselman, H.J. Prediction of the level and duration of shear stress exposure that induces subhemolytic damage to erythrocytes. Biorheology 2016, 53, 237–249. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Suriany, S.; Ponce, D.; Detterich, J.A. Red blood cell mechanical sensitivity improves in patients with sickle cell disease undergoing chronic transfusion after prolonged, subhemolytic shear exposure. Transfusion 2018, 58, 2788–2796. [Google Scholar] [CrossRef]
- Horobin, J.T.; Sabapathy, S.; Simmonds, M.J. Red blood cell tolerance to shear stress above and below the subhemolytic threshold. Biomech. Model. Mechanobiol. 2020, 19, 851–860. [Google Scholar] [CrossRef]
- McNamee, A.P.; Tansley, G.D.; Simmonds, M.J. Sublethal mechanical shear stress increases the elastic shear modulus of red blood cells but does not change capillary transit velocity. Microcirculation 2020, 27, e12652. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Atac, N.; Baskurt, O.K.; Meiselman, H.J.; Yalcin, O. Erythrocyte deformability responses to intermittent and continuous subhemolytic shear stress. Biorheology 2014, 51, 171–185. [Google Scholar] [CrossRef]
- Freitas Leal, J.; Vermeer, H.; Lazari, D.; van Garsse, L.; Brock, R.; Adjobo-Hermans, M.; Bosman, G. The impact of circulation in a heart-lung machine on function and survival characteristics of red blood cells. Artif. Organs 2020, 44, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Buerck, J.P.; Burke, D.K.; Schmidtke, D.W.; Snyder, T.A.; Papavassiliou, D.V.; O’Rear, E.A. Production of erythrocyte microparticles in a sub-hemolytic environment. J. Artif. Organs 2021, 24, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Kuck, L.; Grau, M.; Simmonds, M.J. Recovery time course of erythrocyte deformability following exposure to shear is dependent upon conditioning shear stress. Biorheology 2018, 54, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Vercaemst, L. Hemolysis in cardiac surgery patients undergoing cardiopulmonary bypass: A review in search of a treatment algorithm. J. Extra -Corpor. Technol. 2008, 40, 257–267. [Google Scholar]
- Zhu, Q.; Salehyar, S.; Cabrales, P.; Asaro, R.J. Prospects for Human Erythrocyte Skeleton-Bilayer Dissociation during Splenic Flow. Biophys J. 2017, 113, 900–912. [Google Scholar] [CrossRef] [Green Version]
- McVey, M.J.; Kuebler, W.M.; Orbach, A.; Arbell, D.; Zelig, O.; Barshtein, G.; Yedgar, S. Reduced deformability of stored red blood cells is associated with generation of extracellular vesicles. Transfus. Apher. Sci. 2020, 59, 102851. [Google Scholar] [CrossRef]
- Watanabe, N.; Arakawa, Y.; Sou, A.; Kataoka, H.; Ohuchi, K.; Fujimoto, T.; Takatani, S. Deformability of human red blood cells exposed to a uniform shear stress as measured by a cyclically reversing shear flow generator. Physiol. Meas. 2007, 28, 531–545. [Google Scholar] [CrossRef]
- Matot, I.; Katz, M.; Pappo, O.; Zelig, O.; Corchia, N.; Yedgar, S.; Barshtein, G.; Guerrero, E.B.; Abramovitch, R. Resuscitation With Aged Blood Exacerbates Liver Injury in a Hemorrhagic Rat Model*. Crit. Care Med. 2013, 41, 842–849. [Google Scholar] [CrossRef]
- Parthasarathi, K.; Lipowsky, H.H. Capillary recruitment in response to tissue hypoxia and its dependence on red blood cell deformability. Am. J. Physiol. 1999, 277, H2145–H2157. [Google Scholar] [CrossRef]
- Sakr, Y.; Chierego, M.; Piagnerelli, M.; Verdant, C.; Dubois, M.J.; Koch, M.; Creteur, J.; Gullo, A.; Vincent, J.L.; De Backer, D. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit. Care Med. 2007, 35, 1639–1644. [Google Scholar] [CrossRef]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McHedlishvili, G. Disturbed blood flow structuring as critical factor of hemorheological disorders in microcirculation. Clin. Hemorheol. Microcirc. 1998, 19, 315–325. [Google Scholar] [PubMed]
- Cabrales, P.; Tsai, A.G.; Intaglietta, M. Isovolemic exchange transfusion with increasing concentrations of low oxygen affinity hemoglobin solution limits oxygen delivery due to vasoconstriction. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2212–H2218. [Google Scholar] [CrossRef] [Green Version]
- Grover, G.J.; Loegering, D.J. Effect of splenic sequestration of erythrocytes on splenic clearance function and susceptibility to septic peritonitis. Infect. Immun. 1982, 36, 96–102. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Lu, L.; Li, X.; Buffet, P.A.; Dao, M.; Karniadakis, G.E.; Suresh, S. Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders. Proc. Natl. Acad. Sci. USA 2018, 115, 9574–9579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pivkin, I.V.; Peng, Z.; Karniadakis, G.E.; Buffet, P.A.; Dao, M.; Suresh, S. Biomechanics of red blood cells in human spleen and consequences for physiology and disease. Proc. Natl. Acad. Sci. USA 2016, 113, 7804–7809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barshtein, G.; Pries, A.R.; Goldschmidt, N.; Zukerman, A.; Orbach, A.; Zelig, O.; Arbell, D.; Yedgar, S. Deformability of transfused red blood cells is a potent determinant of transfusion-induced change in recipient’s blood flow. Microcirculation 2016, 23, 479–486. [Google Scholar] [CrossRef]
- Barshtein, G.; Gural, A.; Zelig, O.; Arbell, D.; Yedgar, S. Preparation of packed red blood cell units in the blood bank: Alteration in red blood cell deformability. Transfus. Apher. Sci. 2020, 59, 102738. [Google Scholar] [CrossRef]
- Barshtein, G.; Bergelson, L.; Dagan, A.; Gratton, E.; Yedgar, S. Membrane lipid order of human red blood cells is altered by physiological levels of hydrostatic pressure. Am. J. Physiol. 1997, 272, H538–H543. [Google Scholar] [CrossRef] [Green Version]
- Stukelj, R.; Schara, K.; Bedina-Zavec, A.; Sustar, V.; Pajnic, M.; Paden, L.; Krek, J.L.; Kralj-Iglic, V.; Mrvar-Brecko, A.; Jansa, R. Effect of shear stress in the flow through the sampling needle on concentration of nanovesicles isolated from blood. Eur. J. Pharm. Sci. 2017, 98, 17–29. [Google Scholar] [CrossRef]
- Orbach, A.; Zelig, O.; Yedgar, S.; Barshtein, G. Biophysical and Biochemical Markers of Red Blood Cell Fragility. Transfus. Med. Hemother. 2017, 44, 183–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barshtein, G.; Gural, A.; Manny, N.; Zelig, O.; Yedgar, S.; Arbell, D. Storage-induced damage to red blood cell mechanical properties can be only partially reversed by rejuvenation. Transfus. Med. Hemother. Off. Organ Dtsch. Ges. Transfus. Immunhamatol. 2014, 41, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.; Raval, J.S.; Waters, J.H.; Yazer, M.H.; Kameneva, M.V. Effect of blood bank storage on the rheological properties of male and female donor red blood cells. Clin. Hemorheol. Microcirc. 2014, 56, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Kameneva, M.V.; Antaki, J.F.; Konishi, H.; Whalen, J.J.; Kerrigan, J.P.; Watach, M.J.; Kormos, R.L.; Griffith, B.P.; Borovetz, H.S. Effect of perfluorochemical emulsion on blood trauma and hemorheology. ASAIO J. 1994, 40, M576-9. [Google Scholar] [CrossRef] [PubMed]
- Raval, J.S.; Waters, J.H.; Seltsam, A.; Scharberg, E.A.; Richter, E.; Daly, A.R.; Kameneva, M.V.; Yazer, M.H. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010, 99, 325–331. [Google Scholar] [CrossRef]
- Raval, J.S.; Waters, J.H.; Seltsam, A.; Scharberg, E.A.; Richter, E.; Kameneva, M.V.; Yazer, M.H. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011, 100, 418–421. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Karadimas, D.G.; Velentzas, A.D.; Anastasopoulou, V.I.; Papageorgiou, E.G.; Stamoulis, K.; Papassideri, I.S.; Kriebardis, A.G.; Antonelou, M.H. Early and Late-Phase 24 h Responses of Stored Red Blood Cells to Recipient-Mimicking Conditions. Front. Physiol. 2022, 13, 907497. [Google Scholar] [CrossRef]
- Yazer, M.H.; Waters, J.H.; Elkin, K.R.; Rohrbaugh, M.E.; Kameneva, M.V. A comparison of hemolysis and red cell mechanical fragility in blood collected with different cell salvage suction devices. Transfusion 2008, 48, 1188–1191. [Google Scholar] [CrossRef]
- Ziegler, L.A.; Olia, S.E.; Kameneva, M.V. Red Blood Cell Mechanical Fragility Test for Clinical Research Applications. Artif. Organs 2017, 41, 678–682. [Google Scholar] [CrossRef]
- Barshtein, G.; Pajic-Lijakovic, I.; Gural, A. Deformability of Stored Red Blood Cells. Front. Physiol. 2021, 12, 722896. [Google Scholar] [CrossRef]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 126, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Ahn, K.H.; Lee, S.J.; Sun, K.; Goedhart, P.T.; Hardeman, M.R. Shear induced damage of red blood cells monitored by the decrease of their deformability. Korea-Aust. Rheol. J. 2004, 16, 141–146. [Google Scholar]
- Meram, E.; Yilmaz, B.D.; Bas, C.; Atac, N.; Yalcin, O.; Meiselman, H.J.; Baskurt, O.K. Shear stress-induced improvement of red blood cell deformability. Biorheology 2013, 50, 165–176. [Google Scholar] [CrossRef]
- Meybohm, P.; Choorapoikayil, S.; Wessels, A.; Herrmann, E.; Zacharowski, K.; Spahn, D.R. Washed cell salvage in surgical patients: A review and meta-analysis of prospective randomized trials under PRISMA. Medicine 2016, 95, e4490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salaria, O.N.; Barodka, V.M.; Hogue, C.W.; Berkowitz, D.E.; Ness, P.M.; Wasey, J.O.; Frank, S.M. Impaired red blood cell deformability after transfusion of stored allogeneic blood but not autologous salvaged blood in cardiac surgery patients. Anesth. Analg. 2014, 118, 1179–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vonk, A.B.; Muntajit, W.; Bhagirath, P.; van Barneveld, L.J.; Romijn, J.W.; de Vroege, R.; Boer, C. Residual blood processing by centrifugation, cell salvage or ultrafiltration in cardiac surgery: Effects on clinical hemostatic and ex-vivo rheological parameters. Blood Coagul. Fibrinolysis 2012, 23, 622–628. [Google Scholar] [CrossRef]
- Yokoyama, N.; Sakota, D.; Nagaoka, E.; Takatani, S. Alterations in red blood cell volume and hemoglobin concentration, viscoelastic properties, and mechanical fragility caused by continuous flow pumping in calves. Artif. Organs 2011, 35, 791–799. [Google Scholar] [CrossRef]
- Kozlova, E.; Sergunova, V.; Sherstyukova, E.; Gudkova, O.; Kozlov, A.; Inozemtsev, V.; Lyapunova, S.; Chernysh, A. Topological Relationships Cytoskeleton-Membrane Nanosurface-Morphology as a Basic Mechanism of Total Disorders of RBC Structures. Int. J. Mol. Sci. 2022, 23, 2045. [Google Scholar] [CrossRef]
- Kozlova, E.; Chernysh, A.; Manchenko, E.; Sergunova, V.; Moroz, V. Nonlinear Biomechanical Characteristics of Deep Deformation of Native RBC Membranes in Normal State and under Modifier Action. Scanning 2018, 2018, 1810585. [Google Scholar] [CrossRef]






| Parameters * | After Salvage | Before Salvage | p Values | ||
|---|---|---|---|---|---|
| Average | ±SD | Average | ±SD | ||
| UDFC, % | 3.23 | 1.75 | 2.31 | 1.60 | > 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsohar, T.; Beyth, S.; Gural, A.; Arbell, D.; Yedgar, S.; Barshtein, G. Double-Facet Effect of Artificial Mechanical Stress on Red Blood Cell Deformability: Implications for Blood Salvage. Appl. Sci. 2022, 12, 9951. https://doi.org/10.3390/app12199951
Tsohar T, Beyth S, Gural A, Arbell D, Yedgar S, Barshtein G. Double-Facet Effect of Artificial Mechanical Stress on Red Blood Cell Deformability: Implications for Blood Salvage. Applied Sciences. 2022; 12(19):9951. https://doi.org/10.3390/app12199951
Chicago/Turabian StyleTsohar, Tamir, Shaul Beyth, Alexander Gural, Dan Arbell, Saul Yedgar, and Gregory Barshtein. 2022. "Double-Facet Effect of Artificial Mechanical Stress on Red Blood Cell Deformability: Implications for Blood Salvage" Applied Sciences 12, no. 19: 9951. https://doi.org/10.3390/app12199951
APA StyleTsohar, T., Beyth, S., Gural, A., Arbell, D., Yedgar, S., & Barshtein, G. (2022). Double-Facet Effect of Artificial Mechanical Stress on Red Blood Cell Deformability: Implications for Blood Salvage. Applied Sciences, 12(19), 9951. https://doi.org/10.3390/app12199951







