Impact of Plant Growth Regulators to Development of the Second Generation Energy Crop Miscanthus × giganteus Produced Two Years in Marginal Post-Military Soil
Abstract
:1. Introduction
- −
- To test the impact of three commonly used PGRs applied in the Miscanthus spp. production scheme, i.e., Regoplant, Stimpo, and Charkor, on dry biomass value during two vegetation seasons;
- −
- To analyze the impact and value of three main factors involved in the production cycle: soil, PGRs, and year of cultivation on plant development.
2. Materials and Methods
2.1. Soil Characteristics
2.2. Design of the Pot Experiment and Characteristics of PGRs
2.3. Measuring of Biological Parameters and Dry Biomass Value
2.4. Statistical Analysis
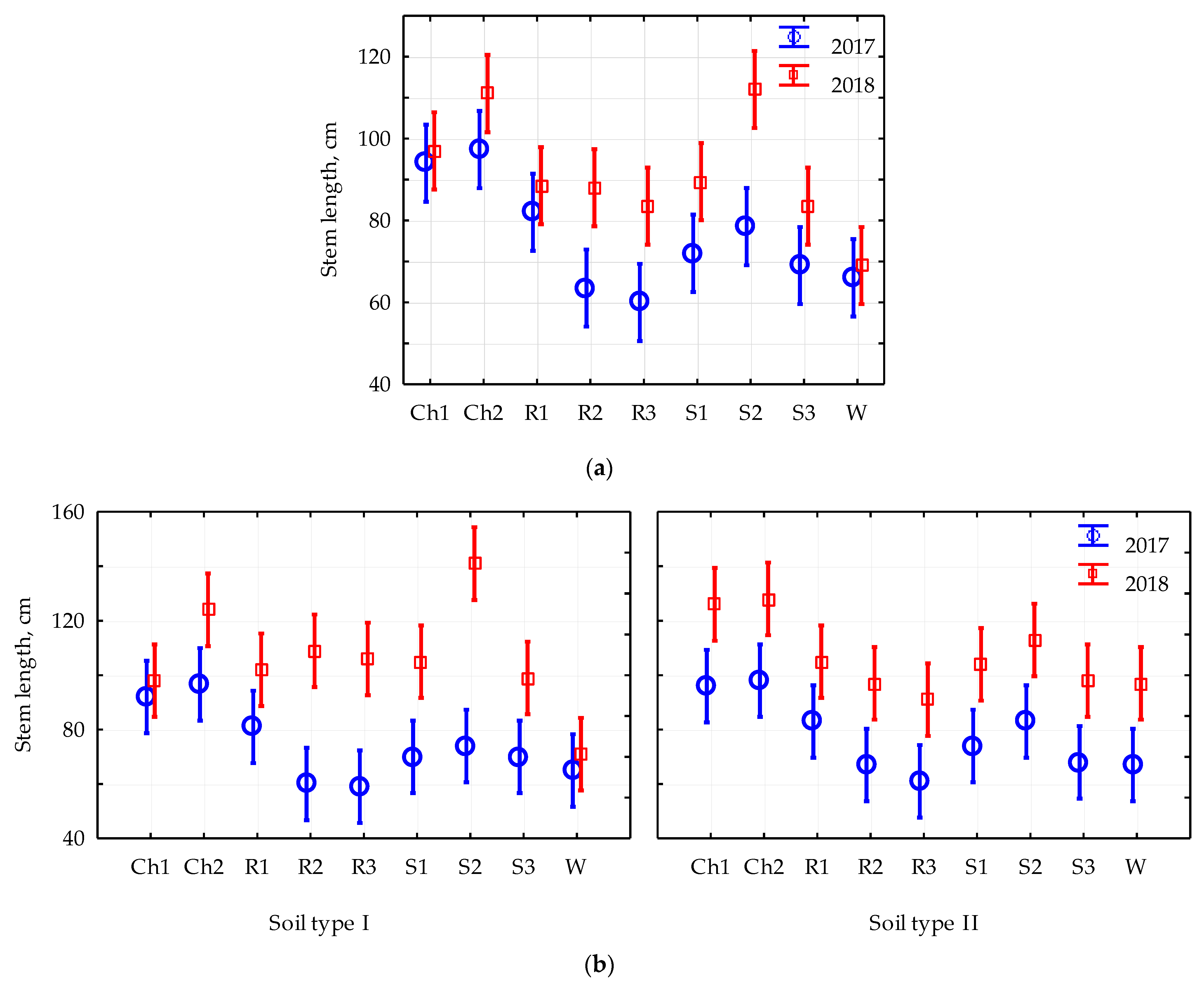
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Bálványos, I.; Kursinszki, L.; Szõke, É. The effect of plant growth regulators on biomass formation and lobeline production of Lobelia inflata L. hairy root cultures. Plant Growth Regul. 2001, 34, 339–345. [Google Scholar] [CrossRef]
- Jeong, S.; Moon, H.S.; Shin, D.; Nam, K. Survival of introduced phosphate-solubilizing bacteria (PSB) and their impact on microbial community structure during the phytoextraction of Cd-contaminated soil. J. Hazard. Mater. 2013, 263, 441–449. [Google Scholar] [CrossRef]
- Kang, Y.; Lee, S.-H.; Lee, J. Development of a selective medium for the fungal pathogen cylindrocarpon destructans using radicicol. Plant Pathol. J. 2014, 30, 432–436. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kaur, M. Plant hormones synthesized by microorganisms and their role in biofertilizer–A review article. Int. J. Adv. Res. 2017, 5, 1753–1766. [Google Scholar] [CrossRef] [Green Version]
- Ponomarenko, S.P.; Terek, O.I.; Grytsaenko, Z.M.; Babayants, O.V.; Moiseeva, T.V.; Wenxiu, H.; Eakin, D. Bioregulation of growth and development of plants: Plant growth regulators in crop science. In Bioregulyatsiya Mikrobnorastitel’nykh System [Bioregulation of Microbial-Plant Systems]; Ponomarenko, S.P., Iutynska, H.O., Eds.; Nichlava: Kyiv, Ukraine, 2010; pp. 251–291. (In Russian) [Google Scholar]
- Tsygankova, V.A.; Stefanovska, T.R.; Galkin, A.P.; Ponomarenko, S.P.; Blume, Y.B. Inducing effect of PGRs on small regulatory si/miRNA in resistance to sugar beet cyst nematode. Commun. Agric. Appl. Biol. Sci. 2012, 77, 779–787. [Google Scholar]
- Tsygankova, V.A.; Andrusevych, Y.V.; Babayants, Y.V.; Ponomarenko, S.P.; Medkow, A.I.; Galkin, A.P. Stimulation of plant immune protection against pathogenic fungi, pests and nematodes with growth regulators. Physiol. Biochem. Cultiv. Plants 2013, 45, 138–147. [Google Scholar]
- Ponomarenko, S.P.; Stefanovska, T.R.; Medkow, A.I.; Kapriy, M. Bioregulators of plant development in growing biofuel crops. In Proceedings of the 17th International Scientific Conference Sakharov Readings: Enviornmental Problems of the XXI Century, II, Minsk, Belarus, 18–19 May 2017; pp. 40–42. [Google Scholar]
- Blyuss, K.B.; Fatehi, F.; Tsygankova, V.A.; Biliavska, L.O.; Iutynska, G.O.; Yemets, A.I.; Blume, Y.B. RNAi-based biocontrol of wheat nematodes using natural poly-component biostimulants. Front. Plant Sci. 2019, 10, 483. [Google Scholar] [CrossRef]
- Kvak, V.; Stefanovska, T.; Pidlisnyuk, V.; Alasmary, Z.; Kharytonov, M. The long-term assessment of Miscanthus × gigantheus cultivation in the forest-steppe zone of Ukraine. INMATEH Agric. Eng. 2018, 54, 113–121. [Google Scholar]
- Roik, M.; Sinchenko, V.; Purkin, V.; Kvak, V.; Humentik, M. Miscanthus in Ukraine; FOP Yamchinskiy Press: Kyiv, Ukraine, 2019; ISBN 978-617-7804-11-5. [Google Scholar]
- Geletukha, G.G.; Zheliezna, T.A.; Tryboi, O.V.; Bashtovyi, A.I. Analysis of criteria for the sustainable development of bioenergy. Ind. Heat Eng. 2016, 38, 47–55. [Google Scholar] [CrossRef]
- Vorobey, V.; Melekh, Y.; Gudz, N. Using of Biomass of the Energy Crops in the Northern Regions of Ukraine. Analytical Research Review; Agency for Economical Development PPV Knowledge Networks: Lviv, Ukraine, 2018. [Google Scholar]
- Pidlisnyuk, V.; Shapoval, P.; Zgorelec, Ž.; Stefanovska, T.; Zhukov, O. Multiyear phytoremediation and dynamic of foliar metal(loid)s concentration during application of Miscanthus × giganteus Greef et Deu to polluted soil from Bakar, Croatia. Environ. Sci. Pollut. Res. 2020, 27, 31446–31457. [Google Scholar] [CrossRef]
- Malinská, H.; Pidlisnyuk, V.; Nebeská, D.; Erol, A.; Medžová, A.; Trögl, J. Physiological response of Miscanthus x giganteus to plant growth regulators in nutritionally poor soil. Plants 2020, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Pidlisnyuk, V.; Herts, A.; Khomenchuk, V.; Mamirova, A.; Kononchuk, O.; Ust’ak, S. Dynamic of morphological and physiological parameters and variation of soil characteristics during Miscanthus × giganteus cultivation in the diesel-contaminated land. Agronomy 2021, 11, 798. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Kiesel, A.; Hastings, A.; Iqbal, Y. Miscanthus. In Perennial Grasses for Bioenergy and Bioproducts; Alexopoulou, E., Ed.; Elsevier Inc. Academic Press: Cambridge, MA, USA, 2018; pp. 35–59. ISBN 978-0-12-812900-5. [Google Scholar]
- Ben Fradj, N.; Rozakis, S.; Borzęcka, M.; Matyka, M. Miscanthus in the European bio-economy: A network analysis. Ind. Crops Prod. 2020, 148, 112281. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.; Pidlisnyuk, V.; Erickson, L.E. Miscanthus biomass for alternative energy production. In Phytotechnology with Biomass Production; Erickson, L.E., Pidlisnyuk, V., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 177–200. ISBN 978-0-367-52280-3. [Google Scholar]
- Jayaraman, K.; Gökalp, I. Pyrolysis, combustion and gasification characteristics of miscanthus and sewage sludge. Energy Convers. Manag. 2015, 89, 83–91. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Newton, R.; Mamirova, A. Miscanthus biochar value chain–A review. J. Environ. Manag. 2021, 290, 112611. [Google Scholar] [CrossRef] [PubMed]
- Kharytonov, M.; Pidlisnyuk, V.; Stefanovska, T.; Babenko, M.; Martynova, N.; Rula, I. The estimation of Miscanthus×giganteus’ adaptive potential for cultivation on the mining and post-mining lands in Ukraine. Environ. Sci. Pollut. Res. 2019, 26, 2974–2986. [Google Scholar] [CrossRef]
- Żołnowski, A.C.; Wyszkowski, M.; Rolka, E.; Sawicka, M. Mineral materials as a neutralizing agent used on soil contaminated with copper. Materials 2021, 14, 6830. [Google Scholar] [CrossRef]
- Fei, H.; Crouse, M.; Papadopoulos, Y.A.; Vessey, J.K. Improving biomass yield of giant Miscanthus by application of beneficial soil microbes and a plant biostimulant. Can. J. Plant Sci. 2020, 100, 29–39. [Google Scholar] [CrossRef]
- Alasmary, Z.; Todd, T.; Hettiarachchi, G.M.; Stefanovska, T.; Pidlisnyuk, V.; Roozeboom, K.; Erickson, L.; Davis, L.; Zhukov, O. Effect of soil treatments and amendments on the nematode community under Miscanthus growing in a lead contaminated military site. Agronomy 2020, 10, 1727. [Google Scholar] [CrossRef]
- Zinchenko, O. Evaluation of the influence of plant growth regulators on theintensity of photosynthesis, survival, morphological parameters of Miscanthus × giganteus. Sci. Work. Inst. Bioenergy Crop. Sugar Beets 2013, 19, 47–51. [Google Scholar]
- Kim, H.S.; Zhang, G.; Juvik, J.A.; Widholm, J.M. Miscanthus×giganteus plant regeneration: Effect of callus types, ages and culture methods on regeneration competence. GCB Bioenergy 2010, 2, 192–200. [Google Scholar] [CrossRef]
- Guo, H.P.; Shao, R.X.; Hong, C.T.; Hu, H.K.; Zheng, B.S.; Zhang, Q.X. Rapid in vitro propagation of bioenergy crop Miscanthus sacchariflorus. Appl. Mech. Mater. 2012, 260–261, 181–186. [Google Scholar] [CrossRef]
- Ślusarkiewicz-Jarzina, A.; Ponitka, A.; Cerazy-Waliszewska, J.; Wojciechowicz, M.K.; Sobańska, K.; Jeżowski, S.; Pniewski, T. Effective and simple in vitro regeneration system of Miscanthus sinensis, M. × giganteus and M. sacchariflorus for planting and biotechnology purposes. Biomass Bioenergy 2017, 107, 219–226. [Google Scholar] [CrossRef]
- Padhye, S.R.; Groninger, J.K. Influence of benzyladenine, trinexapac-ethyl, or uniconazole applications on height and tillering of six ornamental grasses. Horttechnology 2009, 19, 737–742. [Google Scholar] [CrossRef]
- Katelevskij, V.; Gumentyk, M.; Kharytonov, M. Plant growth stimulants influence on Miscanthus × giganteus biomass indexes in forest—Steppe zone of Ukraine. Sci. Pap. Ser. A Agron. 2020, LXIII, 341–345. [Google Scholar]
- Helios, W.; Kotecki, A. Wpływ Terminu I Miejsca Pobierania Sadzonek Oraz Regulatorów Wzrostu Na Zdolność Regeneracyjną Jedno-I Dwuwęzłowych Pędów Miscanthus × Giganteus (Greef Etdeu). Zesz. Nauk. Uniw. Przyr. We Wrocławiu Rol. 2014, 111, 41–50. [Google Scholar]
- Schmidt, C.S.; Mrnka, L.; Frantík, T.; Motyka, V.; Dobrev, P.I.; Vosátka, M. Combined effects of fungal inoculants and the cytokinin-like growth regulator thidiazuron on growth, phytohormone contents and endophytic root fungi in Miscanthus × giganteus. Plant Physiol. Biochem. 2017, 120, 120–131. [Google Scholar] [CrossRef]
- Podan, I.I.; Dzhura, N.M. Influence of oil pollution and humates on growth of miscanthus plants. Ecol. Sci. 2019, 2, 182–186. [Google Scholar] [CrossRef]
- Nebeská, D.; Pidlisnyuk, V.; Stefanovska, T.; Trögl, J.; Shapoval, P.; Popelka, J.; Cerný, J.; Medkow, A.; Kvak, V.; Malinská, H. Impact of plant growth regulators and soil properties on Miscanthus × giganteus biomass parameters and uptake of metals in military soils. Rev. Environ. Health 2019, 34, 283–291. [Google Scholar] [CrossRef]
- Winkler, B.; Mangold, A.; von Cossel, M.; Clifton-Brown, J.; Pogrzeba, M.; Lewandowski, I.; Iqbal, Y.; Kiesel, A. Implementing miscanthus into farming systems: A review of agronomic practices, capital and labour demand. Renew. Sustain. Energy Rev. 2020, 132, 110053. [Google Scholar] [CrossRef]
- Gerwin, W.; Repmann, F.; Galatsidas, S.; Vlachaki, D.; Gounaris, N.; Baumgarten, W.; Volkmann, C.; Keramitzis, D.; Kiourtsis, F.; Freese, D. Assessment and quantification of marginal lands for biomass production in Europe using soil-quality indicators. Soil 2018, 4, 267–290. [Google Scholar] [CrossRef] [Green Version]
- DSTU4287:2004 Soil Quality; Sampling. State Enterprice “Ukrainian Research and Training Center of Standardization. Certification and Quality, UkrNDNC”: Kyiv, Ukraine, 2005; p. 9.
- DSTU:ISO:11464:2007 Soil Quality; Pretreatment of Samples for Physicochemical Analysis. DP “UkrNDNC”: Kyiv, Ukraine, 2007; p. 12.
- DSTU:ISO:10390:2001 Soil Quality; Determination of pH. State Enterprice “Ukrainian Research and Training Center of Standardization. Certification and Quality”, UkrNDNC: Kyiv, Ukraine, 2002; p. 10.
- DSTU:4115:2002 Soils; Determination of Mobile Phosphorus and Potassium Compounds by the Modified Chirikov’s Method. State Enterprice “Ukrainian Research and Training Center of Standardization. Certification and Quality”, UkrNDNC: Kyiv, Ukraine, 2003; p. 12.
- DSTU:7861:2015 Soil Quality; Determination of Exchanges Calcium, Magnesium, Sodium and Potassium in Soil According to Shollenberger in NSC ISSAR Named after Sokolovsky Modification. State Enterprice “Ukrainian Research and Training Center of Standardization. Certification and Quality”, UkrNDNC: Kyiv, Ukraine, 2016; p. 12.
- DSTU:7863-2015 Soil Quality; Determination of Alkalinehydrolysed Nitrogen by the Cornfield Method. State Enterprice “Ukrainian Research and Training Center of Standardization. Certification and Quality”, UkrNDNC: Kyiv, Ukraine, 2015; p. 12.
- DSTU:8347-2015 Soil Quality; Determination of Available Sulphur in Modification of NSC IGA Name Sokolovsky. State Enterprice “Ukrainian Research and Training Center of Standardization. Certification and Quality”, UkrNDNC: Kyiv, Ukraine, 2015; p. 12.
- DSTU:7632-2014 Soils. Method for Determination of Organic Matter; State Enterprice “Ukrainian Research and Training Center of Standardization. Certification and Quality”, UkrNDNC: Kyiv, Ukraine, 2014; p. 15.
- DSTU:4730:2007 Soil Quality; The Soil Granulometric Composition Analysis by Pipette Method in Modification of N.A. Kachinsly. State Enterprice “Ukrainian Research and Training Center of Standardization. Certification and Quality”, UkrNDNC: Kyiv, Ukraine, 2008; p. 14.
- Hodzic, D.; Hodzic, A.; Bajramovic, E. Latin square experiment design in R. IOP Conf. Ser. Mater. Sci. Eng. 2019, 477, 012019. [Google Scholar] [CrossRef]
- GOST 12.1.007:76 Occupational safety standards system; Noxious Substances. Classification and General Safety Requirements. Standart is in Force since 1 January 1977. Interstate Council for Standardization, Metrology and Certification (ISC): Moscow, Russia, 1977.
- Zinchenko, O.; Zinchenko, V.; Ponomarenko, S. Environmental aspects of the cultivation of giant miscanthus, potato and oat. In Phytotechnology woth Biomass Production for Revitalization of Land Contamoinated or Damaged as a Results of the Military Activities; NULES: Kyiv, Ukraine, 2016; pp. 102–105. [Google Scholar]
- Technical conditions of Ukraine 20.2-31168762-005:2012; Plant Grow Regulator “Stimpo”. CSM: Kyiv, Ukraine, 2020; p. 39.
- Technical conditions of Ukraine 20.2-31168762-006:2012; Plant Grow Regulator “Regoplant”. CSM: Kyiv, Ukraine, 2014; p. 39.
- Technical conditions of Ukraine 24.2-03563790-041-2001; Plant Grow Regulator “Charkor”. CSM: Kyiv, Ukraine, 2016; p. 25.
- Certificate No. 21-1652-01-01 2023-01-31; Standard for input Production for Use in Organic Agriculture and Processing (Based on the Equivalent eu Organic Production & Processing Standard for Third Countries). Agrobiotech: Kyiv, Ukraine, 2021.
- Ponomarenko, S.P.; Grizaenko, Z.M.; Babayantz, O.B. Plant Growth Regulators. Recommendations for Utilization; Agrobiotech: Kyiv, Ukraine, 2015. [Google Scholar]
- Statistics; Version 13; Data Analysis Software System; TIBCO Software Inc.: Palo Alto, CA, USA, 2021; Available online: http://Statistica.io (accessed on 1 September 2021).
- Leonard, M.; Pisani-Ferry, J.; Shapiro, J.; Tagliapietra, S.; Wolff, G. The geopolitics of the European Green Deal. Policy Contrib. Bruegel 2021, 4, 1–23. Available online: https://www.bruegel.org/2021/02/the-geopolitics-of-the-european-green-deal (accessed on 10 January 2022). [CrossRef]
- Briens, C.; Piskorz, J.; Berruti, F. Biomass valorization for fuel and chemicals production–A review. Int. J. Chem. React. Eng. 2008, 6. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; van der Linden, G.C.; Schwarz, K.-U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.-L.; Dolstra, O.; Donnison, I.S.; et al. Progress on optimizing miscanthus biomass production for the European bioeconomy: Results of the EU FP7 project OPTIMISC. Front. Plant Sci. 2016, 7, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Alasmary, Z.; Hettiarachchi, G.M.; Roozenboom, K.; Davis, L.A.; Erickson, L.E.; Pidlisnyuk, V.; Stefanovska, T.; Trogl, J. Phytostabilization of a contaminated military site using Miscanthus and soil amendments. J. Environ. Qual. 2021, 50, 1220–1232. [Google Scholar] [CrossRef]
- Mamirova, A.; Pidlisnyuk, V.; Amirbekov, A.; Ševců, A.; Nurzhanova, A. Phytoremediation potential of Miscanthus sinensis And. in organochlorine pesticides contaminated soil amended by Tween 20 and Activated carbon. Environ. Sci. Pollut. Res. 2021, 28, 16092–16106. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J.; Popławska, A. The possibility of using sewage sludge for energy crop cultivation exemplified by reed canary grass and giant miscanthus. Soil Sci. Annu. 2019, 70, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Pranaw, K.; Pidlisnyuk, V.; Trogl, J.; Malinska, H. Bioprospecting of a novel Plant Growth-Promoting Bacterium Bacillus Altitudinis KP-14 for enhancing Miscanthusxgignateus growth in metals contaminated soil. Biology 2020, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Kvak, V. Optimization of Elements of Technology for Production Miscanthus for Energy Fuel in the Western Part of Ukrainian Forest-Step. Ph.D. Thesis, Institute of Bioenergy Crops and Sugar Beets, Kyiv, Ukraine, 24 March 2014. [Google Scholar]
- Tikhonenko, D.; Gorin, M.; Laktionov, M.; Kanivec, V.; Medvedev, V.; Baluk, S.; Buligin, S.; Truskaveckii, R.; Kanash, O.; Degtyar’ov, V. Soil Science; Tikhonenko, D., Ed.; Higher Education: Kyiv, Ukraine, 2005; ISBN 966-8081-37-4. [Google Scholar]
- GOST:12248.6-2020 Interstate Standard. Soils; Methods for Determination of Swelling and Shrinking Characteristics. Interstate Council for Standardization, Metrology and Certification (ISC): Moscow, Russia, 2020; p. 12.
- Jones, L.; Banks, V.; Jefferson, I. Chapter 8 Swelling and shrinking soils. Geol. Soc. Lond. Eng. Geol. Spec. Publ. 2020, 29, 223–242. [Google Scholar] [CrossRef]

| Parameter | Soil I | Soil II | Standard | Method | Apparatus |
|---|---|---|---|---|---|
| pH | 7.1 | 6.1 | DSTU ISO 10390:2001 [41] | pH (KCl) [41] | pH meter pH-150MИ |
| Available P, mg kg−1 | 50 | 20 | DSTU 4115:2002 [42] | Chirikov [42] | ULAB 102UV |
| Available K, mg kg−1 | 58 | 73 | DSTU 4115:2002 [42] | Chirikov [42] | Flame photometerCL-378 (ELICO) |
| Available Ca, mmol-equivalent 100 g−1 | 17.1 | 15.6 | DSTU 7861:2015 [43] | CINAO [43] | Titration method (chemical beaker, magnetic stirrer, pipettes) |
| Available Mg, mmol-equivalent 100 g−1 | 1.5 | 1.9 | DSTU 7861:2015 [43] | CINAO [43] | Titration method (chemical beaker, magnetic stirrer, pipettes) |
| Alkaline hydrolyzed N, mg kg−1 | 120 | 130 | DSTU 7863:2015 [44] | Cornfield [44] | Titration method (chemical beaker, magnetic stirrer, pipettes) |
| Available S, mg kg−1 | 0.2 | 0.1 | DSTU 8347:2015 [45] | Sokolovsky [45] | ULAB 102UV (ULAB) |
| Organic matter, % | 11.2 | 10.9 | DSTU 7632:2014 [46] | Tyurin [46] | ULAB 102UV (ULAB) |
| Soil | Hygroscopic Moisture, % | Soil Fractions (in mm), Content (in %) | Sum of Particles Less than 0.01 mm % | |||||
|---|---|---|---|---|---|---|---|---|
| Physical Sand | Physical Clay (<0.01) | |||||||
| Sand | Silt | Clay | ||||||
| 1.00–0.25 | 0.25–0.05 | 0.05–0.01 | 0.01–0.005 | 0.005–0.001 | <0.001 | |||
| I | 3.52 | 0.94 | 4.55 | 22.24 | 5.56 | 22.93 | 43.78 | 72.28 |
| II | 3.08 | 2.57 | 2.94 | 29.18 | 6.95 | 16.68 | 41.69 | 65.31 |
| PGR Title | Stimpo | Regoplant | Charkor |
|---|---|---|---|
| Standard | TU U 20.2-31168762-005:2012 [51] | TU U 20.2-31168762-006:2012 [52] | TU U 24.2-03563790-041-2001 [53] |
| Content | Natural | Natural | Synthetic |
| Mode of action | Promotes accelerated cell division and the development of a powerful root system, increases the leaf area and chlorophyll content, reduces phytotoxic action of pesticides, enhances host plant tolerance to pathogenic organisms | Promotes accelerated cell division, rhyzogenesis, development of symbiotic microflora in the root system, intensification of photosynthetic activity and development of leaf surface, decrease in phytotoxic action of pesticides, enhances host plant tolerance to pathogenic organisms | Accelerates cell division, rhizogenesis, development of symbiotic microflora in the root system, enhances photosynthetic activity and development of the leaf surface. High-performance stimulant of root formation. Increases plant resistance to abiotic stress |
| Composition | PGR emistim C: emistim C: metabolic product of Cylindrocarpon obtusiusculum—analogues of phytohormones of auxin, cytokinin nature, saturated and unsaturated fatty acids, amino acids, carbohydrates | PGR emistim C: emistim C: metabolic product of Cylindrocarpon obtusiusculum—analogues of phytohormones of auxin, cytokinin nature, saturated and unsaturated fatty acids, amino acids, carbohydrates | PGR emistim C: emistim C: metabolic product of Cylindrocarpon obtusiusculum—analogues of phytohormones of auxin, cytokinin nature, saturated and unsaturated fatty acids, amino acids, carbohydrates |
| Natural complex aversectin C, a metabolic product of actinomycetes Streptomyces avermytilis. Microelements: boric acid, copper sulphuric acid (II) 5-water, ammonium, molybdenum acid, manganese (II) chloride 4-water, potassium iodide | Natural complex aversectin C, a metabolic product of actinomycetes Streptomyces avermytilis. Universal micro fertilizer “Reakom-ZERNO”: Composition of biogenic microelements | 2,6-Dimethylpyridine-N- oxide with a synthetic analogue of phytohormone with 1-naphthyl-acetic acid. Empirical formula C19 H19 NO3. Structural formula:  |
| Factor | Number of Stems, Radj2 = 0.78, F = 15.35, p < 0.001 | Number of Shoots, Radj2 = 0.66, F = 6.87, p < 0.001 | Length of Roots, Radj2 = 0.94, F = 45.46, p < 0.001 | Length of Stems, cm, Radj2 = 0.71, F = 8.49, p < 0.001 | ||||
|---|---|---|---|---|---|---|---|---|
| F-Ratio | p-Level | F-Ratio | p-Level | F-Ratio | p-Level | F-Ratio | p-Level | |
| Year | 254.22 | <0.001 | 79.61 | <0.001 | 479.91 | <0.001 | 48.50 | <0.001 |
| Soil | 46.69 | <0.001 | 1.29 | 0.26 | 31.05 | <0.001 | 34.82 | <0.001 |
| PGR | 4.11 | <0.001 | 14.55 | <0.001 | 102.06 | <0.001 | 13.72 | <0.001 |
| Year × Soil | 48.86 | <0.001 | 0.03 | 0.87 | 13.48 | <0.001 | 54.22 | <0.001 |
| Year × PGR | 3.54 | <0.001 | 2.87 | <0.001 | 29.27 | <0.001 | 2.44 | 0.02 |
| Soil × PGR | 0.44 | 0.89 | 0.93 | 0.49 | 1.19 | 0.32 | 1.62 | 0.14 |
| Year × Soil × PGR | 0.37 | 0.93 | 1.57 | 0.15 | 0.83 | 0.58 | 2.19 | 0.04 |
| Effect | F-Ratio | p-Level |
|---|---|---|
| Year | 0.01 | 0.91 |
| Soil | 0.35 | 0.56 |
| PGR | 4.26 | <0.001 |
| Year × Soil | 0.74 | 0.39 |
| Year × PGR | 2.25 | 0.03 |
| Soil × PGR | 1.58 | 0.15 |
| Year × Soil × PGR | 1.34 | 0.24 |
| Length of stems | 18.44 | <0.001 |
| Length of roots | 0.08 | 0.77 |
| Number of shoots | 0.16 | 0.69 |
| Number of stems | 0.00 | 0.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pidlisnyuk, V.; Stefanovska, T.; Zhukov, O.; Medkow, A.; Shapoval, P.; Stadnik, V.; Sozanskyi, M. Impact of Plant Growth Regulators to Development of the Second Generation Energy Crop Miscanthus × giganteus Produced Two Years in Marginal Post-Military Soil. Appl. Sci. 2022, 12, 881. https://doi.org/10.3390/app12020881
Pidlisnyuk V, Stefanovska T, Zhukov O, Medkow A, Shapoval P, Stadnik V, Sozanskyi M. Impact of Plant Growth Regulators to Development of the Second Generation Energy Crop Miscanthus × giganteus Produced Two Years in Marginal Post-Military Soil. Applied Sciences. 2022; 12(2):881. https://doi.org/10.3390/app12020881
Chicago/Turabian StylePidlisnyuk, Valentina, Tatyana Stefanovska, Olexander Zhukov, Artem Medkow, Pavlo Shapoval, Vitalii Stadnik, and Martyn Sozanskyi. 2022. "Impact of Plant Growth Regulators to Development of the Second Generation Energy Crop Miscanthus × giganteus Produced Two Years in Marginal Post-Military Soil" Applied Sciences 12, no. 2: 881. https://doi.org/10.3390/app12020881