The Effect of Terpenoid Compounds on the Formation of Advanced Glycation Endproducts (AGEs) in Model Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Experimental Model of Protein Glycation
2.3. Non-Specific Markers of Maillard Reaction
2.3.1. Color Development Assay
2.3.2. Assessment of the Maillard Reaction Evolution by Ultraviolet–Visible (UV-Vis) Spectroscopy
2.3.3. Measurement of Fluorescent AGEs Formation
2.4. HPLC Determination of Lysine
2.4.1. Preparation of Solutions
2.4.2. Derivatization Procedure
2.4.3. Liquid Chromatography
2.5. Statistical Analysis and Response Surface Methodology
3. Results
3.1. Non-Specific Markers of Maillard Reaction
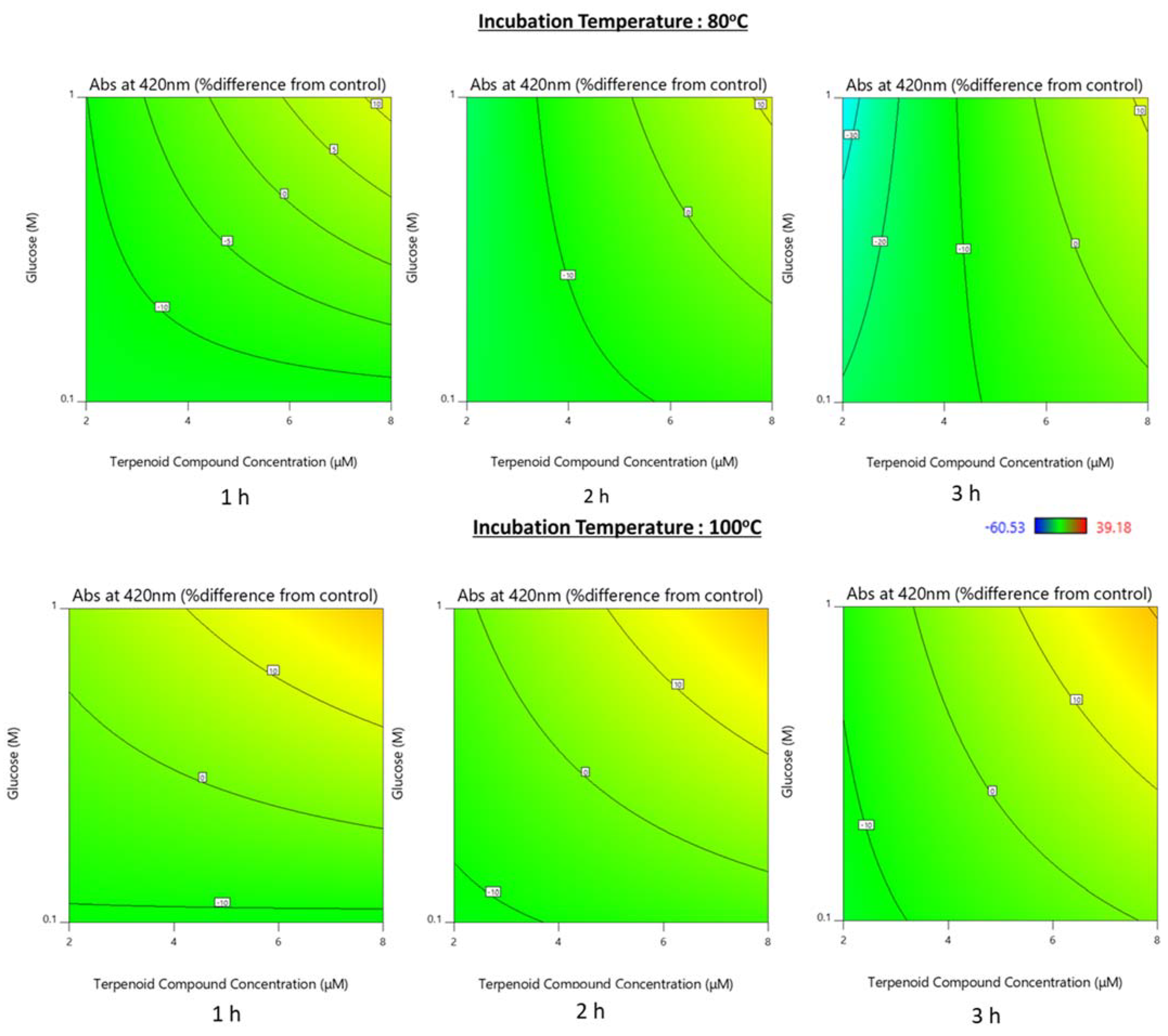
3.1.1. Color Development
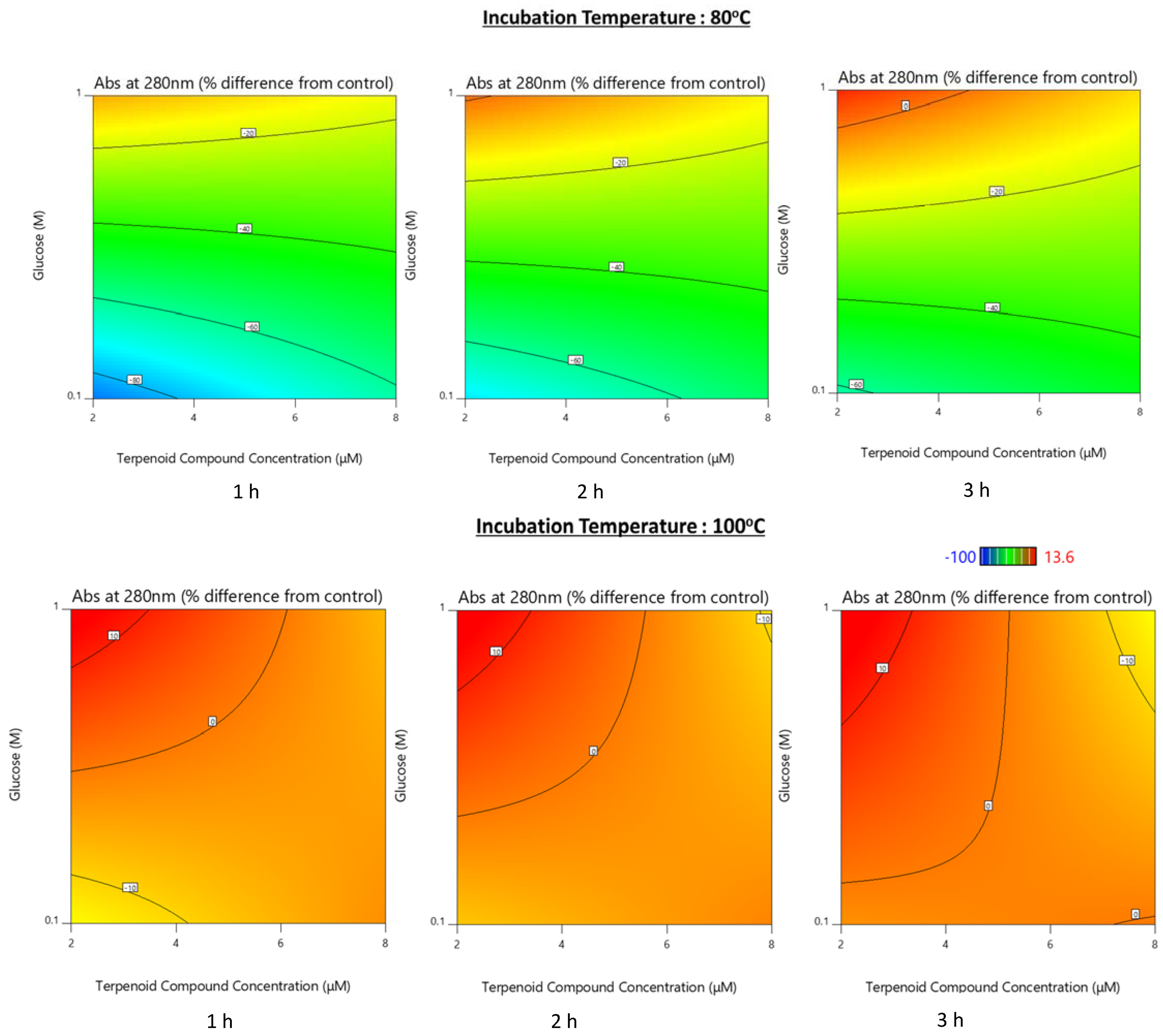
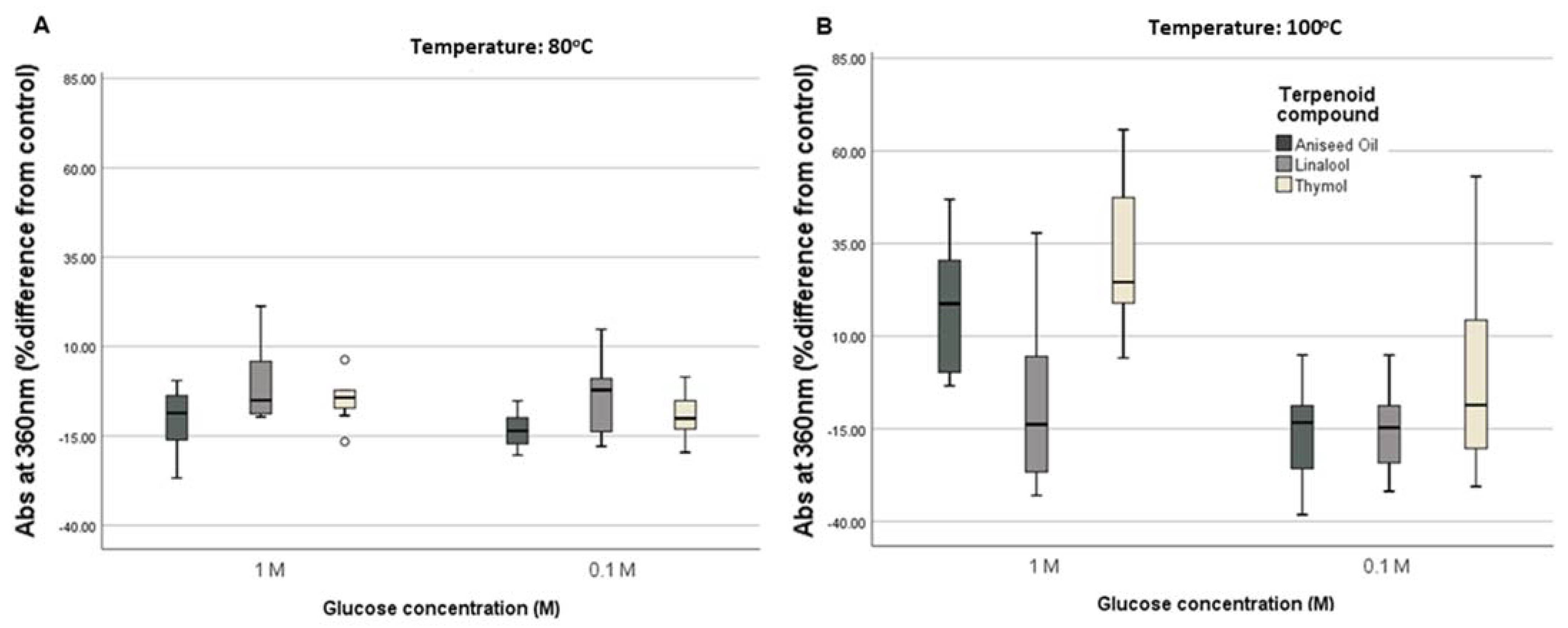
3.1.2. Assessment of the Maillard Reaction Evolution by Ultraviolet–Visible (UV–Vis) Spectroscopy
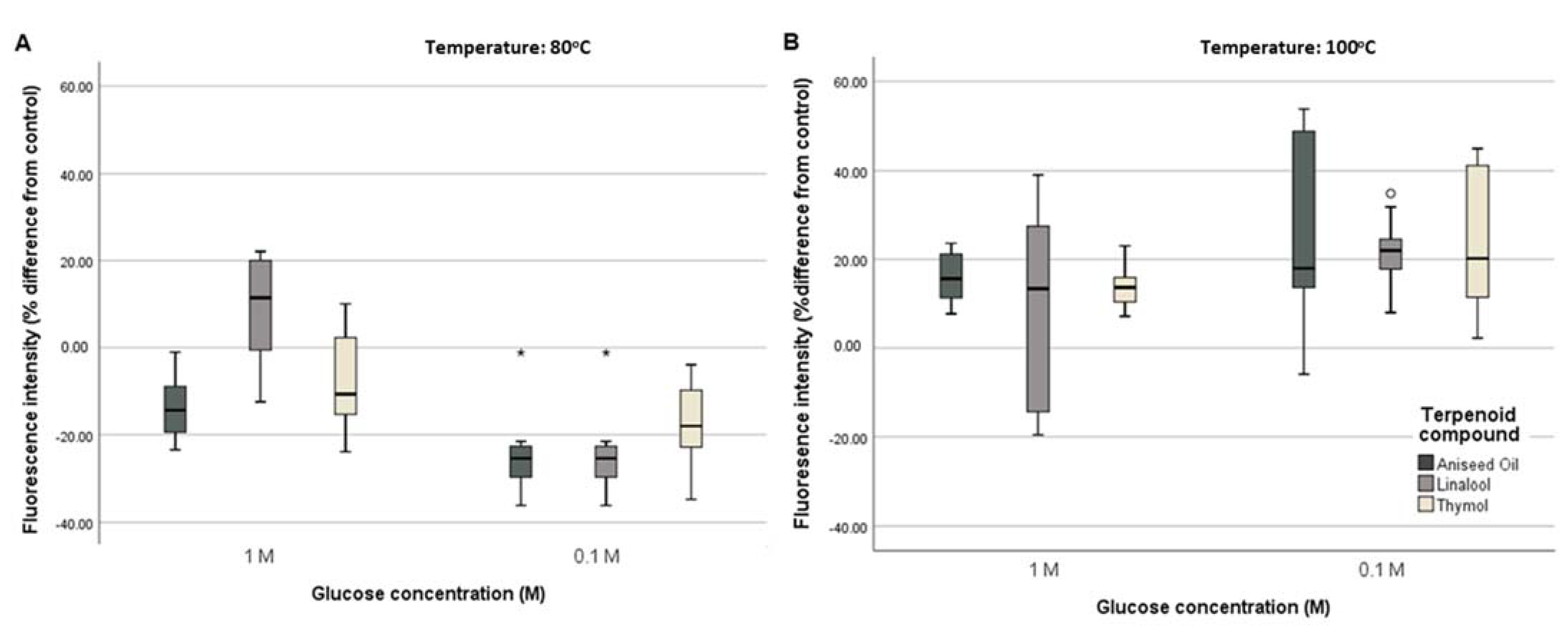
3.1.3. Measurement of Fluorescent AGEs Formation
3.2. Specific Markers of Maillard Reaction—HPLC Determination of Lysine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Naeem, A.; Abbas, T.; Ali, T.M.; Hasnain, A. Essential oils: Brief background and uses. Ann. Short Rep. 2018, 1, 6. [Google Scholar]
- Graßmann, J. Terpenoids as Plant Antioxidants. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 2005; Volume 72, pp. 505–535. ISBN 978-0-12-709872-2. [Google Scholar]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A review of their potential antioxidant activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Ruberto, G.; Baratta, M.T. Antioxidant activity of selected essential oil components in two lipid model systems. Food Chem. 2000, 69, 167–174. [Google Scholar] [CrossRef]
- Navarro, M.; Morales, F.J. In vitro investigation on the antiglycative and carbonyl trapping activities of hydroxytyrosol. Eur. Food Res. Technol. 2016, 242, 1101–1110. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, K.; Schröter, D.; Schreiner, M.; Grune, T. Dietary advanced glycation end products and their relevance for human health. Ageing Res. Rev. 2018, 47, 55–66. [Google Scholar] [CrossRef]
- Peng, X.; Ma, J.; Chen, F.; Wang, M. Naturally occurring inhibitors against the formation of advanced glycation end-products. Food Funct. 2011, 2, 289–301. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Interplay between the maillard reaction and lipid peroxidation in biochemical systems. Ann. N. Y. Acad. Sci. 2005, 1043, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Van Der Fels-Klerx, H.J.; Van Boekel, M.A.J.S. Nϵ-(carboxymethyl)lysine: A review on analytical methods, formation, and occurrence in processed food, and health impact. Food Rev. Int. 2014, 30, 36–52. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, T.; Sun, D.-W. Advanced glycation end-products (AGEs) in foods and their detecting techniques and methods: A review. Trends Food Sci. Technol. 2018, 82, 32–45. [Google Scholar] [CrossRef]
- Delgado-Andrade, C. Carboxymethyl-lysine: Thirty years of investigation in the field of AGE formation. Food Funct. 2016, 7, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Liu, H.; Wang, J.; Sun, B. Inhibitory effect of phenolic compounds and plant extracts on the formation of advance glycation end products: A comprehensive review. Food Res. Int. 2020, 130, 108933. [Google Scholar] [CrossRef]
- Uribarri, J.; Del Castillo, M.D.; De La Maza, M.P.; Filip, R.; Gugliucci, A.; Luevano-Contreras, C.; Macías-Cervantes, M.H.; Markowicz Bastos, D.H.M.; Medrano, A.; Menini, T.; et al. Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 2015, 6, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Meeprom, A.; Sompong, W.; Chan, C.B.; Adisakwattana, S. Isoferulic Acid, a new anti-glycation agent, inhibits fructose- and glucose-mediated protein glycation in vitro. Molecules 2013, 18, 6439–6454. [Google Scholar] [CrossRef] [Green Version]
- Sadowska-Bartosz, I.; Bartosz, G. Prevention of protein glycation by natural compounds. Molecules 2015, 20, 3309–3334. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-Anun, S. Cinnamic acid and its derivatives inhibit fructose-mediated protein glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, X.; Zhang, D.; Wang, J.; Wang, S.; Sun, B. Effects of highland barley bran extract rich in phenolic acids on the formation of Nε-Carboxymethyllysine in a biscuit model. J. Agric. Food Chem. 2018, 66, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Zhang, D.; Liu, J.; Wang, J.; Wang, S.; Sun, B. Baijiu vinasse extract scavenges glyoxal and inhibits the formation of Nε-Carboxymethyllysine in dairy food. Molecules 2019, 24, 1526. [Google Scholar] [CrossRef] [Green Version]
- Sadowska-Bartosz, I.; Galiniak, S.; Bartosz, G. Kinetics of Glycoxidation of Bovine Serum Albumin by Glucose, Fructose and Ribose and Its Prevention by Food Components. Molecules 2014, 19, 18828–18849. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Ma, H.; Frost, L.; Yuan, T.; Dain, J.A.; Seeram, N.P. Pomegranate phenolics inhibit formation of advanced glycation endproducts by scavenging reactive carbonyl species. Food Funct. 2014, 5, 2996–3004. [Google Scholar] [CrossRef]
- Ho, S.-C.; Chang, P.-W.; Tong, H.-T.; Yu, P.-Y. Inhibition of fluorescent advanced glycation end-products and N-Carboxymethyllysine formation by several floral herbal infusions. Int. J. Food Prop. 2014, 17, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Navarro, M.; Morales, F.J. Mechanism of reactive carbonyl species trapping by hydroxytyrosol under simulated physiological conditions. Food Chem. 2015, 175, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Joglekar, M.M.; Panaskar, S.N.; Chougale, A.D.; Kulkarni, M.J.; Arvindekar, A.U. A novel mechanism for antiglycative action of limonene through stabilization of protein conformation. Mol. BioSyst. 2013, 9, 2463–2472. [Google Scholar] [CrossRef] [PubMed]
- Chawla, H.M.; Sahu, S.N. Effect of spice essential oils on maillard browning model reaction of glucose and glycine: An uv-visible and reverse phase hplc analysis. J. Food Sci. Technol. 2007, 44, 602–606. [Google Scholar]
- Kamatou, G.P.P.; Viljoen, A.M. Linalool–a review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1934578X0800300727. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wu, C.-J.; Yu, S.-J. Impact of microwave-assisted heating on the ph value, color, and flavor compounds in glucose-ammonium model system. Food Bioprocess Technol. 2018, 11, 1248–1258. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Seiquer, I.; Haro, A.; Castellano, R.; Navarro, M.P. Development of the maillard reaction in foods cooked by different techniques. Intake of maillard-derived compounds. Food Chem. 2010, 122, 145–153. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Ren, S.; Zhang, Z.; Wang, Y.; Song, R. Optimization of a precolumn OPA derivatization HPLC assay for monitoring of l-Asparagine depletion in serum during l-Asparaginase therapy. J. Chromatogr. Sci. 2018, 56, 794–801. [Google Scholar] [CrossRef] [Green Version]
- KNAUER Determination of Amino Acids by UHPLC with Automated OPADerivatization by the Autosampler. Available online: https://www.knauer.net/Application/application_notes/vbs0029n_opa_amino_acids_uhplc_1310.pdf (accessed on 9 November 2021).
- Phenomenex HPLC Application #23092: Amino Acid Analysis Using OPA on Kinetex 5u EVO C18 150 × 4.6 mm. Available online: https://www.phenomenex.com/Application/Detail/23092 (accessed on 9 November 2021).
- Pukelsheim, F. Optimal Design of Experiments; SIAM: New York, NY, USA, 2006; ISBN 0-89871-604-7. [Google Scholar]
- Vlassopoulos, A.; Lean, M.E.J.; Combet, E. Protein–phenolic interactions and inhibition of glycation–combining a systematic review and experimental models for enhanced physiological relevance. Food Funct. 2014, 5, 2646–2655. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, N.; Fogliano, V. Cooking, industrial processing and caloric density of foods. Curr. Opin. Food Sci. 2017, 14, 98–102. [Google Scholar] [CrossRef]






| Run | A: Time | B: Terpenoid Compound Concentration | C: Glucose Concentration | D: Temperature | E: Terpenoid Compound |
|---|---|---|---|---|---|
| Units | Hr | μΜ | M | °C | - |
| 1 | 2 | 4 | 0.1 | 100 | Linalool |
| 2 | 3 | 4 | 1 | 100 | Linalool |
| 3 | 2 | 2 | 1 | 80 | Linalool |
| 4 | 2 | 8 | 0.1 | 100 | Linalool |
| 5 | 2 | 6 | 0.1 | 80 | Thymol |
| 6 | 1 | 8 | 0.1 | 100 | Thymol |
| 7 | 3 | 8 | 1 | 100 | Aniseed Oil |
| 8 | 1 | 2 | 1 | 80 | Linalool |
| 9 | 2 | 6 | 1 | 100 | Thymol |
| 10 | 2 | 6 | 1 | 100 | Thymol |
| 11 | 3 | 4 | 0.1 | 80 | Linalool |
| 12 | 3 | 8 | 0.1 | 80 | Aniseed Oil |
| 13 | 1 | 6 | 0.1 | 80 | Linalool |
| 14 | 2 | 6 | 0.1 | 80 | Thymol |
| 15 | 3 | 6 | 0.1 | 100 | Thymol |
| 16 | 1 | 8 | 0.1 | 80 | Thymol |
| 17 | 1 | 6 | 1 | 100 | Linalool |
| 18 | 1 | 4 | 1 | 80 | Thymol |
| 19 | 3 | 2 | 0.1 | 100 | Aniseed Oil |
| 20 | 1 | 6 | 1 | 80 | Linalool |
| 21 | 2 | 2 | 0.1 | 100 | Thymol |
| 22 | 2 | 6 | 0.1 | 100 | Aniseed Oil |
| 23 | 2 | 2 | 0.1 | 100 | Thymol |
| 24 | 1 | 2 | 0.1 | 80 | Aniseed Oil |
| 25 | 3 | 2 | 1 | 80 | Thymol |
| 26 | 3 | 4 | 1 | 100 | Linalool |
| 27 | 3 | 4 | 1 | 80 | Aniseed Oil |
| 28 | 1 | 2 | 0.1 | 100 | Linalool |
| 29 | 1 | 8 | 1 | 80 | Aniseed Oil |
| 30 | 2 | 6 | 0.1 | 100 | Aniseed Oil |
| 31 | 2 | 6 | 1 | 80 | Aniseed Oil |
| 32 | 1 | 2 | 1 | 100 | Aniseed Oil |
| 33 | 3 | 8 | 1 | 80 | Linalool |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlassopoulos, A.; Mikrou, T.; Papantoni, A.; Papadopoulos, G.; Kapsokefalou, M.; Mallouchos, A.; Gardeli, C. The Effect of Terpenoid Compounds on the Formation of Advanced Glycation Endproducts (AGEs) in Model Systems. Appl. Sci. 2022, 12, 908. https://doi.org/10.3390/app12020908
Vlassopoulos A, Mikrou T, Papantoni A, Papadopoulos G, Kapsokefalou M, Mallouchos A, Gardeli C. The Effect of Terpenoid Compounds on the Formation of Advanced Glycation Endproducts (AGEs) in Model Systems. Applied Sciences. 2022; 12(2):908. https://doi.org/10.3390/app12020908
Chicago/Turabian StyleVlassopoulos, Antonis, Theano Mikrou, Artemis Papantoni, Georgios Papadopoulos, Maria Kapsokefalou, Athanasios Mallouchos, and Chrysavgi Gardeli. 2022. "The Effect of Terpenoid Compounds on the Formation of Advanced Glycation Endproducts (AGEs) in Model Systems" Applied Sciences 12, no. 2: 908. https://doi.org/10.3390/app12020908
APA StyleVlassopoulos, A., Mikrou, T., Papantoni, A., Papadopoulos, G., Kapsokefalou, M., Mallouchos, A., & Gardeli, C. (2022). The Effect of Terpenoid Compounds on the Formation of Advanced Glycation Endproducts (AGEs) in Model Systems. Applied Sciences, 12(2), 908. https://doi.org/10.3390/app12020908






