Biomechanical Analysis of Staples for Epiphysiodesis
Abstract
:1. Introduction
- Numerical approach—(the main subject of this paper).
- Experimental approach (used and described only marginally here).
2. Materials and Methods
2.1. Finite Element Analysis
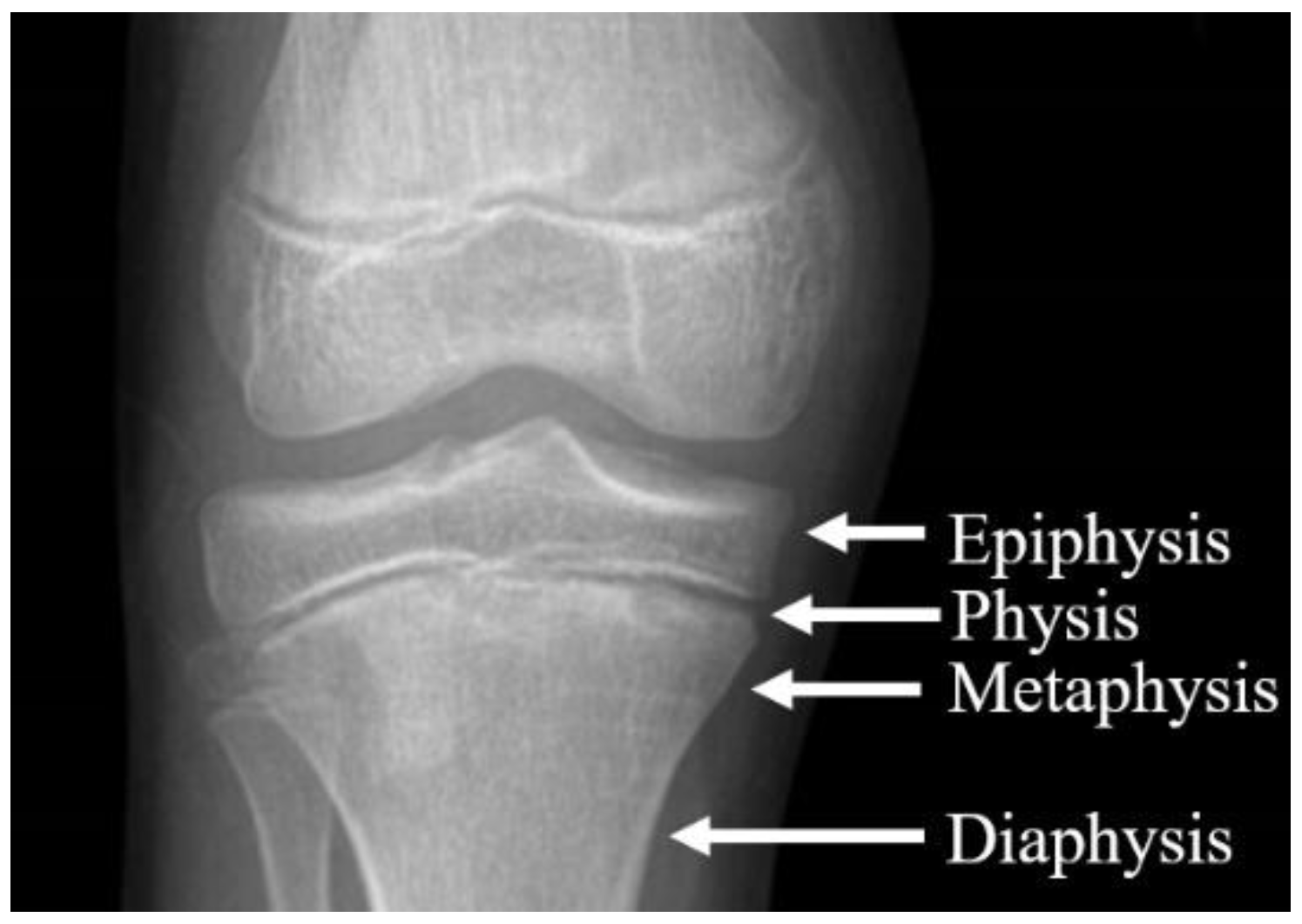
2.1.1. CAD and FEM Model
2.1.2. Boundary Conditions
2.2. Experiment
3. Results of FEA
4. Results of the Experiment
5. Discussion
6. Conclusions
Future Development
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fillingham, Y.A.; Kogan, M. Chapter 21—Epiphysiodesis for Limb Length Discrepancy and Angular Deformity. In Book Case Competencies in Orthopaedic Surgery; Frank, R.M., Forsythe, B., Provencher, M.T., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 195–207. ISBN 978-0-323-39038-5. [Google Scholar] [CrossRef]
- Eastwood, D.M.; Sanghrajka, A.P. Guided Growth: Recent Advances in a Deep-Rooted Concept. J. Bone Jt. Surg. Br. 2011, 93, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S. Blount Disease: An Update. Orthop. Clin. N. Am. 2014, 46. [Google Scholar] [CrossRef]
- Halo, T.; Frydrýšek, K.; Čepica, D.; Skoupý, O.; Michal, P.; Kraus, Š.; Havlas, V.; Kohut, J. Numerical Simulation of Staples for Epiphysiodesis; Vladimír, F., Ed.; Brno University of Technology Institute of Solid Mechanics, Mechatronics and Biomechanics: Brno, Czech Republic, 2020; pp. 186–189. ISSN 1805-8256. [Google Scholar] [CrossRef]
- Métaizeau, J.P.; Wong-Chung, J.; Bertrand, H.; Pasquier, P. Percutaneous epiphysiodesis using transphyseal screws (PETS). J. Pediatr. Orthop. 1998, 18, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Blount, W.P.; Clarke, G.R. Control of Bone Growth by epiphyseal Stapling; A Preliminary Report. J. Bone Jt. Surg. Am. 1949, 31A-3, 64–478. [Google Scholar]
- Shaibi, S.; Sbihi, Y.; Sdoudi, A.; Choukri, M.A.; Andaloussi, Y.E.; Fadili, M. Pseudarthrosis of Distal Radial Growth Plate Treated with Blount Clip: A Case Report. Int. J. Surg. Case Rep. 2021, 87, 106339. [Google Scholar] [CrossRef]
- Phemister, D.B. Operative Arrest of Longitudinal Growth of Bones in The Treatment of Deformities. J. Bone Jt. Surg. Am. 1933, 15, 1–15. [Google Scholar]
- Stevens, P.M. Guided Growth for Angular Correction: A Preliminary Series Using A Tension Band Plate. J. Pediatr. Orthop. 2007, 27, 253–259. [Google Scholar] [CrossRef]
- Sabharwal, S.; Green, S.; McCarthy, J.; Hamdy, R.C. What’s New in Limb Lengthening and Deformity Correction. J. Bone Jt. Surg. Am. 2011, 93, 213–221. [Google Scholar] [CrossRef]
- Courvoisier, A.; Eid, A.; Merloz, P. Epiphyseal Stapling of the Proximal Tibia for Idiopathic Genu Valgum. J. Child Orthop. 2009, 3, 217–221. [Google Scholar] [CrossRef] [Green Version]
- Jochymek, J.; Turek, J.; Peterková, T.; Urbášek, K. Eight—Figure Plate System for Correction of Angular Deformities of Lower Limbs in Children. Initial Results; Pohybové ústrojí: Ortotika, Praha, 2016; Volume 23, pp. 82–93. [Google Scholar]
- Hub, J. A Study on Topology Optimization of Airplane Air Brake Bracing Beam. In International Conference on Military Technologies (ICMT) 2019; Institute of Electrical and Electronics Engineers Inc.: Brno, Czech Republic, 2019; p. 8870028. ISBN 978-172814593-8. [Google Scholar] [CrossRef]
- Yang, J.C.-S.; Lin, K.-Y.; Lin, H.-H.; Lee, O.K. Biomechanical evaluation of high tibial osteotomy plate with internal support block using finite element analysis. PLoS ONE 2021, 16, e0247412. [Google Scholar] [CrossRef] [PubMed]
- Cofaru, N.F.; Roman, M.D.; Cofaru, I.I.; Oleksik, V.S.; Fleaca, S.R. Medial Opening Wedge High Tibial Osteotomy in Knee Osteoarthritis—A Biomechanical Approach. Appl. Sci. 2020, 10, 8972. [Google Scholar] [CrossRef]
- Joshi, J.; Manral, A.R.; Maurya, S.; Vishnoi, M. Biomechanical analysis of human tibia bone based on FEA. Mater. Today Proc. 2021, 44, 1711–1717. [Google Scholar] [CrossRef]
- Alonso, M.G.; Bertolino, G.; Yawny, A. Mechanobiological Based Long Bone Growth Model for the Design of Limb Deformities Correction Devices. J. Biomech. 2020, 109, 109905. [Google Scholar] [CrossRef]
- Rozehnal, D.; Hub, J.; Konvalinova, B. Innovative Processes in Preparing Models and Measuring them in the Wind Tunnel. In Proceedings of the 2021 8th International Conference on Military Technologies, ICMT 2021, Brno, Czech Republic, 8–11 June 2021; Institute of Electrical and Electronics Engineers Inc.: Brno, Czech Republic, 2021; pp. 1–6, ISBN 978-1-6654-3724-0. [Google Scholar] [CrossRef]
- Hub, J.; Komenda, J.; Vitek, R.; Jedlicka, L. Combined Effect of Pistol Ammunition. In International Conference on Military Technologies (ICMT), 2017; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2017; pp. 54–60. ISBN 978-153861988-9. [Google Scholar] [CrossRef]
- MEDIN, a.s. Instruments and Implants for Traumatology [catalog]. 2018, pp. B.2.1–B.2.2. Available online: https://www.medin.cz/media/cache/file/ce/medin-traumatology-catalogue-2018-10-CS-EN_LQ.pdf (accessed on 20 August 2021).
- Ansys Workbench 2020, Version R2, Software. 2020. Available online: https://aopds.com/ansys-2020-r2/(accessed on 20 August 2021).
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Ding, J.; Wang, F.; Jin, F.; Wu, Z.; Shen, P. Finite Element and Biomechanical Analysis of Risk Factors for Implant Failure during Tension Band Plating. J. Int. Med. Res. 2020, 48, 0300060520972075. [Google Scholar] [CrossRef] [PubMed]
- Bylski-Austrow, D.I.; Wall, E.J.; Rupert, M.P.; Roy, D.R.; Crawford, A.H. Growth Plate Forces in the Adolescent Human Knee: A Radiographic and Mechanical Study of Epiphyseal Staples. J. Pediatric Orthop. 2001, 21, 817–823. [Google Scholar] [CrossRef]
- Ben Achour, A.; Petto, C.; Meißner, H.; Mostofa, A.; Teicher, U.; Ihlenfeldt, S.; Lauer, G. Evaluation of a Method to Measure the Friction Coefficient between Vital Mandibular Bone and Biomedical Materials. Biotribology 2021, 28, 100198. [Google Scholar] [CrossRef]
- “Tibia, 4th Gen., Composite, 17 PCF Solid Foam Core, Medium.” SAWBONES. Available online: https://www.sawbones.com/tibia-medium-left-4th-generation-composite-3401.html (accessed on 12 June 2021).
- “Block, Solid Foam, 10 PCF Laminated with 3 mm Solid Foam 40 PCF. SAWBONES. Available online: https://www.sawbones.com/block-10-solid-foam-1522-01-laminated-w-3mm-40-solid-foam-1522-07-finished-size-170-x-120-x-43mm-thick1522-107.html (accessed on 12 June 2021).
- Lucas, J.F.; Chip Routt, M.L., Jr.; Eastman, J.G. Biomechanical Analysis of Retrograde Superior Ramus Screw Fixation Constructs. J. Orthop. Trauma. 2021, 35, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Murath, S.K.; Uzun, B.; Qelik, S. Comparison of five percutaneous pinning methods for unstable extra-articular distal radius fractures: A mechanical study using sawbones. Jt. Dis. Relat. Surg. 2021, 32, 51–58. [Google Scholar] [CrossRef]
- “50 kN Machines.” Testometric. Available online: https://www.testometric.co.uk/50kn/ (accessed on 5 July 2021).
- Raab, P.; Wild, A.; Seller, K.; Krauspe, R. Correction of Length Discrepancies and Angular Deformities of the Leg by Blount’s Epiphyseal Stapling. Eur. J. Pediatr. 2001, 160, 668–674. [Google Scholar] [CrossRef]
- Schroerlucke, S.; Bertrand, S.; Clapp, J.; Bundy, J.; Gregg, F.O. Failure of Orthofix Eight-Plate for the Treatment of Blount Disease. J. Pediatric Orthop. 2009, 29, 57–60. [Google Scholar] [CrossRef]
- Frydrýšek, K.; Michenková, Š.; Pleva, L.; Koutecký, J.; Fries, J.; Peterek Dědková, K.; Madeja, R.; Trefil, A.; Krpec, P.; Halo, T.; et al. Mechanics of Screw Joints Solved as Beams Placed in a Tangential Elastic Foundation. Appl. Sci. 2021, 11, 5616. [Google Scholar] [CrossRef]
- Theisz, G.; Frydrýšek, K.; Fojtík, F. Medial Plate for Treatment of Distal Tibia Fractures. In Proceedings of the EAN 2015—53rd Conference on Experimental Stress Analysis, Cesky Krumlov, Czech Republic, 1–4 June 2015; Padevet, P., Bittnar, P., Eds.; CTU in Prague: Český Krumlov, Czech Republic; pp. 431–437, ISBN 978-800105735-6. [Google Scholar]
- Pothong, W.; Phinyo, P.; Sirirungruangsarn, Y.; Nabudda, K.; Wongba, N.; Sarntipiphat, C.; Pruksakorn, D. Biomechanical Analysis of Sagittal Plane Pin Placement Configurations for Pediatric Supracondylar Humerus Fractures. Appl. Sci. 2021, 11, 3447. [Google Scholar] [CrossRef]
- Kwon, J.; Ha, M.H.; Lee, M.G. Alternative Pedicle Screw Design via Biomechanical Evaluation. Appl. Sci. 2020, 10, 4746. [Google Scholar] [CrossRef]
- Čada, R.; Frydrýšek, K.; Sejda, F.; Demel, J.; Pleva, L. Analysis of locking self-taping bone screws for angularly stable plates. J. Med. Biol. Eng. 2017, 37, 612–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frydrýšek, K.; Šír, M.; Pleva, L. Strength Analyses of Screws for Femoral Neck Fractures. J. Med. Biol. Eng. 2018, 38, 816–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frydrýšek, K.; Čepica, D.; Halo, T. Biomechanics—Probabilistic Anthropometry Approach for Sitting Human and Seat. In Proceedings of the 57th International Scientific Conference on Experimental Stress Analysis (EAN 2019), Luhacovice, Czech Republic, 13–16 May 2019; pp. 90–96, ISBN 978-80-214-5766-9. [Google Scholar]
- Tai, W.-H.; Peng, H.-T.; Song, C.-Y.; Lin, J.-Z.; Yu, H.-B.; Wang, L.-I. Dynamic Characteristics of Approach Spike Jump Tasks in Male Volleyball Players. Appl. Sci. 2021, 11, 2710. [Google Scholar] [CrossRef]


















| Material | Young’s Modulus (GPa) | Poisson’s Ratio (1) | Yield Strength (MPa) | Ultimate Strength (MPa) |
|---|---|---|---|---|
| 1.4441 | 183 | 0.33 | 690 | 800 |
| Corticalis | 16.1 | 0.3 | ||
| Spongiosis | 0.4 | 0.3 | ||
| Part | Number of FE Elements | Number of FE Nodes | |
|---|---|---|---|
| Tibia | Epiphysis | 66,083 | 102,706 |
| Metaphysis-Diaphysis | 80,183 | 126,033 | |
| Staple | Medial | 132,292 | 438,008 |
| Lateral | 132,581 | 442,394 | |
| Total | 411,139 | 1,109,141 | |
| Value | Place | |
|---|---|---|
| Maximal Equivalent von Mises Stress | 682.39 MPa | Radius of medial staple, outside of tibia; see Figure 13 and Figure 14 |
| Maximal Displacement | 0.2 mm | Apex of medial staple, inside of tibia; see Figure 15 |
| Average distance between the proximal and distal bone segment of the tibia | 0.3 mm | Epiphysis, inside of tibia, see Figure 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frydrýšek, K.; Čepica, D.; Halo, T.; Skoupý, O.; Pleva, L.; Madeja, R.; Pometlová, J.; Losertová, M.; Koutecký, J.; Michal, P.; et al. Biomechanical Analysis of Staples for Epiphysiodesis. Appl. Sci. 2022, 12, 614. https://doi.org/10.3390/app12020614
Frydrýšek K, Čepica D, Halo T, Skoupý O, Pleva L, Madeja R, Pometlová J, Losertová M, Koutecký J, Michal P, et al. Biomechanical Analysis of Staples for Epiphysiodesis. Applied Sciences. 2022; 12(2):614. https://doi.org/10.3390/app12020614
Chicago/Turabian StyleFrydrýšek, Karel, Daniel Čepica, Tomáš Halo, Ondřej Skoupý, Leopold Pleva, Roman Madeja, Jana Pometlová, Monika Losertová, Jan Koutecký, Pavel Michal, and et al. 2022. "Biomechanical Analysis of Staples for Epiphysiodesis" Applied Sciences 12, no. 2: 614. https://doi.org/10.3390/app12020614
APA StyleFrydrýšek, K., Čepica, D., Halo, T., Skoupý, O., Pleva, L., Madeja, R., Pometlová, J., Losertová, M., Koutecký, J., Michal, P., Havlas, V., Kraus, Š., Ďurica, D., Dědková, K. P., Pagáč, M., Krpec, P., & Osemlak, P. (2022). Biomechanical Analysis of Staples for Epiphysiodesis. Applied Sciences, 12(2), 614. https://doi.org/10.3390/app12020614









