Boron Nitride Nanotubes for Curcumin Delivery as an Anticancer Drug: A DFT Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model System
2.2. Computational Details
2.3. Loading Efficiency of Curcumin Molecules
3. Results and Discussion
3.1. Molecular Geometry and Adsorption Energy
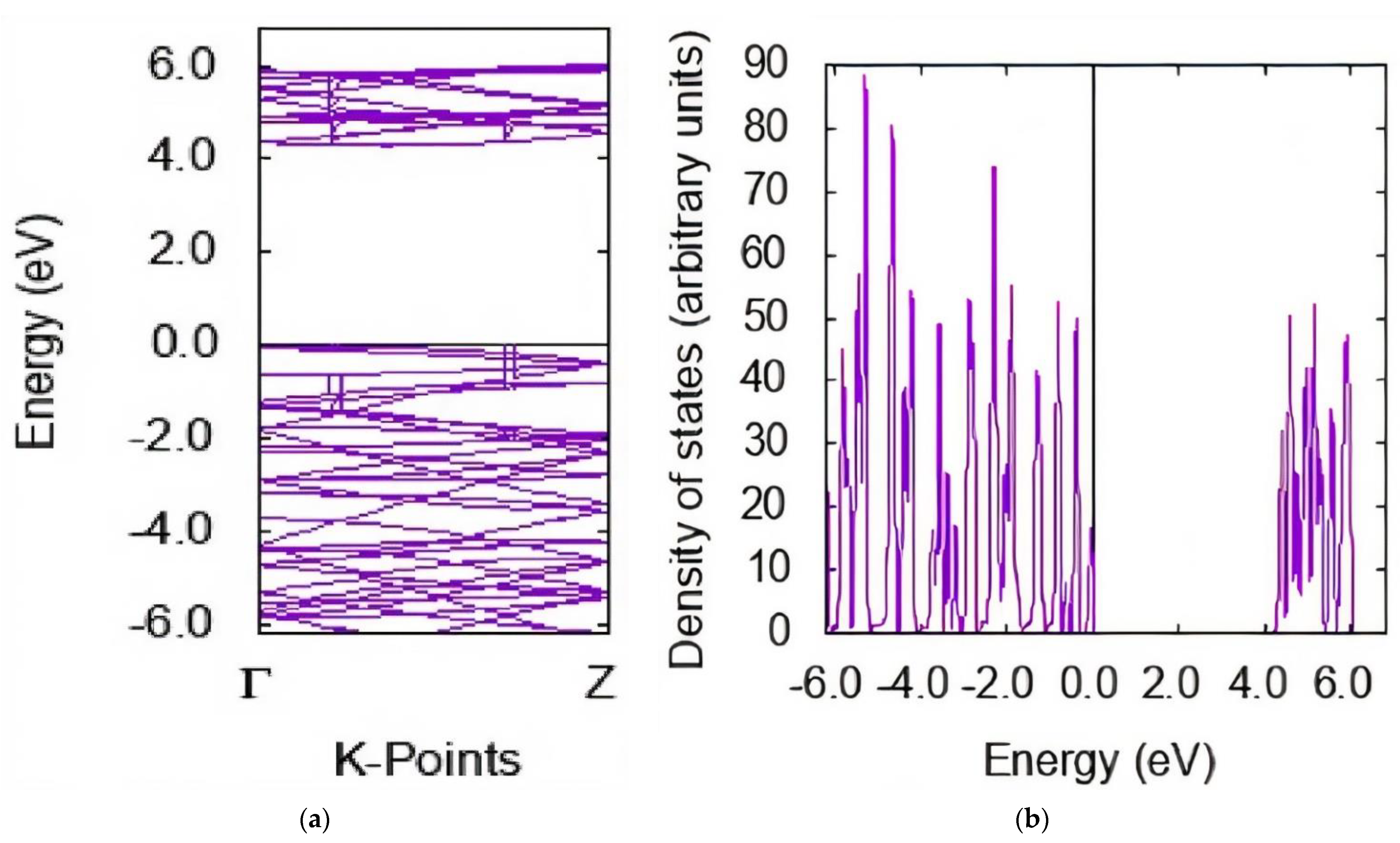
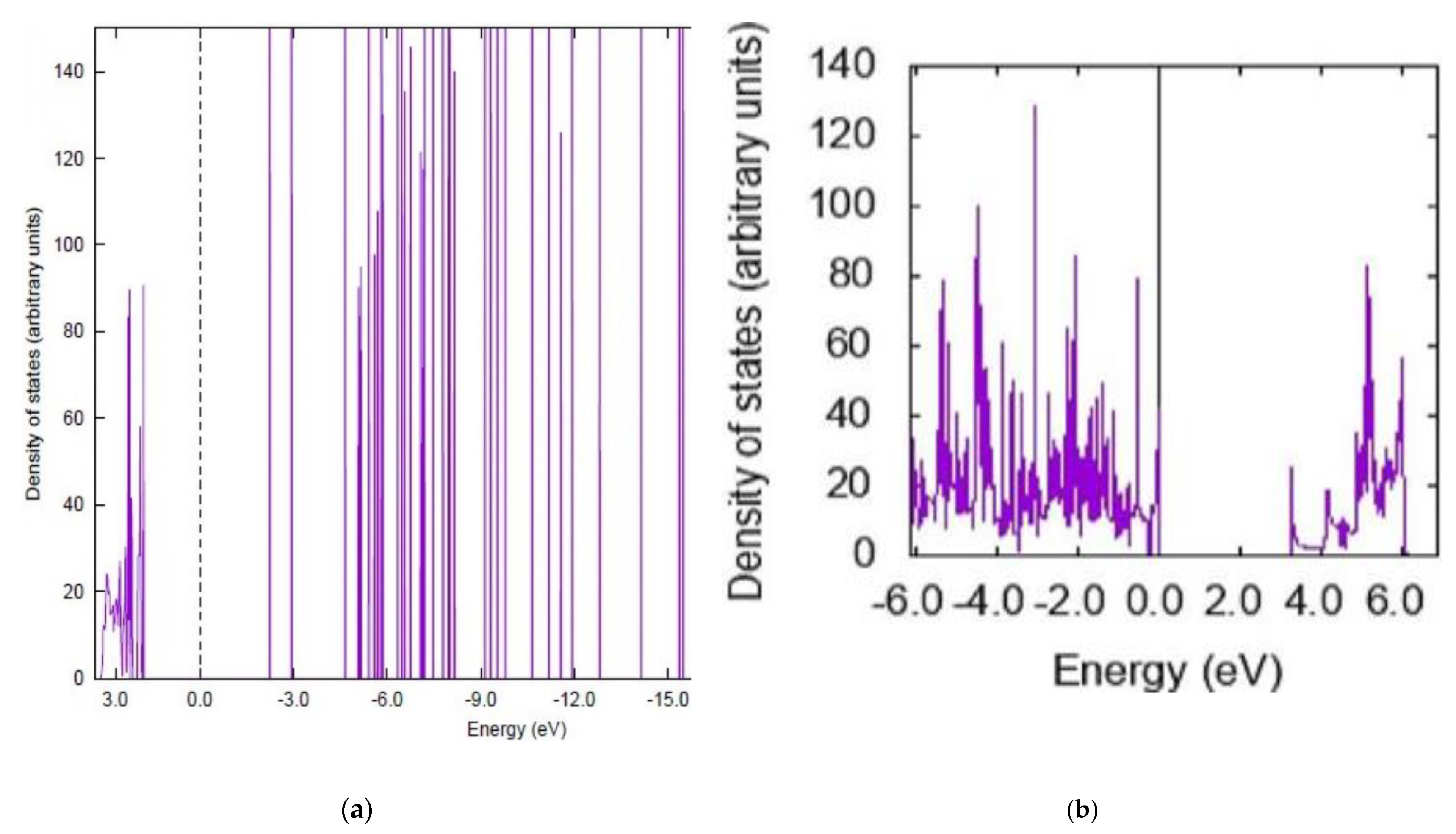
3.2. Electronic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostoglou, N.; Tampaxis, C.; Charalambopoulou, G.; Constantinides, G.; Ryzhkov, V.; Doumanidis, C.; Matovic, B.; Mitterer, C.; Rebholz, C. Boron nitride nanotubes versus carbon nanotubes: A thermal stability and oxidation behavior study. Nanomaterials 2020, 10, 2435. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, N. Joining between Boron Nitride Nanocones and Nanotubes. Adv. Math. Phys. 2020, 2020. [Google Scholar] [CrossRef]
- Yanar, N.; Yang, E.; Park, H.; Son, M.; Choi, H. Boron nitride nanotube (BNNT) membranes for energy and environmental applications. Membranes 2020, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Guan, J.; Lu, P.; Mihailov, S.J.; Kingston, C.T.; Simard, B. Boron nitride nanotubes for optical fiber chemical sensing applications. IEEE Sens. Lett. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Panigrahi, P.; Kumar, A.; Bae, H.; Lee, H.; Ahuja, R.; Hussain, T. Capacity enhancement of polylithiated functionalized boron nitride nanotubes: An efficient hydrogen storage medium. Phys. Chem. Chem. Phys. 2020, 22, 15675–15682. [Google Scholar] [CrossRef] [PubMed]
- Dymova, M.A.; Taskaev, S.Y.; Richter, V.A.; Kuligina, E.V. Boron neutron capture therapy: Current status and future perspectives. Cancer Commun. 2020, 40, 406–421. [Google Scholar] [CrossRef] [PubMed]
- Khalili, N.P.; Moradi, R.; Kavehpour, P.; Islamzada, F. Boron nitride nanotube clusters and their hybrid nanofibers with polycaprolacton: Thermo-pH sensitive drug delivery functional materials. Eur. Polym. J. Vol. 2020, 127, 109585. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.J.; Ahn, S.; Koh, B. Purification of boron nitride nanotubes enhances biological application properties. Int. J. Mol. Sci. 2020, 21, 1529. [Google Scholar] [CrossRef] [Green Version]
- Joy, J.; George, E.; Haritha, P.; Thomas, S.; Anas, S. An overview of boron nitride based polymer nanocomposites. J. Polym. Sci. 2020, 55, 3115–3141. [Google Scholar] [CrossRef]
- Maurya, M.; Sappidi, P.K.; Singh, J.K. Selective Separation of CO2 from Flue Gas Using Carbon and Boron Nitride Nanotubes as a Membrane. Energy Fuels 2020, 34, 7223–7231. [Google Scholar] [CrossRef]
- Mohsennia, M.; Rakhshi, M.; Rasa, H. A computational study on interactions of Ni- and Pt-doped boron nitride nano tubes with NH3 in presence and absence of electric fields. Comput. Theor. Chem. 2018, 1136, 1–9. [Google Scholar] [CrossRef]
- Marko, Š.; Snežana, M.; Marijana K., I.; Jasmina, N.; Malcolm, W.; Zoltan, K.; Jelena, T. Comparing the adsorption performance of multiwalled carbon nanotubes oxidized by varying degrees for removal of low levels of copper, nickel and chromium(VI) from aqueous solutions. Water 2020, 12, 723. [Google Scholar] [CrossRef] [Green Version]
- Zarghami Dehaghani, M.; Yousefi, F.; Sajadi, S.M.; Tajammal Munir, M.; Abida, O.; Habibzadeh, S.; Mashhadzadeh, A.; Rabiee, N. Mostafavi, E.; Saeb, M.R. Theoretical encapsulation of fluorouracil (5-fu) anti-cancer chemotherapy drug into carbon nanotubes (cnt) and boron nitride nanotubes (bnnt). Molecules 2021, 26, 4920. [Google Scholar] [CrossRef]
- Jha, R.; Singh, A.; Sharma, P.K.; Fuloria, N.K. Smart carbon nanotubes for drug delivery system: A comprehensive study. J. Drug Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- Sohrabi, N.; Alihosseini, A.; Pirouzfar, V.; Pedram, M.Z. Analysis of dynamics targeting CNT-based drug delivery through lung cancer cells: Design, simulation, and computational approach. Membranes 2020, 10, 283. [Google Scholar] [CrossRef]
- Pham, D.T.; Tiyaboonchai, W. Fibroin nanoparticles: A promising drug delivery system. Drug Deliv. 2020, 27, 431–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghahremani, S.; Samadizadeh, M.; Khaleghian, M.; Shiraz, N.Z. Theoretical study of encapsulation of Floxuridine anticancer drug into BN (9,9-7) nanotube for medical application. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 293–306. [Google Scholar] [CrossRef]
- Genchi, G.G.; Rocca, A.; Grillone, A.; Marino, A.; Ciofani, G. Boron nitride nanotubes in nanomedicine: Historical and future perspectives. In Boron Nitride Nanotubes in Nanomedicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 201–217. [Google Scholar]
- Rathore, S.; Mukim, M.; Sharma, P.; Devi, S.; Nagar, J.C.; Khalid, M. Curcumin: A Review for Health Benefits. Int. J. Res. Rev. 2020, 7, 273–290. [Google Scholar]
- Reda, F.M.; El-Saadony, M.T.; Elnesr, S.S.; Alagawany, M.; Tufarelli, V. Effect of dietary supplementation of biological curcumin nanoparticles on growth and carcass traits, antioxidant status, immunity and caecal microbiota of Japanese quails. Animals 2020, 10, 754. [Google Scholar] [CrossRef]
- Manoharan, Y.; Haridas, V.; Vasanthakumar, K.C.; Muthu, S.; Thavoorullah, F.F.; Shetty, P. Curcumin: A Wonder Drug as a Preventive Measure for COVID19 Management. Indian J. Clin. Biochem. 2020, 35, 373–375. [Google Scholar] [CrossRef]
- Akram, M.; Riaz, M.; Wadood, A.W.C.; Hazrat, A.; Mukhtiar, M.; Ahmad Zakki, S.; Daniyal, M.; Shariati, M.A.; Said Khan, F.; Zainab, R. Medicinal plants with anti-mutagenic potential. Biotechnol. Biotechnol. Equip. 2020, 34, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Sharifi, S.; Fathi, N.; Memar, M.Y.; Hosseiniyan Khatibi, S.M.; Khalilov, R.; Negahdari, R.; Zununi Vahed, S.; Maleki Dizaj, S. Anti-microbial activity of curcumin nanoformulations: New trends and future perspectives. Phyther. Res. 2020, 34, 1926–1946. [Google Scholar] [CrossRef] [PubMed]
- Olotu, F.; Agoni, C.; Soremekun, O.; Soliman, M.E.S. An Update on the Pharmacological Usage of Curcumin: Has it Failed in the Drug Discovery Pipeline. Cell Biochem. Biophys. 2020, 78, 267–289. [Google Scholar] [CrossRef]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [Green Version]
- Zahedi, H.; Hosseinzadeh-Attar, M.-J.; Sahebkar, A.; Ranjbar, S.H.; Najafi, A.; Hosseini, S.; Qorbani, M.; Ahmadi, A.; Ardehali, S.H.; Moravvej, H.; et al. Therapeutic effects of supplementation with Curcuminoids in critically ill patients receiving enteral nutrition: A randomized controlled trial protocol. J. Diabetes Metab. Disord. 2020, 19, 1609–1614. [Google Scholar] [CrossRef]
- Bresciani, L.; Favari, C.; Calani, L.; Francinelli, V.; Riva, A.; Petrangolini, G.; Allegrini, P.; Mena, P.; Del Rio, D. The effect of formulation of curcuminoids on their metabolism by human colonic microbiota. Molecules 2020, 25, 940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhang, N.; Hao, Y.; Wang, Y.; Jia, S.; Zhang, H.; Zhang, Y.; Zhang, Z. Formulation of curcumin delivery with functionalized single-walled carbon nanotubes: Characteristics and anticancer effects in vitro. Drug Deliv. 2014, 21, 379–387. [Google Scholar] [CrossRef]
- Chen, S.; Li, Q.; McClements, D.J.; Han, Y.; Dai, L.; Mao, L.; Gao, Y. Co-delivery of curcumin and piperine in zein-carrageenan core-shell nanoparticles: Formation, structure, stability and in vitro gastrointestinal digestion. Food Hydrocoll. 2020, 99, 105334. [Google Scholar] [CrossRef]
- Hosseinzadeh, B.; Beni, A.S.; Eskandari, R.; Karami, M.; Khorram, M. Interaction of propylthiouracil, an anti-thyroid drug with boron nitride nanotube: A DFT study. Adsorption 2020, 26, 1385–1396. [Google Scholar] [CrossRef]
- Sukhender; Pravesh, P.; Mohan, L.; Verma, A.S. First principles calculations for electronic, optical and magnetic properties of full heusler compounds. East Eur. J. Phys. 2020, 2020, 111–121. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baseggio, O.; Pietro, B.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; De Gironcoli, S.; Delugas, P.; Ferrari Ruffino, F. Quantum ESPRESSO toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasfi, A.; Awwad, F.; Ayesh, A.I. DNA sequencing via Z-shaped graphene nano ribbon field effect transistor decorated with nanoparticles using first-principle transport simulations. New J. Phys. 2020, 22, 063004. [Google Scholar] [CrossRef]
- Legesse, M.; Rashkeev, S.N.; Saidaoui, H.; el Mellouhi, F.; Ahzi, S.; Alharbi, F.H. Band gap tuning in aluminum doped two-dimensional hexagonal boron nitride. Mater. Chem. Phys. 2020, 250, 123176. [Google Scholar] [CrossRef]
- Shokri, A.; Yazdani, A.; Rahimi, K. Possible bandgap values of graphene-like ZnO in density functional theory corrected by the Hubbard U term and HSE hybrid functional. Mater. Today Commun. 2020, 22, 100756. [Google Scholar] [CrossRef]
- Mojarrab, M.; Ashhadi, M. Tight-binding method for the electronic and optical properties of C and BN nanotubes. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2020, 261, 114671. [Google Scholar] [CrossRef]
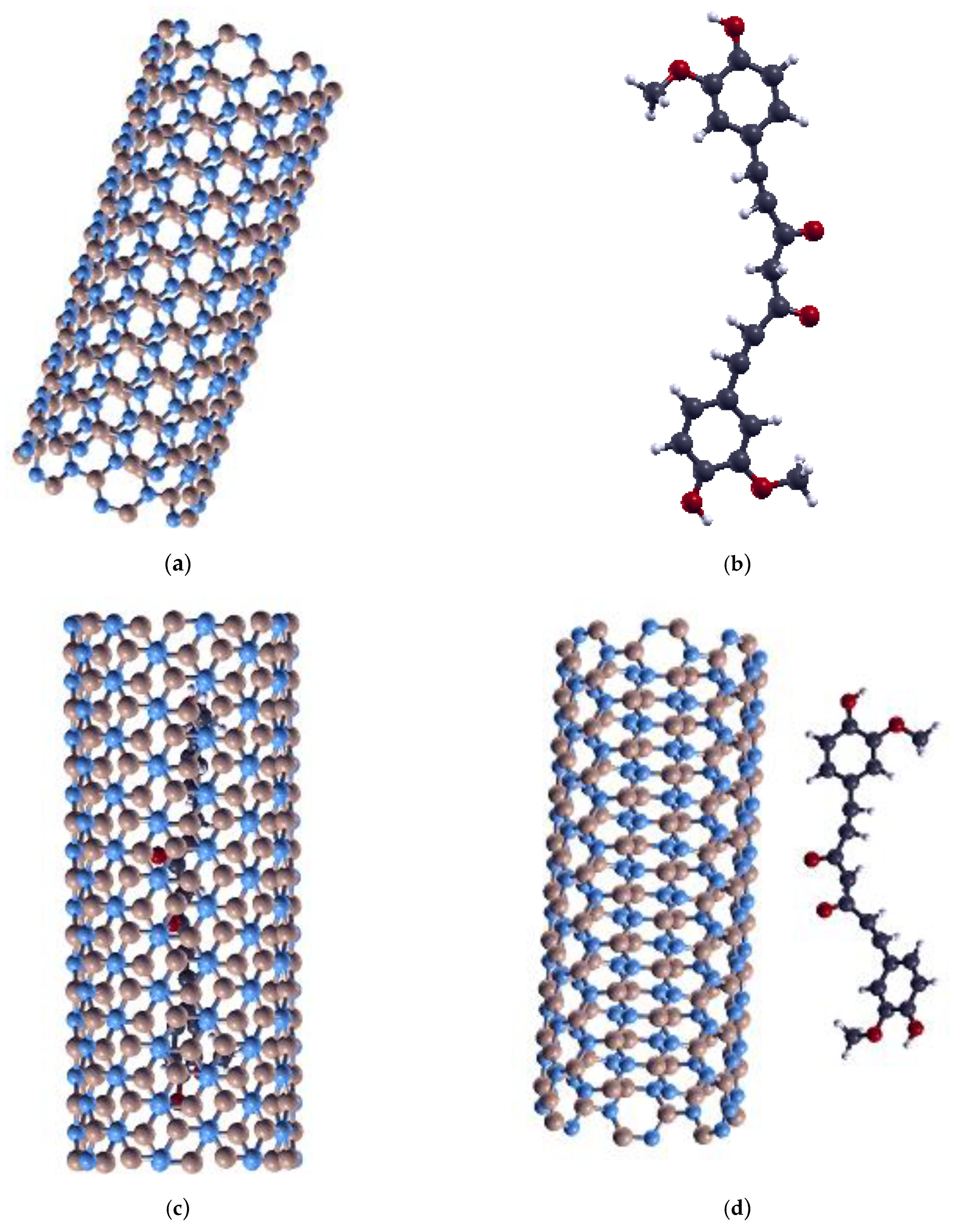
| BNNT Configuration | Diameter (nm) | Bandgap (eV) |
|---|---|---|
| (5,5) | 9.872 | 4.71 |
| Element | Pseudopotential Configuration | Valence Configuration |
|---|---|---|
| N | [He] 2s2 2p3 3d | 2s1 2p2 |
| B | 1s2 2s2 2p1 3d2 | 2s1 2p2 |
| C | [He] 2s2 2p2 3d2 | 2s1 2p2 |
| O | [He] 2s2 2p4 3d2 | 2s1 p2 |
| H | 1s1 2p1 | 1s1 |
| System | BNNT Type | Drug Position | Number of Atoms |
|---|---|---|---|
| (nm) Diameter | B N | ||
| Curcumin-(5,5) BNNT | (5,5) 7.191 | outside | 50 50 |
| Curcumin-(5,5) BNNT | (5,5) 7.191 | inside | 50 50 |
| System | Position | Eads (kcal/mol) LDA + DFT | EHOMO (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|---|---|
| Curcumin | −5.03 | −1.22 | 3.81 | ||
| (5,5) BNNT | −5.68 | −0.96 | 4.71 | ||
| Curcumin-(5,5) BNNT | outside | −15.15 | −4.84 | −1.22 | 3.62 |
| Curcumin-(5,5) BNNT | inside | −16.91 | −4.94 | −1.29 | 3.65 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nafiu, S.; Apalangya, V.A.; Yaya, A.; Sabi, E.B. Boron Nitride Nanotubes for Curcumin Delivery as an Anticancer Drug: A DFT Investigation. Appl. Sci. 2022, 12, 879. https://doi.org/10.3390/app12020879
Nafiu S, Apalangya VA, Yaya A, Sabi EB. Boron Nitride Nanotubes for Curcumin Delivery as an Anticancer Drug: A DFT Investigation. Applied Sciences. 2022; 12(2):879. https://doi.org/10.3390/app12020879
Chicago/Turabian StyleNafiu, Suleiman, Vitus Atanga Apalangya, Abu Yaya, and Edward Benjamin Sabi. 2022. "Boron Nitride Nanotubes for Curcumin Delivery as an Anticancer Drug: A DFT Investigation" Applied Sciences 12, no. 2: 879. https://doi.org/10.3390/app12020879
APA StyleNafiu, S., Apalangya, V. A., Yaya, A., & Sabi, E. B. (2022). Boron Nitride Nanotubes for Curcumin Delivery as an Anticancer Drug: A DFT Investigation. Applied Sciences, 12(2), 879. https://doi.org/10.3390/app12020879







