Removal Behavior of Heavy Metals from Aqueous Solutions via Microbially Induced Carbonate Precipitation Driven by Acclimatized Sporosarcina pasteurii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Culture Media
2.3. Factors Affecting Growth of S. pasteurii
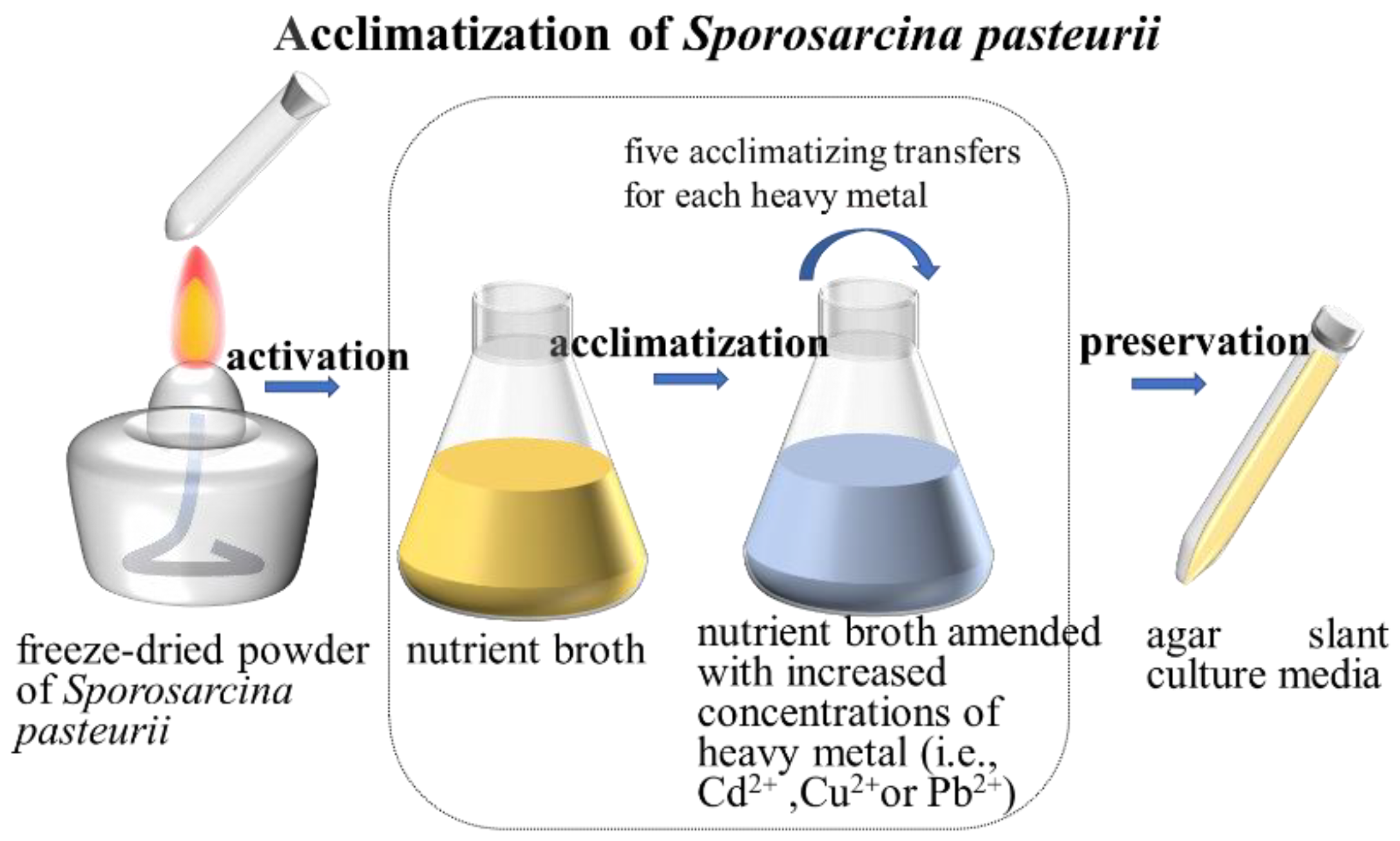
2.4. Acclimatization of S. pasteurii Using Heavy-Metal-Amended Nutrient Broths
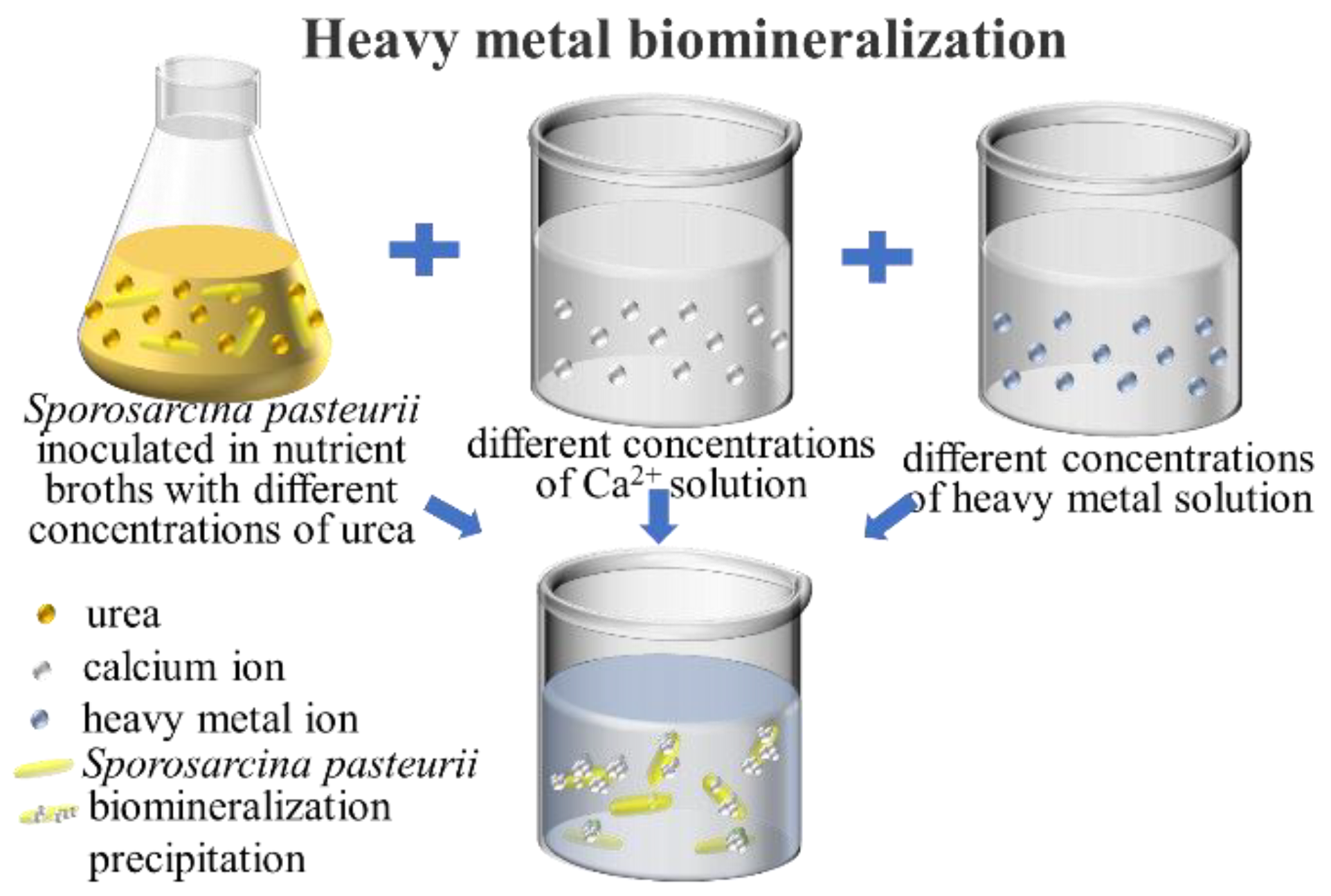
2.5. Removal of Heavy Metals from Aqueous Solutions Using Acclimatized S. pasteurii
3. Results and Discussion
3.1. Growth Characteristic of S. pasteurii
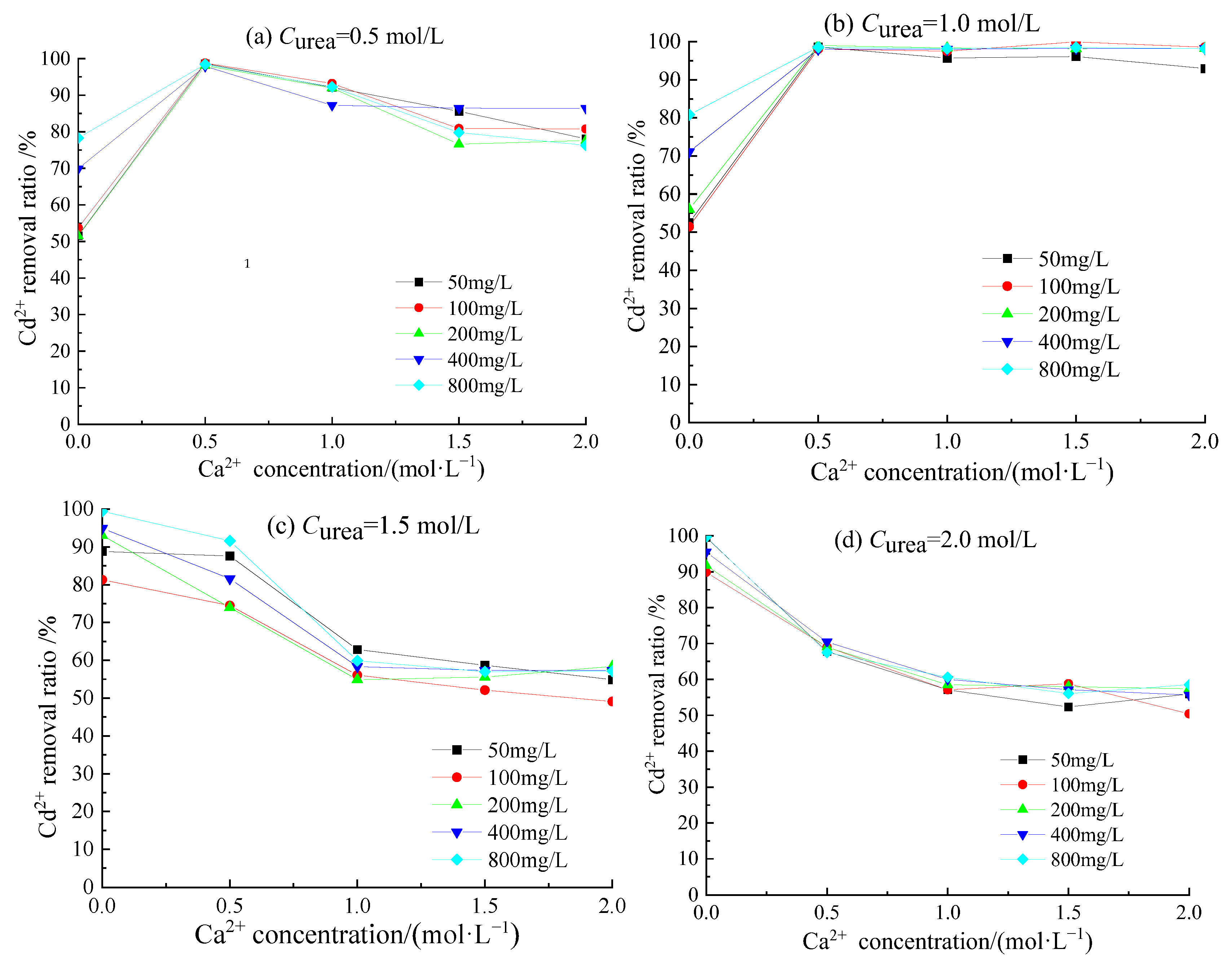
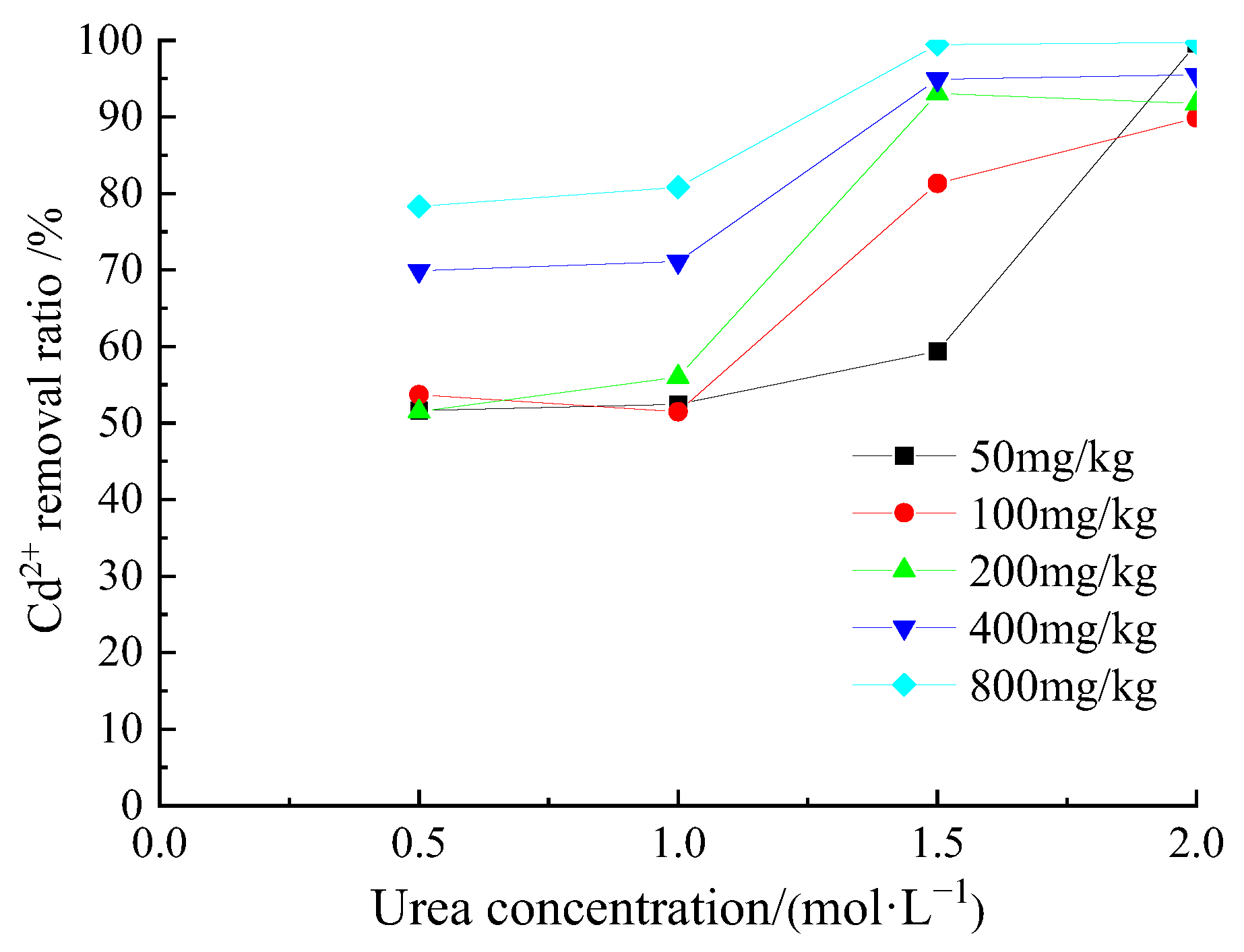
3.2. Removal Behavior of Cd2+ by Acclimatized S. pasteurii
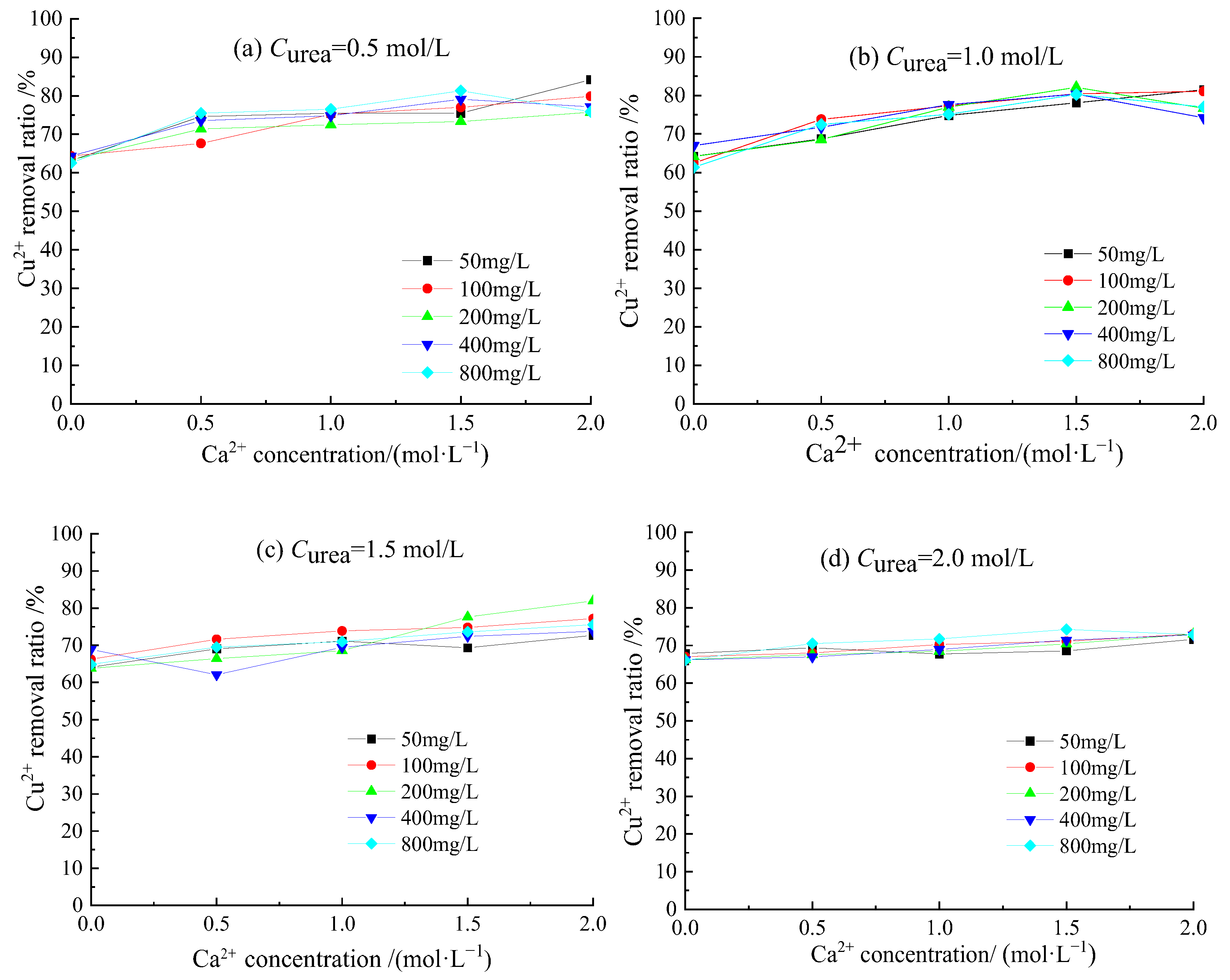
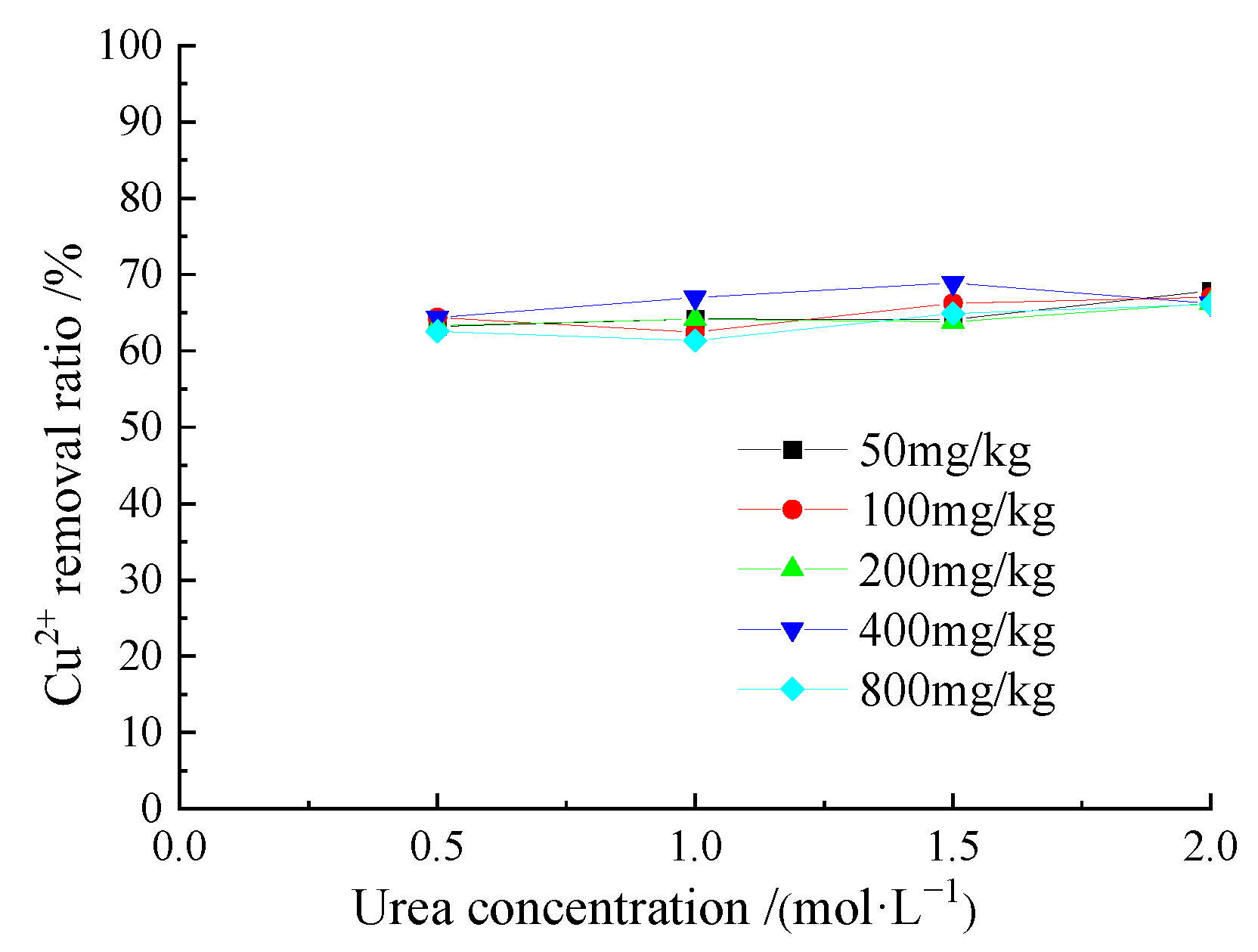
3.3. Removal Behavior of Cu2+ by Acclimatized S. pasteurii
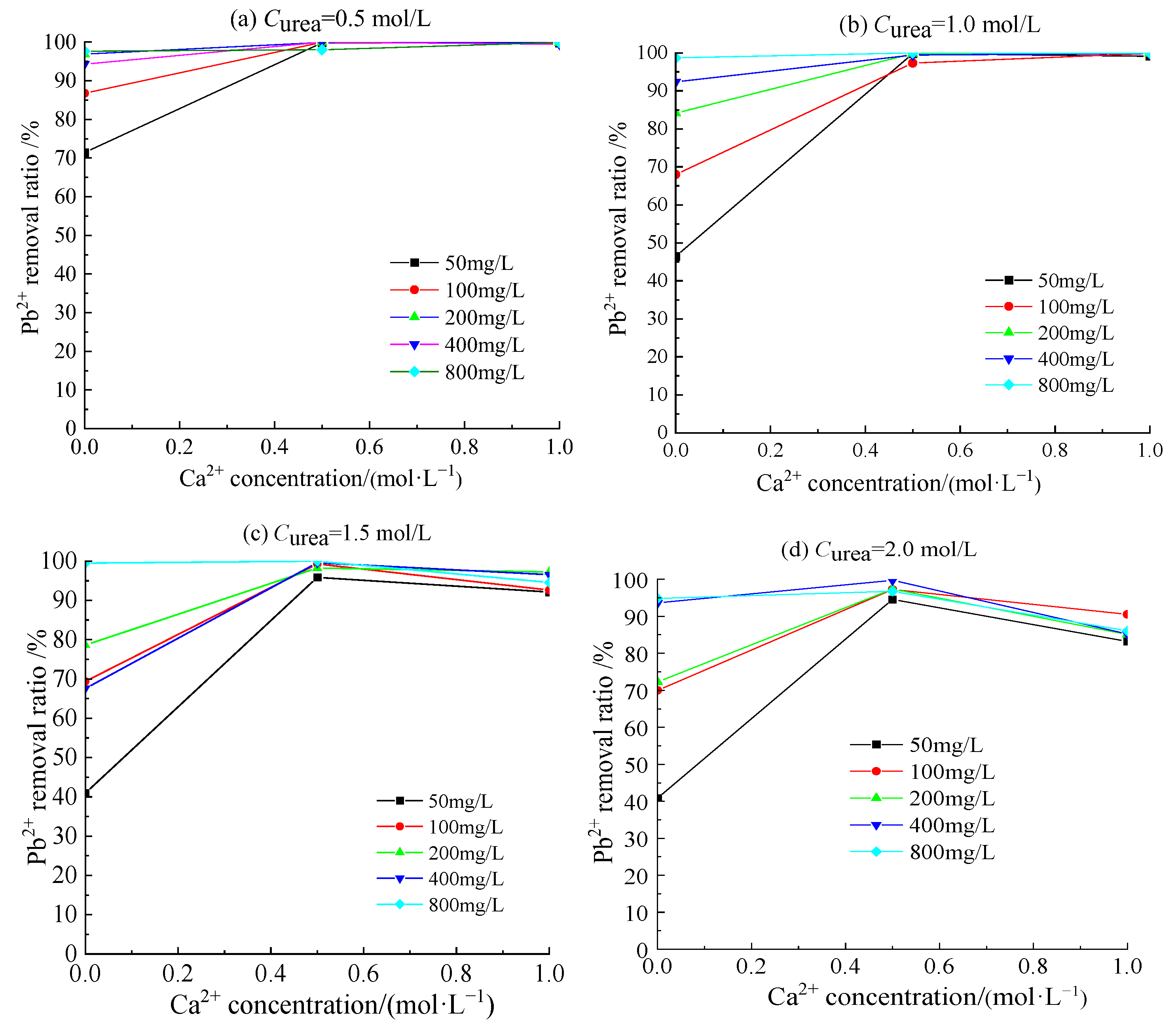
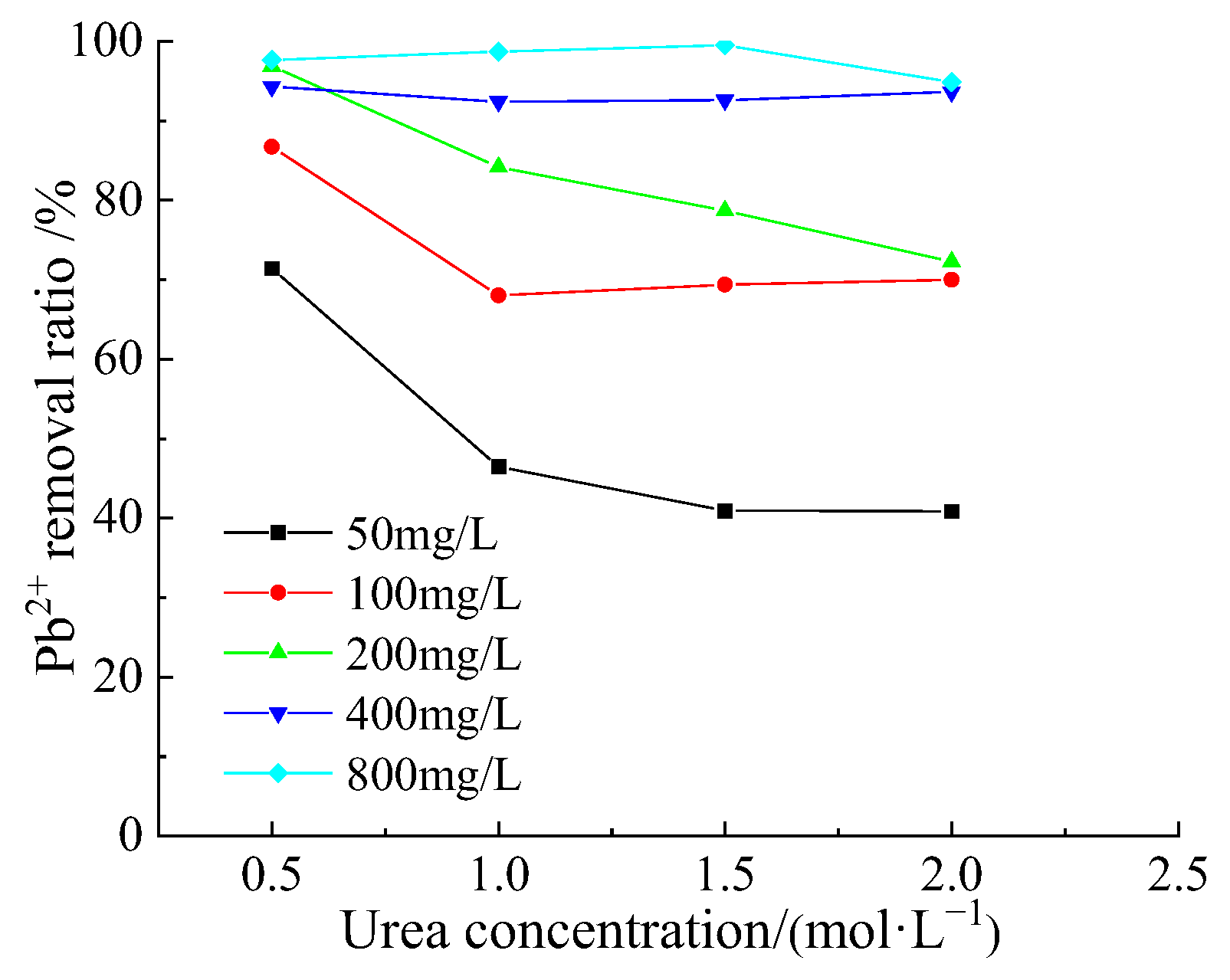
3.4. Removal Behavior of Pb2+ by Acclimatized S. pasteurii
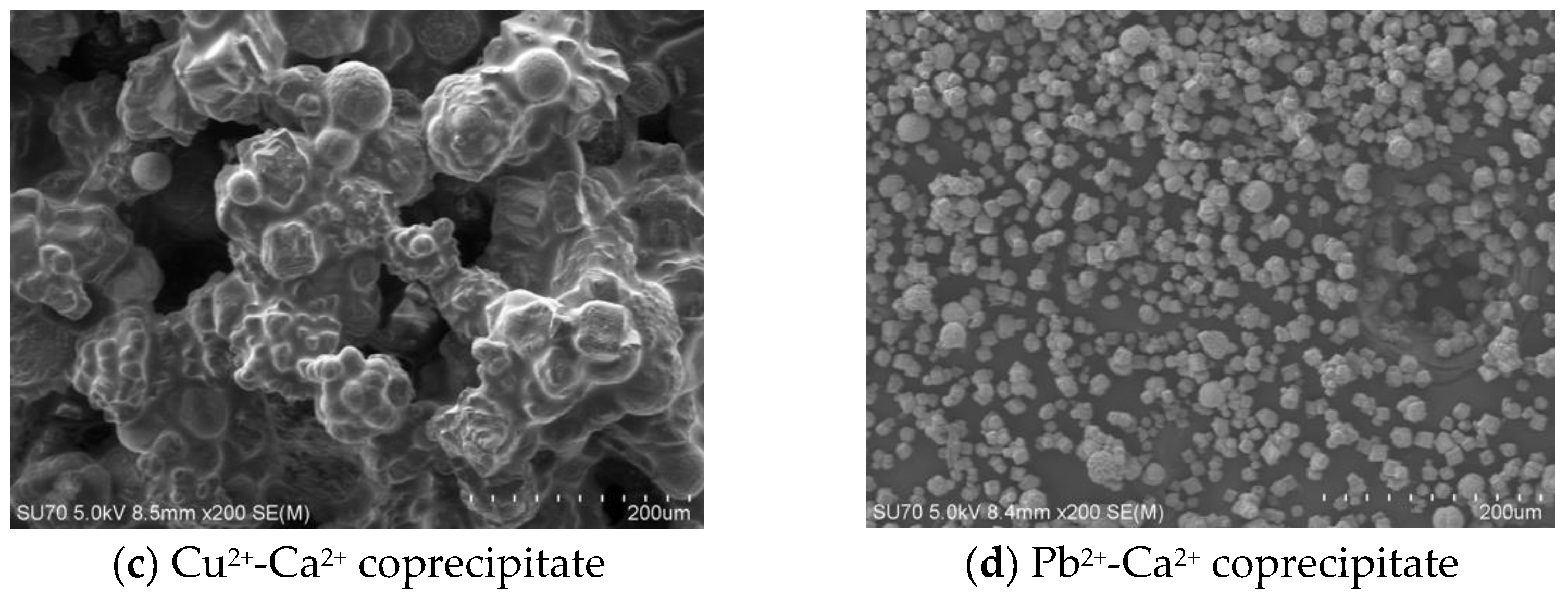
3.5. Biomineral Morphology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Zhang, R.; Cui, M.; Lai, H. Experimental Investigation on Bioremediation of Heavy Metal Contaminated Solution by Sporosarcina pasteurii under Some Complex Conditions. Water 2022, 14, 595. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Nie, J.; Hu, N.; Ding, D.; Han, F.X. Microbially Induced Carbonate Precipitation Techniques for the Remediation of Heavy Metal and Trace Element–Polluted Soils and Water. Water Air Soil Pollut. 2021, 232, 1–15. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, S.S.; Zhang, M.; Tsang, Y.F.; Kim, K.-H. Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 2019, 125, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, J.; Jin, B.; Li, Y.; Shi, Z. Assessment of the potential health risks of heavy metals in soils in a coastal industrial region of the Yangtze River Delta. Environ. Sci. Pollut. Res. 2017, 24, 19816–19826. [Google Scholar] [CrossRef]
- Hu, B.; Shao, S.; Ni, H.; Fu, Z.; Hu, L.; Zhou, Y.; Min, X.; She, S.; Chen, S.; Huang, M.; et al. Current status, spatial features, health risks, and potential driving factors of soil heavy metal pollution in China at province level. Environ. Pollut. 2020, 266, 114961. [Google Scholar] [CrossRef]
- Iqbal, A.; Jalees, M.I.; Farooq, M.U.; Cevik, E.; Bozkurt, A. Superfast adsorption and high-performance tailored membrane filtration by engineered Fe-Ni-Co nanocomposite for simultaneous removal of surface water pollutants. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 652, 129751. [Google Scholar] [CrossRef]
- Iqbal, A.; Cevik, E.; Bozkurt, A.; Asiri, S.M.M.; Alagha, O.; Qahtan, T.F.; Jalees, M.I.; Farooq, M.U. Ultrahigh adsorption by regenerable iron-cobalt core-shell nanospheres and their synergetic effect on nanohybrid membranes for removal of malachite green dye. J. Environ. Chem. Eng. 2022, 10, 107968. [Google Scholar] [CrossRef]
- Wu, Y.; Li, H.; Li, Y. Biomineralization Induced by Cells of Sporosarcina pasteurii: Mechanisms, Applications and Challenges. Microorganisms 2021, 9, 2396. [Google Scholar] [CrossRef]
- Song, M.; Ju, T.; Meng, Y.; Han, S.; Lin, L.; Jiang, J. A review on the applications of microbially induced calcium carbonate precipitation in solid waste treatment and soil remediation. Chemosphere 2021, 290, 133229. [Google Scholar] [CrossRef]
- Arias, D.; Cisternas, L.A.; Rivas, M. Biomineralization Mediated by Ureolytic Bacteria Applied to Water Treatment: A Review. Crystals 2017, 7, 345. [Google Scholar] [CrossRef]
- Chuo, S.C.; Mohamed, S.F.; Mohd Setapar, S.H.; Ahmad, A.; Jawaid, M.; Wani, W.A.; Yaqoob, A.A.; Ibrahim, M.N.M. Insights into the Current Trends in the Utilization of Bacteria for Microbially Induced Calcium Carbonate Precipitation. Materials 2020, 13, 4993. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Edittrich, M. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Görgen, S.; Benzerara, K.; Skouri-Panet, F.; Gugger, M.; Chauvat, F.; Cassier-Chauvat, C. The diversity of molecular mechanisms of carbonate biomineralization by bacteria. Discov. Mater. 2020, 1, 1–20. [Google Scholar] [CrossRef]
- Yin, T.; Lin, H.; Dong, Y.; Li, B.; He, Y.; Liu, C.; Chen, X. A novel constructed carbonate-mineralized functional bacterial consortium for high-efficiency cadmium biomineralization. J. Hazard. Mater. 2020, 401, 123269. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, A.; Wilkinson, S.; Moy, C.K. MICP as a potential sustainable technique to treat or entrap contaminants in the natural environment: A review. Environ. Sci. Ecotechnol. 2021, 6, 100096. [Google Scholar] [CrossRef]
- Phillips, A.J.; Gerlach, R.; Lauchnor, E.; Mitchell, A.C.; Cunningham, A.B.; Spangler, L. Engineered applications of ureolytic biomineralization: A review. Biofouling 2013, 29, 715–733. [Google Scholar] [CrossRef] [Green Version]
- Silva-Castro, G.A.; Uad, I.; Rivadeneyra, A.; Vilchez, J.I.; Martin-Ramos, D.; González-López, J.; Rivadeneyra, M.A. Carbonate Precipitation of Bacterial Strains Isolated from Sediments and Seawater: Formation Mechanisms. Geomicrobiol. J. 2013, 30, 840–850. [Google Scholar] [CrossRef]
- Fujita, Y.; Redden, G.D.; Ingram, J.C.; Cortez, M.M.; Ferris, F.; Smith, R.W. Strontium incorporation into calcite generated by bacterial ureolysis. Geochim. Et Cosmochim. Acta 2004, 68, 3261–3270. [Google Scholar] [CrossRef] [Green Version]
- Lauchnor, E.G.; Schultz, L.N.; Bugni, S.; Mitchell, A.C.; Cunningham, A.B.; Gerlach, R. Bacterially Induced Calcium Carbonate Precipitation and Strontium Coprecipitation in a Porous Media Flow System. Environ. Sci. Technol. 2013, 47, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Mugwar, A.J.; Harbottle, M.J. Toxicity effects on metal sequestration by microbially-induced carbonate precipitation. J. Hazard. Mater. 2016, 314, 237–248. [Google Scholar] [CrossRef]
- Mitchell, A.C.; Ferris, F.G. The coprecipitation of Sr into calcite precipitates induced by bacterial ureolysis in artificial groundwater: Temperature and kinetic dependence. Geochim. Et Cosmochim. Acta 2005, 69, 4199–4210. [Google Scholar] [CrossRef]
- Gebru, K.A.; Kidanemariam, T.G.; Gebretinsae, H.K. Bio-cement production using microbially induced calcite precipitation (MICP) method: A review. Chem. Eng. Sci. 2021, 238, 116610. [Google Scholar] [CrossRef]
- Zhan, M.; Pan, G.; Wang, Y.; Fu, M.; Lu, X. Recycled aggregate mortar enhanced by microbial calcite precipitation. Mag. Concr. Res. 2020, 72, 622–633. [Google Scholar] [CrossRef]
- Dhami, N.K.; Ereddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balam, N.H.; Mostofinejad, D.; Eftekhar, M. Effects of bacterial remediation on compressive strength, water absorption, and chloride permeability of lightweight aggregate concrete. Constr. Build. Mater. 2017, 145, 107–116. [Google Scholar] [CrossRef]
- Vijay, K.; Murmu, M.; Deo, S.V. Bacteria based self healing concrete–A review. Constr. Build. Mater. 2017, 152, 1008–1014. [Google Scholar] [CrossRef]
- Al-Salloum, Y.; Hadi, S.; Abbas, H.; Almusallam, T.; Moslem, M. Bio-induction and bioremediation of cementitious composites using microbial mineral precipitation–A review. Constr. Build. Mater. 2017, 154, 857–876. [Google Scholar] [CrossRef]
- De Jong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2013, 63, 287–301. [Google Scholar] [CrossRef] [Green Version]
- Chu, J.; Ivanov, V.; Stabnikov, V.; Li, B. Microbial method for construction of an aquaculture pond in sand. Géotechnique 2013, 63, 871–875. [Google Scholar] [CrossRef]
- Salifu, E.; MacLachlan, E.; Iyer, K.R.; Knapp, C.W.; Tarantino, A. Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: A preliminary investigation. Eng. Geol. 2016, 201, 96–105. [Google Scholar] [CrossRef]
- Xiao, P.; Liu, H.; Xiao, Y.; Stuedlein, A.; Evans, T.M. Liquefaction resistance of bio-cemented calcareous sand. Soil Dyn. Earthq. Eng. 2018, 107, 9–19. [Google Scholar] [CrossRef]
- Duo, L.; Kan-Liang, T.; Hui-Li, Z.; Yu-Yao, W.; Kang-Yi, N.; Shi-Can, Z. Experimental investigation of solidifying desert aeolian sand using microbially induced calcite precipitation. Constr. Build. Mater. 2018, 172, 251–262. [Google Scholar] [CrossRef]
- Sharaky, A.M.; Mohamed, N.; Elmashad, M.E.; Shredah, N.M. Application of microbial biocementation to improve the physico-mechanical properties of sandy soil. Constr. Build. Mater. 2018, 190, 861–869. [Google Scholar] [CrossRef]
- Sasaki, T.; Kuwano, R. Undrained cyclic triaxial testing on sand with non-plastic fines content cemented with microbially induced CaCO3. Soils Found. 2016, 56, 485–495. [Google Scholar] [CrossRef]
- Fang, L.; Niu, Q.; Cheng, L.; Jiang, J.; Yu, Y.-Y.; Chu, J.; Achal, V.; You, T. Ca-mediated alleviation of Cd2+ induced toxicity and improved Cd2+ biomineralization by Sporosarcina pasteurii. Sci. Total Environ. 2021, 787, 147627. [Google Scholar] [CrossRef]
- Duarte-Nass, C.; Rebolledo, K.; Valenzuela, T.; Kopp, M.; Jeison, D.; Rivas, M.; Azócar, L.; Torres-Aravena, Á.; Ciudad, G. Application of microbe-induced carbonate precipitation for copper removal from copper-enriched waters: Challenges to future industrial application. J. Environ. Manag. 2019, 256, 109938. [Google Scholar] [CrossRef]
- Warren, P.A.M.L.A. Microbially Mediated Calcium Carbonate Precipitation: Implications for Interpreting Calcite Precipitation and for Solid-Phase Capture of Inorganic Contaminants. Geomicrobiol. J. 2001, 18, 93–115. [Google Scholar] [CrossRef]
- Li, M.; Cheng, X.; Guo, H. Heavy metal removal by biomineralization of urease producing bacteria isolated from soil. Int. Biodeterior. Biodegrad. 2013, 76, 81–85. [Google Scholar] [CrossRef]
- Jiang, N.-J.; Liu, R.; Du, Y.-J.; Bi, Y.-Z. Microbial induced carbonate precipitation for immobilizing Pb contaminants: Toxic effects on bacterial activity and immobilization efficiency. Sci. Total Environ. 2019, 672, 722–731. [Google Scholar] [CrossRef]
- Jalilvand, N.; Akhgar, A.; Alikhani, H.A.; Rahmani, H.A.; Rejali, F. Removal of Heavy Metals Zinc, Lead, and Cadmium by Biomineralization of Urease-Producing Bacteria Isolated from Iranian Mine Calcareous Soils. J. Soil Sci. Plant Nutr. 2019, 20, 206–219. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, S.; Roh, Y. Effect of Divalent Cations (Cu, Zn, Pb, Cd, and Sr) on Microbially Induced Calcium Carbonate Precipitation and Mineralogical Properties. Front. Microbiol. 2021, 12, 646748. [Google Scholar] [CrossRef] [PubMed]
- Dhami, N.K.; Quirin, M.E.C.; Mukherjee, A. Carbonate biomineralization and heavy metal remediation by calcifying fungi isolated from karstic caves. Ecol. Eng. 2017, 103, 106–117. [Google Scholar] [CrossRef]
- Lapierre, F.M.; Schmid, J.; Ederer, B.; Ihling, N.; Huber, R. Revealing nutritional requirements of MICP-relevant Sporosarcina pasteurii DSM33 for growth improvement in chemically defined and complex media. Sci. Rep. 2020, 10, 22448. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-H.; Han, S.-H.; Shin, Y.; Oh, S.J.; So, J.-S. Bioremediation of Cd by Microbially Induced Calcite Precipitation. Appl. Biochem. Biotechnol. 2013, 172, 1929–1937. [Google Scholar] [CrossRef]
- Chada, V.G.R.; Hausner, D.B.; Strongin, D.R.; Rouff, A.A.; Reeder, R.J. Divalent Cd and Pb uptake on calcite {1014} cleavage faces: An XPS and AFM study. J. Colloid Interface Sci. 2005, 288, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lian, F.; Zhu, L. Biosorption of divalent Pb, Cd and Zn on aragonite and calcite mollusk shells. Environ. Pollut. 2011, 159, 1763–1768. [Google Scholar] [CrossRef]
- Kumari, D.; Qian, X.-Y.; Pan, X.; Achal, V.; Li, Q.; Gadd, G.M. Microbially-induced Carbonate Precipitation for Immobilization of Toxic Metals. Adv. Appl. Microbio. 2016, 94, 79–108. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhang, B.; Ge, Q.; Yang, X. Calcium carbonate precipitation induced by calcifying bacteria in culture experiments: Influence of the medium on morphology and mineralogy. Int. Biodeterior. Biodegrad. 2018, 134, 83–92. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; González-Muñoz, M.T. Influence of Substrate Mineralogy on Bacterial Mineralization of Calcium Carbonate: Implications for Stone Conservation. Appl. Environ. Microbiol. 2012, 78, 4017–4029. [Google Scholar] [CrossRef] [Green Version]
- Omoregie, A.I.; Ngu, L.H.; Ong, D.E.L.; Nissom, P.M. Low-cost cultivation of Sporosarcina pasteurii strain in food-grade yeast extract medium for microbially induced carbonate precipitation (MICP) application. Biocatal. Agric. Biotechnol. 2018, 17, 247–255. [Google Scholar] [CrossRef]
- Xu, J.; Du, Y.; Jiang, Z.; She, A. Effects of Calcium Source on Biochemical Properties of Microbial CaCO3 Precipitation. Front. Microbiol. 2015, 6, 1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulos, R.A.; Zhang, F.; Tjandra, E.S.; Martin, A.; Spagnoli, D.; Raston, C.L. Spinning up the polymorphs of calcium carbonate. Sci. Rep. 2014, 4, 3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, A.C.; Ferris, F.G. The Influence of Bacillus pasteurii on the Nucleation and Growth of Calcium Carbonate. Geomicrobiol. J. 2006, 23, 213–226. [Google Scholar] [CrossRef]
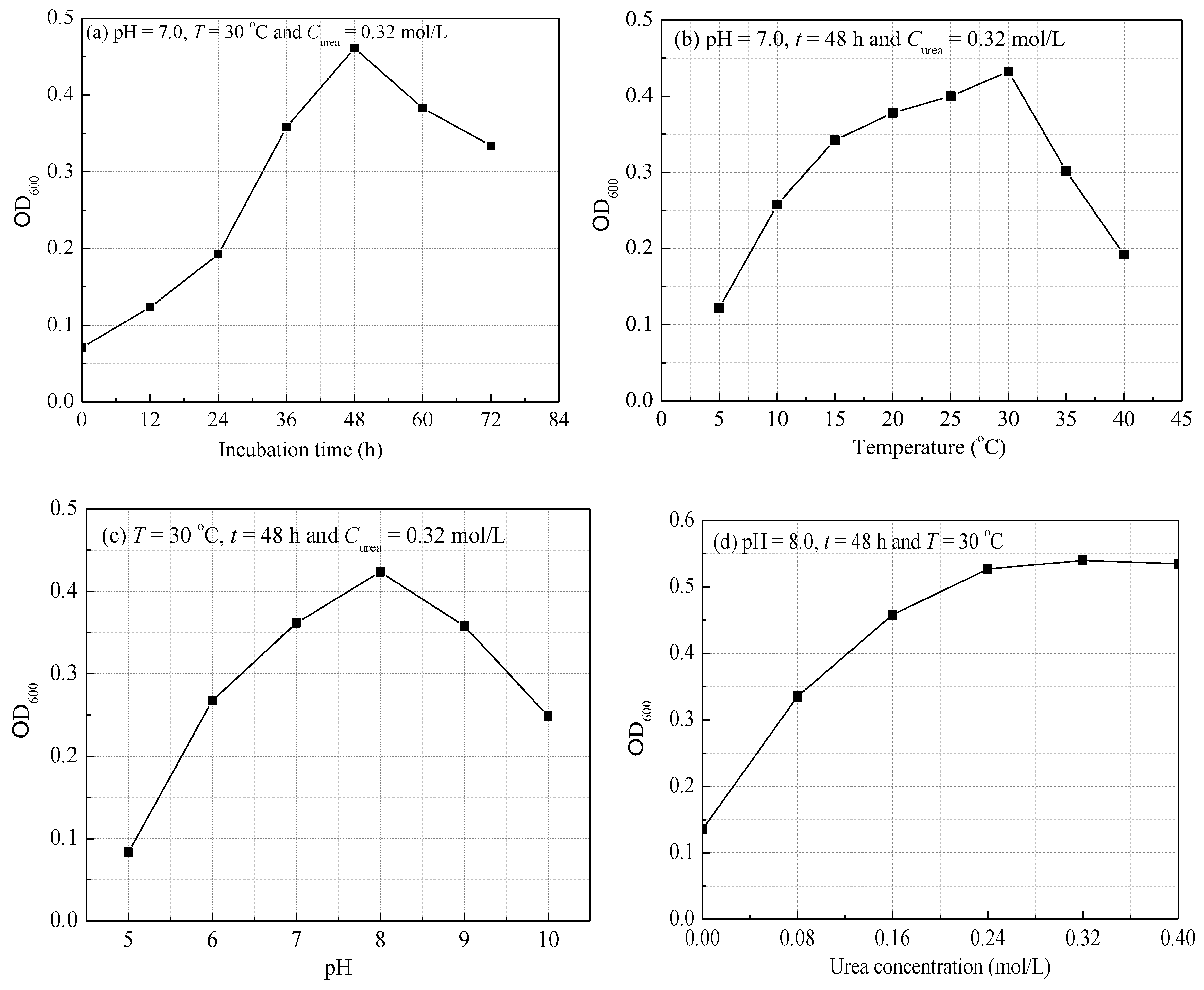


| Group | pH | T (°C) | t (h) | Curea (mol/L) |
|---|---|---|---|---|
| 1 | 7.0 | 30 | 0, 12, 24, 36, 48, 60, 72 | 0.32 |
| 2 | 7.0 | 10, 15, 20, 25, 30, 35, 40, 45 | 48 | 0.32 |
| 3 | 5.0, 6.0, 7.0, 8.0, 9.0, 10.0 | 30 | 48 | 0.32 |
| 4 | 8.0 | 30 | 48 | 0, 0.08, 0.16, 0.32, 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Y.; Tang, J.; Li, K. Removal Behavior of Heavy Metals from Aqueous Solutions via Microbially Induced Carbonate Precipitation Driven by Acclimatized Sporosarcina pasteurii. Appl. Sci. 2022, 12, 9958. https://doi.org/10.3390/app12199958
Li X, Wang Y, Tang J, Li K. Removal Behavior of Heavy Metals from Aqueous Solutions via Microbially Induced Carbonate Precipitation Driven by Acclimatized Sporosarcina pasteurii. Applied Sciences. 2022; 12(19):9958. https://doi.org/10.3390/app12199958
Chicago/Turabian StyleLi, Xinxin, Yan Wang, Jiajie Tang, and Keke Li. 2022. "Removal Behavior of Heavy Metals from Aqueous Solutions via Microbially Induced Carbonate Precipitation Driven by Acclimatized Sporosarcina pasteurii" Applied Sciences 12, no. 19: 9958. https://doi.org/10.3390/app12199958
APA StyleLi, X., Wang, Y., Tang, J., & Li, K. (2022). Removal Behavior of Heavy Metals from Aqueous Solutions via Microbially Induced Carbonate Precipitation Driven by Acclimatized Sporosarcina pasteurii. Applied Sciences, 12(19), 9958. https://doi.org/10.3390/app12199958





