Nanotechnology in Plant Metabolite Improvement and in Animal Welfare
Abstract
:1. Introduction
2. Silver-Nanoparticles in Crop Improvement
3. Silver-Nanoparticles in Plant Secondary Metabolite Enhancement
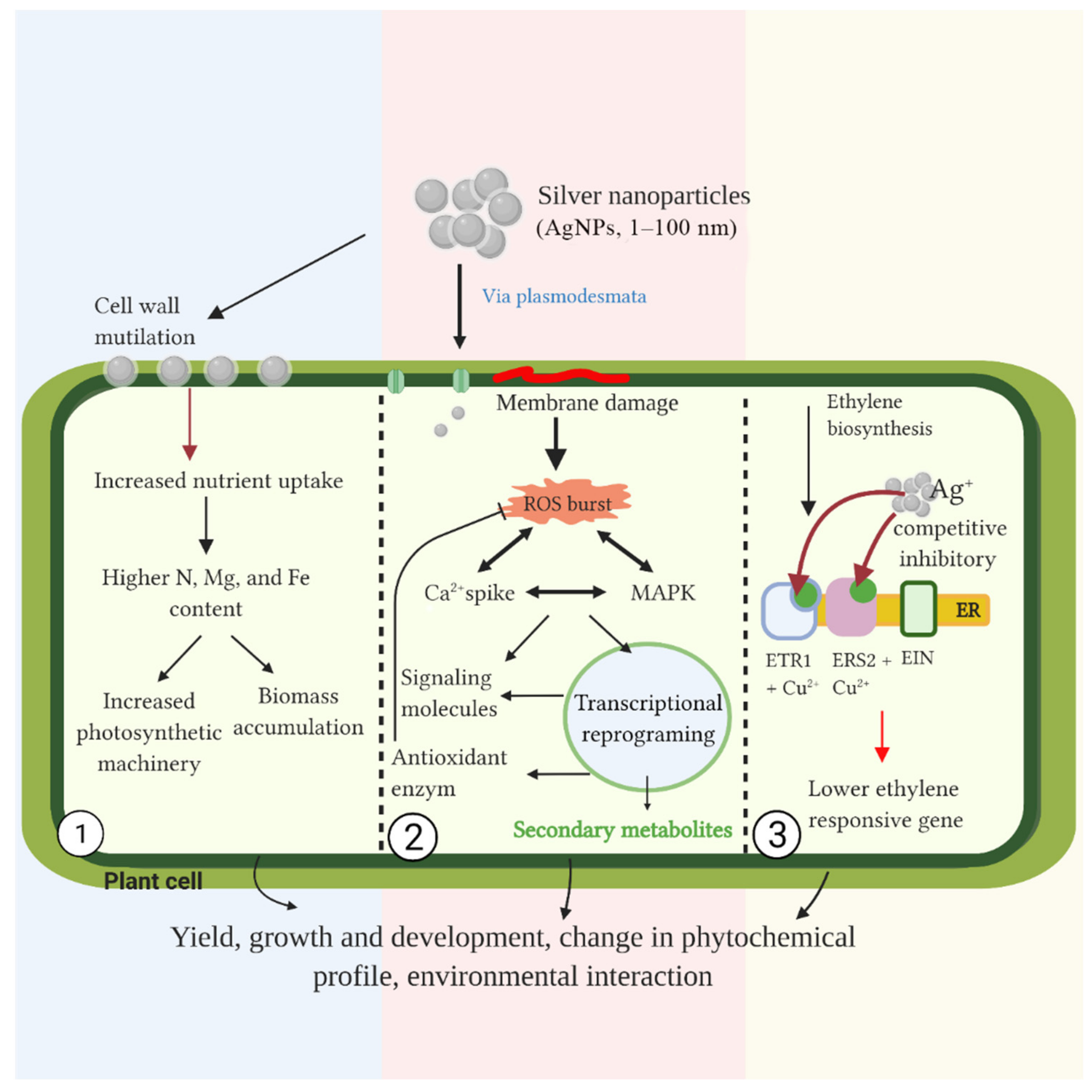
4. Possible Mechanism of AgNPs in Plant Cells
5. Nanomaterials: The Future Goal in Many Multi-Faceted Fields of Animal Welfare
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ali, A.M.A.; El-Nour, M.E.A.M.; Yagi, S.M. Callus Induction, Direct and Indirect Organogenesis of Ginger (Zingiber Officinale Rosc). Afr. J. Biotechnol. 2016, 15, 2106–2114. [Google Scholar] [CrossRef] [Green Version]
- Bidabadi, S.S.; Jain, S.M. Cellular, Molecular, and Physiological Aspects of In Vitro Plant Regeneration. Plants 2020, 9, 702. [Google Scholar] [CrossRef] [PubMed]
- Kasilingam, T.; Raman, G.; Sundramoorthy, N.; Supramaniam, G.; Mohtar, S.; Avin, F. A Review on In Vitro Regeneration of Ginger: Tips and Highlights. Eur. J. Med. Plants 2018, 23, 1–8. [Google Scholar] [CrossRef]
- Singh, C.R. Review on Problems and Its Remedy in Plant Tissue Culture. Asian J. Biol. Sci. 2018, 11, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, S.P.; Tapia, M.A.M.; Vega, M.E.G.; Ardisana, E.F.H.; Medina, J.A.C.; Zamora, G.L.F.; Bustamante, D.V. Nanotechnology and Plant Tissue Culture. In Plant Nanobionics; Nanotechnology in the Life Sciences; Prasad, R., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 333–370. ISBN 978-3-030-12495-3. [Google Scholar]
- Acemi, R.K.; Acemi, A.; Cakir, M.; Polat, E.G.; Ozen, F. Preliminary screening the antioxidant potential of in vitro-propagated Amsonia orientalis: An example to sustainable use of rare medicinal plants in pharmaceutical studies. Sustain. Chem. Pharm. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Oseni, O.M.; Pande, V.; Nailwal, T.K. A Review on Plant Tissue Culture, A Technique for Propagation and Conservation of Endangered Plant Species. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 3778–3786. [Google Scholar] [CrossRef]
- Ali, A.; Mohammad, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z.-R. Silver Nanoparticles Elicited in Vitro Callus Cultures for Accumulation of Biomass and Secondary Metabolites in Caralluma tuberculata. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.S.; Taha, H.S.; Aly, U.I.; El-Shabrawi, H.M.; Gaber, E.-S.I. In Vitro Propagation of Ginger (Zingiber Officinale Rosco). J. Genet. Eng. Biotechnol. 2011, 9, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Chandran, H.; Meena, M.; Barupal, T.; Sharma, K. Plant Tissue Culture as a Perpetual Source for Production of Industrially Important Bioactive Compounds. Biotechnol. Rep. 2020, 26, e00450. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Kim, D.H.; Gopal, J.; Sivanesan, I. Nanomaterials in Plant Tissue Culture: The Disclosed and Undisclosed. RSC Adv. 2017, 7, 36492–36505. [Google Scholar] [CrossRef] [Green Version]
- Jadid, N.; Kurniawan, E.; Himayani, C.E.S.; Prasetyowati, I.; Purwani, K.I.; Muslihatin, W.; Hidayati, D.; Tjahjaningrum, I.T.D. An Ethnobotanical Study of Medicinal Plants Used by the Tengger Tribe in Ngadisari Village, Indonesia. PLoS ONE 2020, 15, e0235886. [Google Scholar] [CrossRef]
- Jadid, N.; Rachman, R.Y.; Hartanti, S.R.; Abdulgani, N.; Wikanta, W.; Muslihatin, W. Methanol Extract of Piper Retrofractum Vahl. Potentially Mediates Mast Cell Stabilization. Int. J. Pharm. Bio Sci. 2016, 7, 379–383. [Google Scholar]
- Salmerón-Manzano, E.; Garrido-Cardenas, J.A.; Manzano-Agugliaro, F. Worldwide Research Trends on Medicinal Plants. Int. J. Environ. Res. Public Health 2020, 17, 3376. [Google Scholar] [CrossRef]
- Abdi, G.; Salehi, H.; Khosh-Khui, M. Nano Silver: A Novel Nanomaterial for Removal of Bacterial Contaminants in Valerian (Valeriana officinalis L.) Tissue Culture. Acta Physiol. Plant 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Bhoite, H.A.; Palshikar, G.S. Plant Tissue Culture: A Review. World J. Pharm. Sci. 2014, 2, 565–572. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A.; Wahab, P.E.M.; Halim, M.R.A. Effect of Different Light Intensities on Total Phenolics and Flavonoids Synthesis and Anti-Oxidant Activities in Young Ginger Varieties (Zingiber officinale Roscoe). Int. J. Mol. Sci. 2010, 11, 3885–3897. [Google Scholar] [CrossRef] [Green Version]
- Angelova, Z.; Georgiev, S.; Roos, W. Elicitation of Plants. Biotechnol. Biotechnol. Equip. 2006, 20, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Patel, H.; Krishnamurthy, R.J. Elicitors in plant tissue culture. Pharmacogn. Phytochem. 2013, 2, 6. [Google Scholar]
- Chung, I.-M.; Rekha, K.; Rajakumar, G.; Thiruvengadam, M. Elicitation of Silver Nanoparticles Enhanced the Secondary Metabolites and Pharmacological Activities in Cell Suspension Cultures of Bitter Gourd. 3 Biotech 2018, 8, 412. [Google Scholar] [CrossRef]
- Jadczak, P.; Kulpa, D.; Bihun, M.; Przewodowski, W. Positive Effect of AgNPs and AuNPs in in Vitro Cultures of Lavandula Angustifolia Mill. Plant Cell Tissue Organ Cult. 2019, 139, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Dabuwar Benjamin, E.; Adamu Ishaku, G.; Andrew Peingurta, F.; Samuel Afolabi, A. Callus Culture for the Production of Therapeutic Compounds. Am. J. Plant Biol. 2019, 4, 76–84. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dandin, V.S.; Zhong, J.-J.; Paek, K.-Y. Strategies for Enhanced Production of Plant Secondary Metabolites from Cell and Organ Cultures. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.-Y., Murthy, H.N., Zhong, J.-J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 471–508. ISBN 978-94-017-9222-6. [Google Scholar]
- Kokina, I.; Mickeviča, I.; Jahundoviča, I.; Ogurcovs, A.; Krasovska, M.; Jermaļonoka, M.; Mihailova, I.; Tamanis, E.; Gerbreders, V. Plant Explants Grown on Medium Supplemented with Fe3O4 Nanoparticles Have a Significant Increase in Embryogenesis. J. Nanomater. 2017, 2017, 4587147. [Google Scholar] [CrossRef] [Green Version]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarmast, M.K.; Salehi, H. Silver Nanoparticles: An Influential Element in Plant Nanobiotechnology. Mol. Biotechnol. 2016, 58, 441–449. [Google Scholar] [CrossRef]
- Salachna, P.; Byczyńska, A.; Zawadzińska, A.; Piechocki, R.; Mizielińska, M. Stimulatory Effect of Silver Nanoparticles on the Growth and Flowering of Potted Oriental Lilies. Agronomy 2019, 9, 610. [Google Scholar] [CrossRef] [Green Version]
- Castro-González, C.G.; Sánchez-Segura, L.; Gómez-Merino, F.C.; Bello-Bello, J.J. Exposure of Stevia (Stevia Rebaudiana B.) to Silver Nanoparticles in Vitro: Transport and Accumulation. Sci. Rep. 2019, 9, 10372. [Google Scholar] [CrossRef]
- Risuleo, G. Biological properties of a partially purified component of Neem oil: An updated and revised work. In Nuts and Seeds in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 67–72. [Google Scholar] [CrossRef]
- Kumari, P.; Luqman, S.; Meena, A. Application of the combinatorial approaches of medicinal and aromatic plants with nanotechnology and its impacts on healthcare. DARU J. Pharm. Sci. 2019, 27, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Simberg, D.S.; Weisman, Y.; Talmon, Y. DOTAP (and Other Cationic Lipids): Chemistry, Biophysics, and Transfection. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 257–319. [Google Scholar] [CrossRef]
- Lonez, C.; Vandenbranden, M.; Ruysschaert, J.-M. Cationic Liposomal Lipids: From Gene Carriers to Cell Signaling. Prog. Lipid Res. 2008, 47, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436. [Google Scholar] [CrossRef] [Green Version]
- Stefanutti, E.; Papacci, F.; Sennato, S.; Bombelli, C.; Viola, I.; Bonincontro, A.; Bordi, F.; Mancini, G.; Gigli, G.; Risuleo, G. Cationic Liposomes Formulated with DMPC and a Gemini Surfactant Traverse the Cell Membrane without Causing a Significant Bio-Damage. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 2646–2655. [Google Scholar] [CrossRef] [Green Version]
- Cosimati, R.; Milardi, G.L.; Bombelli, C.; Bonincontro, A.; Bordi, F.; Mancini, G.; Risuleo, G. Interactions of DMPC and DMPC/Gemini Liposomes with the Cell Membrane Investigated by Electrorotation. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 352–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Mesa, C.; Risuleo, G. On Concept of Hybrid. Colloids Interfaces 2021, 5, 33–53. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In Vitro Plant Tissue Culture: Means for Production of Biological Active Compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Migdam Elsheikh, A.; Mohamed Daffalla, H.; Mohamed Khalfala, M. In Vitro Micropropagation of the Ornamental Plant Dieffenbachia—A Review. Univers. J. Plant Sci. 2013, 1, 91–99. [Google Scholar] [CrossRef]
- Gupta, N.; Jain, V.; Joseph, M.R.; Devi, S. A Review on Micropropagation Culture Method. Asian J. Pharm. Res. Dev. 2020, 8, 86–93. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, Y.; Zheng, K.; Hu, X.; Zhu, M.; Deng, X.; Xi, R. An Efficient Micropropagation Protocol for an Endangered Ornamental Tree Species (Magnolia sirindhorniae Noot. & Chalermglin) and Assessment of Genetic Uniformity through DNA Markers. Sci. Rep. 2019, 9, 9634. [Google Scholar] [CrossRef] [Green Version]
- Rashmi, R.; Trivedi, M.P. Callus Induction and Callogenic Response of Rauvolfia Serpentina and Catharanthus Roseus By Using Various Growth Hormone Concentrations Singly and in Combination. Int. J. Sci. Eng. Res. 2014, 5, 17. [Google Scholar]
- Bello-Bello, J.J.; Chavez-Santoscoy, R.A.; Lecona-Guzmán, C.A.; Bogdanchikova, N.; Salinas-Ruíz, J.; Gómez-Merino, F.C.; Pestryakov, A. Hormetic Response by Silver Nanoparticles on In Vitro Multiplication of Sugarcane (Saccharum Spp. Cv. Mex 69-290) Using a Temporary Immersion System. Dose-Response 2017, 15, 155932581774494. [Google Scholar] [CrossRef] [Green Version]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-Dependent Phytotoxicity of Nanoparticles to Plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Zuverza-Mena, N.; Armendariz, R.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of Silver Nanoparticles on Radish Sprouts: Root Growth Reduction and Modifications in the Nutritional Value. Front. Plant Sci. 2016, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M. Elicitation of Medicinally Important Antioxidant Secondary Metabolites with Silver and Gold Nanoparticles in Callus Cultures of Prunella vulgaris L. Appl. Biochem. Biotechnol. 2016, 180, 1076–1092. [Google Scholar] [CrossRef] [PubMed]
- Manickavasagam, M.; Pavan, G.; Vasudevan, V. A Comprehensive Study of the Hormetic Influence of Biosynthesized AgNPs on Regenerating Rice Calli of Indica Cv. IR64. Sci. Rep. 2019, 9, 8821. [Google Scholar] [CrossRef]
- Nghia, L.T.; Tung, H.T.; Huy, N.P.; Luan, V.Q.; Nhut, D.T. The Effects of Silver Nanoparticles on Growth of Chrysanthemum Morifolium Ramat. Cv. “JIMBA” in Different Cultural Systems. Vietnam J. Sci. Technol. 2017, 55, 503. [Google Scholar] [CrossRef]
- Fouad, A.; Hegazy, A.E.; Azab, E.; Khojah, E.; Kapiel, T. Boosting of Antioxidants and Alkaloids in Catharanthus roseus Suspension Cultures Using Silver Nanoparticles with Expression of CrMPK3 and STR Genes. Plants 2021, 10, 2202. [Google Scholar] [CrossRef]
- Khan, A.K.; Kousar, S.; Tungmunnithum, D.; Hano, T.; Abbasi, B.H.; Anjum, S. Nano-elicitation as an effective strategy for in vitro production of industrially important flavonoids. App. Sci. 2021, 11, 1694. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-J.; Sang, W.; Guk, J.; Gun, D. The Silver Nanoparticle (Nano-Ag): A New Model for Antifungal Agents. In Silver Nanoparticles; Pozo, D., Ed.; IntechOpen: London, UK, 2010; ISBN 978-953-307-028-5. [Google Scholar]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory Effect of Silver Nanoparticles (AgNPs) on Rice Seedling Growth: An Insight from Antioxidative Enzyme Activities and Gene Expression Patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Ali, H.; Khan, M.A.; Ullah, N.; Khan, R.S. Impacts of Hormonal Elicitors and Photoperiod Regimes on Elicitation of Bioactive Secondary Volatiles in Cell Cultures of Ajuga Bracteosa. J. Photochem. Photobiol. B Biol. 2018, 183, 242–250. [Google Scholar] [CrossRef]
- La Mesa, C.; Corbo, A.; Gkouvi, A.; Risuleo, G. Bio-Active Principles from the Animal and Plant Kingdom: A Review. Adv. Res. Org. Inorg. Chem. (AROIC) 2020, 1, 1–12. [Google Scholar]
- Akash Bhat, P.B. Silver Nanoparticles for Enhancement of Accumulation of Capsaicin in Suspension Culture of Capsicum Sp. J. Exp. Sci. 2016, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, M.; Shujait Ali, S.; Khan, A.; Wei, D.-Q. Sustainable Production of Biomass and Industrially Important Secondary Metabolites in Cell Cultures of Selfheal (Prunella vulgaris L.) Elicited by Silver and Gold Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2553–2561. [Google Scholar] [CrossRef] [Green Version]
- Kruszka, D.; Sawikowska, A.; Kamalabai Selvakesavan, R.; Krajewski, P.; Kachlicki, P.; Franklin, G. Silver Nanoparticles Affect Phenolic and Phytoalexin Composition of Arabidopsis thaliana. Sci. Total Environ. 2020, 716, 135361. [Google Scholar] [CrossRef] [PubMed]
- Thangavelu, R.M.; Gunasekaran, D.; Jesse, M.I.; SU, M.R.; Sundarajan, D.; Krishnan, K. Nanobiotechnology Approach Using Plant Rooting Hormone Synthesized Silver Nanoparticle as “Nanobullets” for the Dynamic Applications in Horticulture—An in Vitro and Ex Vitro Study. Arab. J. Chem. 2018, 11, 48–61. [Google Scholar] [CrossRef] [Green Version]
- Sarmast, M.K.; Niazi, A.; Salehi, H.; Abolimoghadam, A. Silver Nanoparticles Affect ACS Expression in Tecomella Undulata in Vitro Culture. Plant Cell Tissue Organ Cult. 2015, 121, 227–236. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; Prinsi, B.; Marsoni, M.; Espen, L.; Bracale, M. Morphological and Proteomic Responses of Eruca Sativa Exposed to Silver Nanoparticles or Silver Nitrate. PLoS ONE 2013, 8, e68752. [Google Scholar] [CrossRef] [Green Version]
- Do, D.G.; Dang, T.K.T.; Nguyen, T.H.T.; Nguyen, T.D.; Tran, T.T.; Hieu, D.D. Effects of Nano Silver on the Growth of Banana (Musa Spp.) Cultured in Vitro. J. Viet. Environ. 2018, 10, 92–98. [Google Scholar] [CrossRef]
- Timoteo, C.; de, O.; Paiva, R.; dos Reis, M.V.; Claro, P.I.C.; da Silva, D.P.C.; Marconcini, J.M.; de Oliveira, J.E. Silver Nanoparticles in the Micropropagation of Campomanesia Rufa (O. Berg) Nied. Plant Cell Tissue Organ Cult. 2019, 137, 359–368. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Chavez-Santoscoy, R.A.; Bogdanchikova, N.; Pérez-Sato, J.A.; Morales-Ramos, V.; Bello-Bello, J.J. Antimicrobial and Hormetic Effects of Silver Nanoparticles on in Vitro Regeneration of Vanilla (Vanilla Planifolia Jacks. Ex Andrews) Using a Temporary Immersion System. Plant Cell Tissue Organ Cult. 2017, 129, 195–207. [Google Scholar] [CrossRef]
- Chung, I.-M.; Rajakumar, G.; Thiruvengadam, M. Effect of Silver Nanoparticles on Phenolic Compounds Production and Biological Activities in Hairy Root Cultures of Cucumis Anguria. Acta Biol. Hung. 2018, 69, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Karakaş, Ö. Effect of Silver Nanoparticles on Production of Indole Alkaloids in Isatis Constricta. Iran. J. Sci. Technol. Trans. Sci. 2020, 44, 621–627. [Google Scholar] [CrossRef]
- Ramezannezhad, R.; Aghdasi, M.; Fatemi, M. Enhanced Production of Cichoric Acid in Cell Suspension Culture of Echinacea Purpurea by Silver Nanoparticle Elicitation. Plant Cell Tissue Organ Cult. 2019, 139, 261–273. [Google Scholar] [CrossRef]
- Ghanati, F.; Bakhtiarian, S. Effect of Methyl Jasmonate and Silver Nanoparticles on Production of Secondary Metabolites by Calendula officinalis L (Asteraceae). Trop. J. Pharm. Res. 2014, 13, 1783. [Google Scholar] [CrossRef] [Green Version]
- Poovitha, S.; Parani, M. In Vitro and in Vivo α-Amylase and α-Glucosidase Inhibiting Activities of the Protein Extracts from Two Varieties of Bitter Gourd (Momordica charantia L.). BMC Complement Altern. Med. 2016, 16, 185. [Google Scholar] [CrossRef] [Green Version]
- Hassan, S.A.M.; Mahfouze, H.A.; Mahfouze, S.A.; Allatif, A.M.A. Genotoxicity Assessment of Nano-Particles on Micropropagated Olive (Olea europaea L.) Plants Using Rapd and Damd Markers. Plant Arch. 2019, 19, 1985–1994. [Google Scholar]
- Zhang, B.; Liu, H.; Ding, X.; Qiu, J.; Zhang, M.; Chu, Z. AtACS8 Plays a Critical Role in the Early Biosynthesis of Ethylene Elicited by Cu2+ Ions. J. Cell Sci. 2018, 131, Jcs202424. [Google Scholar] [CrossRef] [Green Version]
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Vankova, R.; Landa, P.; Podlipna, R.; Dobrev, P.I.; Prerostova, S.; Langhansova, L.; Gaudinovaa, A.; Motkovab, K.; Knirscha, V.; Vanek, T. ZnO nanoparticle effects on hormonal pools in Arabidopsis thaliana. Sci. Total Environ. 2017, 593–594, 535–542. [Google Scholar] [CrossRef]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles Alter Secondary Metabolism in Plants via ROS Burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [Green Version]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef]
- Štefanić, P.P.; Cvjetko, P.; Biba, R.; Domijan, A.-M.; Letofsky-Papst, I.; Tkalec, M.; Šikić, S.; Cindrić, M.; Balen, B. Physiological, Ultrastructural and Proteomic Responses of Tobacco Seedlings Exposed to Silver Nanoparticles and Silver Nitrate. Chemosphere 2018, 209, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xiao, Y.; Jiao, T.; Zhang, Y.; Chen, J.; Gao, Y. Effects of Copper Oxide Nanoparticles on the Growth of Rice (Oryza sativa L.) Seedlings and the Relevant Physiological Responses. Int. J. Environ. Res. Public Health 2020, 17, 1260. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Parvatam, G.; Ravi–shankar, G.A. AgNO3—A Potential Regulator of Ethylene Activity and Plant Growth Modulator. Electron. J. Biotechnol. 2009, 12, 8–9. [Google Scholar] [CrossRef] [Green Version]
- Schaller, G.E.; Binder, B.M. Inhibitors of Ethylene Biosynthesis and Signaling. In Ethylene Signaling; Methods in Molecular Biology; Binder, B.M., Eric Schaller, G., Eds.; Springer: New York, NY, USA, 2017; Volume 1573, pp. 223–235. ISBN 978-1-4939-6852-7. [Google Scholar]
- Sosan, A.; Svistunenko, D.; Straltsova, D.; Tsiurkina, K.; Smolich, I.; Lawson, T.; Subramaniam, S.; Golovko, V.; Anderson, D.; Sokolik, A.; et al. Engineered Silver Nanoparticles Are Sensed at the Plasma Membrane and Dramatically Modify the Physiology of Arabidopsis thaliana Plants. Plant J. 2016, 85, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoid as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [Green Version]
- Khataee, E.; Karimi, F.; Razavi, K. Alkaloids production and antioxidant properties in Catharanthus roseus (L.) G. Don. shoots and study of alkaloid biosynthesis-related gene expression levels in response to methyl jasmonate and putrescine treatments as eco-friendly elicitors. Biol. Futura 2019, 70, 38–46. [Google Scholar] [CrossRef]
- Paeizi, M.; Karimi, F.; Razavi, K. Changes in Medicinal Alkaloids Production and Expression of Related Regulatory and Biosynthetic Genes in Response to Silver Nitrate Combined with Methyl Jasmonate in Catharanthus roseus in Vitro Propagated Shoots. Plant Physiol. Biochem. 2018, 132, 623–632. [Google Scholar] [CrossRef]
- Bombelli, C.; Bordi, F.; Ferro, S.; Giansanti, L.; Jori, G.; Mancini, G.; Mazzuca, C.; Monti, D.; Ricchelli, F.; Sennato, S.; et al. New Cationic Liposomes as Vehicles of m-Tetrahydroxyphenylchlorin in Photodynamic Therapy of Infectious Diseases. Mol. Pharm. 2008, 5, 672–679. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. J. Chem. Soc. Faraday Trans. 2 1976, 72, 1525. [Google Scholar] [CrossRef]
- Bonincontro, A.; Risuleo, G. Electrorotation: A Spectroscopic Imaging Approach to Study the Alterations of the Cytoplasmic Membrane. Adv. Mol. Imagin 2015, 5, 52953. [Google Scholar] [CrossRef] [Green Version]
- Berardi, V.; Aiello, C.; Bonincontro, A.; Risuleo, G. Alterations of the Plasma Membrane Caused by Murine Polyomavirus Proliferation: An Electrorotation Study. J. Membr. Biol. 2009, 229, 19–25. [Google Scholar] [CrossRef]
- Lozano, N.; Pérez, L.; Pons, R.; Pinazo, A. Diacyl Glycerol Arginine-Based Surfactants: Biological and Physicochemical Properties of Catanionic Formulations. Amino Acids 2011, 40, 721–729. [Google Scholar] [CrossRef]
- Kuo, J.-H.S.; Jan, M.-S.; Chang, C.-H.; Chiu, H.-W.; Li, C.-T. Cytotoxicity Characterization of Catanionic Vesicles in RAW 264.7 Murine Macrophage-like Cells. Colloids Surf. B Biointerfaces 2005, 41, 189–196. [Google Scholar] [CrossRef]
- Vlachy, N.; Touraud, D.; Heilmann, J.; Kunz, W. Determining the Cytotoxicity of Catanionic Surfactant Mixtures on HeLa Cells. Colloids Surf. B Biointerfaces 2009, 70, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Aiello, C.; Andreozzi, P.; La Mesa, C.; Risuleo, G. Biological Activity of SDS-CTAB Cat-Anionic Vesicles in Cultured Cells and Assessment of Their Cytotoxicity Ending in Apoptosis. Colloids Surf. B Biointerfaces 2010, 78, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Russo, L.; Berardi, V.; Tardani, F.; La Mesa, C.; Risuleo, G. Delivery of RNA and Its Intracellular Translation into Protein Mediated by SDS-CTAB Vesicles: Potential Use in Nanobiotechnology. BioMed Res. Int. 2013, 2013, 734596. [Google Scholar] [CrossRef]
- Ji, D.-K.; Ménard-Moyon, C.; Bianco, A. Physically-Triggered Nanosystems Based on Two-Dimensional Materials for Cancer Theranostics. Adv. Drug Deliv. Rev. 2019, 138, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Pallela, R.; Kim, S.-K. Applications of Carbon Nanomaterials in Bone Tissue Engineering. J. Biomed. Nanotechnol. 2014, 10, 3105–3123. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical Applications of Carbon Nanomaterials: Drug and Gene Delivery Potentials. J. Cell. Physiol. 2019, 234, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Shtansky, D.V.; Firestein, K.L.; Golberg, D.V. Fabrication and Application of BN Nanoparticles, Nanosheets and Their Nanohybrids. Nanoscale 2018, 10, 17477–17493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, B.D.; Shawky, J.H.; Dahl, K.N.; Davidson, L.A.; Islam, M.F. Distribution of Single Wall Carbon Nanotubes in the Xenopus laevis Embryo after Microinjection: Distribution of Single Wall Carbon Nanotubes in X. laevis Embryo. J. Appl. Toxicol. 2016, 36, 568–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardani, F.; Mesa, C. Dispersability of Carbon Nanotubes in Biopolymer-Based Fluids. Crystals 2015, 5, 74–90. [Google Scholar] [CrossRef] [Green Version]
- Prato, M.; Kostarelos, K.; Bianco, A. Functionalized Carbon Nanotubes in Drug Design and Discovery. Acc. Chem. Res. 2008, 41, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Muzi, L.; Mouchet, F.; Cadarsi, S.; Janowska, I.; Russier, J.; Ménard-Moyon, C.; Risuleo, G.; Soula, B.; Galibert, A.-M.; Flahaut, E.; et al. Examining the Impact of Multi-Layer Graphene Using Cellular and Amphibian Models. 2D Mater. 2016, 3, 025009. [Google Scholar] [CrossRef] [Green Version]
- Bianco, A. Graphene: Safe or Toxic? The Two Faces of the Medal. Angew. Chem. Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Muzi, L.; Tardani, F.; La Mesa, C.; Bonincontro, A.; Bianco, A.; Risuleo, G. Interactions and Effects of BSA-Functionalized Single-Walled Carbon Nanotubes on Different Cell Lines. Nanotechnology 2016, 27, 155704. [Google Scholar] [CrossRef]
- Zanni, E.; De Bellis, G.; Bracciale, M.P.; Broggi, A.; Santarelli, M.L.; Sarto, M.S.; Palleschi, C.; Uccelletti, D. Graphite Nanoplatelets and Caenorhabditis Elegans: Insights from an in Vivo Model. Nano Lett. 2012, 12, 2740–2744. [Google Scholar] [CrossRef]
- Guo, X.; Dong, S.; Petersen, E.J.; Gao, S.; Huang, Q.; Mao, L. Biological Uptake and Depuration of Radio-Labeled Graphene by Daphnia magna. Environ. Sci. Technol. 2013, 47, 12524–12531. [Google Scholar] [CrossRef]
- Pretti, C.; Oliva, M.; Pietro, R.D.; Monni, G.; Cevasco, G.; Chiellini, F.; Pomelli, C.; Chiappe, C. Ecotoxicity of Pristine Graphene to Marine Organisms. Ecotoxicol. Environ. Saf. 2014, 101, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Mouchet, F.; Landois, P.; Datsyuk, V.; Puech, P.; Pinelli, E.; Flahaut, E.; Gauthier, L. International Amphibian Micronucleus Standardized Procedure (ISO 21427-1) for in Vivo Evaluation of Double-Walled Carbon Nanotubes Toxicity and Genotoxicity in Water. Environ. Toxicol. 2011, 26, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Pilaquinga, F.; Morey, J.; Torres, M.; Seqqat, R.; Piña, M.L.N. Silver nanoparticles as a potential treatment against SARS-CoV-2: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2021, 13, e1707. [Google Scholar] [CrossRef] [PubMed]
- Statistics and Research, Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus (accessed on 10 January 2022).
- Bamal, D.; Singh, A.; Chaudhary, G.; Kumar, M.; Singh, M.; Rani, N.; Mundlia, P.; Sehrawat, A.R. Silver Nanoparticles Biosynthesis, Characterization, Antimicrobial Activities, Applications, Cytotoxicity and Safety Issues: An Updated Review. Nanomaterials 2021, 11, 2086. [Google Scholar] [CrossRef] [PubMed]
- Heynick, F. The Original ‘Magic Bullet’ Is 100 Years Old. Br. J. Psychiatry 2009, 195, 456. [Google Scholar] [CrossRef] [PubMed]
| Plant Species | Size (nm) | Concentration | Effects | Ref. |
|---|---|---|---|---|
| Caralluma tuberculata | 40 nm | 30, 60, 90 µg/L | Callus biomass ↑, TPC, and TFC content ↑ | [8] |
| Saccharum spp. (Sugarcane) | 35 ± 15 nm | 25, 50, 100, 200 mg/L | shoot number, shoot length, phenolic compound ↑ | [43] |
| Capsicum spp. | - | 50 ppm | Capsaicin content ↑ | [55] |
| Stevia rebaudiana | 35 ± 15 nm | 25, 50, 100, 200 mg/L | Shoot growth and proliferation | [29] |
| Momordica charantia | 2–12 nm | 0, 1, 5, 10 mg/L | Flavonoid and phenolic ↑, anti-microbials, and bioactivity level ↑ | [21] |
| Prunella vulgaris | 25–35 nm | 30 µg/L (AgNPs/AuNPs) | Increased callus proliferation to 100%, TPC and TFC ↑ | [46,56] |
| Lavandula angustifolia Mill. | 27.5 ± 4.8 nm | 1, 2, 5, 10, 20 and 50 mg/dm3 | Shoot multiplication and oil gland ↑ | [22] |
| Arabidopsis thaliana | 10, 40, 100 nm | 0.5, 1, 5 mg/L | camalexin accumulation ↑ | [57] |
| Oryza sativa cv. IR64 | 1–50 nm | 0, 5, 10, 15, 20 mg/L | Callus induction ↑ | [47] |
| Nicotiana tabacum | 20–140 nm | 0.02 mg/L | Rooting ↑ | [58] |
| Chrysanthemum morifolium | <20 nm | 0.5, 1, 1.5, 2, 3, 5, 7, 10 ppm | Improved number of shoots, plant heigh, and biomass | [48] |
| Tecomella undulata | - | 0, 30, 60, 120 µg/L | 1-aminocyclopropane-1-carboxylic acid synthase (ACS) ↓, growth ↑ | [59] |
| Oryza sativa L. cv. Swarna | 18.16 nm | 0, 10, 20, 40 ppm | shoot length ↑ (1.2 folds), dry weight, chlorophyll content, enzyme related to cell wall protection (CAT, APX, and GR) ↑, CuZnSOD gene ↓. | [52] |
| Eruca sativa | 14 ± 0.3 nm | 0, 0.1, 1, 10, 20, 100 mg/L | ↑ root elongation | [60] |
| Musa spp. | 25–30 nm | 1, 3, 5, 7 ppm | ↑ shoot numbers, shoot length, number of leaves, total chlorophyll content, and fresh/dry weight. | [61] |
| Hylocereus undatus (Haw.) | - | 0, 0.5, 1, 2, 4 and 8 mg/L | Longer roots were observed in concentration of 8 mg/L | [62] |
| Vanilla planifolia | 35 ± 15 nm | 0, 25, 50, 100, 200 mg/L | Microbial contamination ↓, shoot length, biomass, and chlorophyll ↑ | [63] |
| Cucumis anguira | - | 0.5, 1, 2 mg/L | Hairy root biomass, TFC, and TPC ↑ | [64] |
| Isatis constricta | - | 0, 0.25, 0.5, 1, 1.5 and 2 mg/L | Indigo and tryptanthrin production ↑ | [65] |
| Echinacea purpurea | 35 nm | 0, 2 and 4 mg/L | Chicoric acid ↑ | [66] |
| Calendula officinalis | 30–50 nm | 0.4, 0.8, 1.2 mM | Increased saponin content (177% in 0.4 mM AgNPs + 100 µM Metil jasmonate compared to control) | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmawati, M.; Mahfud, C.; Risuleo, G.; Jadid, N. Nanotechnology in Plant Metabolite Improvement and in Animal Welfare. Appl. Sci. 2022, 12, 838. https://doi.org/10.3390/app12020838
Rahmawati M, Mahfud C, Risuleo G, Jadid N. Nanotechnology in Plant Metabolite Improvement and in Animal Welfare. Applied Sciences. 2022; 12(2):838. https://doi.org/10.3390/app12020838
Chicago/Turabian StyleRahmawati, Maulidia, Choirul Mahfud, Gianfranco Risuleo, and Nurul Jadid. 2022. "Nanotechnology in Plant Metabolite Improvement and in Animal Welfare" Applied Sciences 12, no. 2: 838. https://doi.org/10.3390/app12020838






