Structural Characterization and Cardioprotective Effect of Water-Soluble Polysaccharides Extracted from Clematis flammula
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Extraction and Purification of CFPS Fraction from the Aerial Part of C. Flammula
2.3. Preliminary Study of CFPS
2.3.1. Biochemical Composition
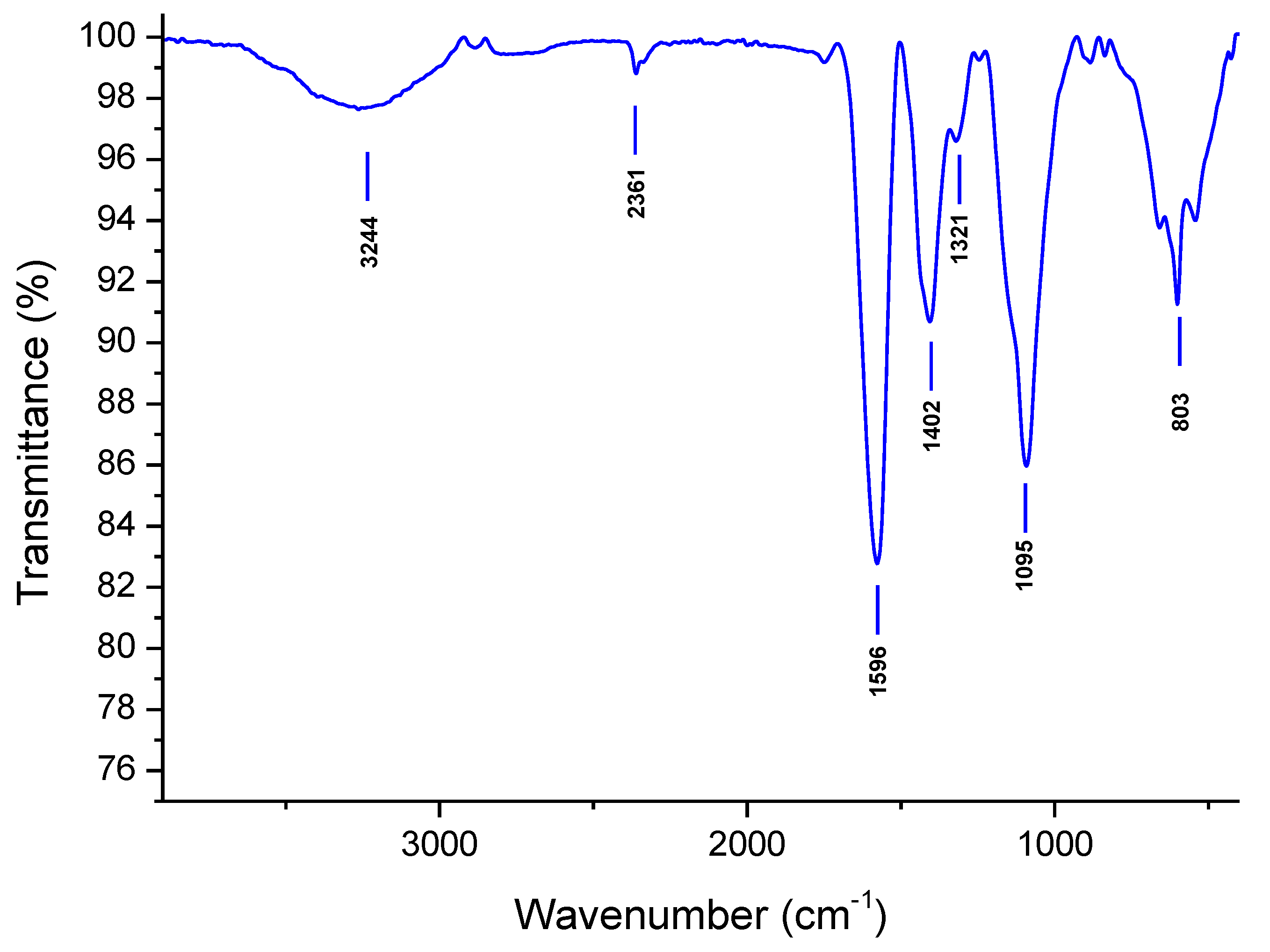
2.3.2. Fourier Transform–Infrared Spectroscopy (FT-IR) Analysis
2.3.3. Monosaccharide Composition Analysis with GC/MS
2.3.4. Molecular Structural Characteristics
- Determination by HPLC-RID
- Determination by SEC-MALS
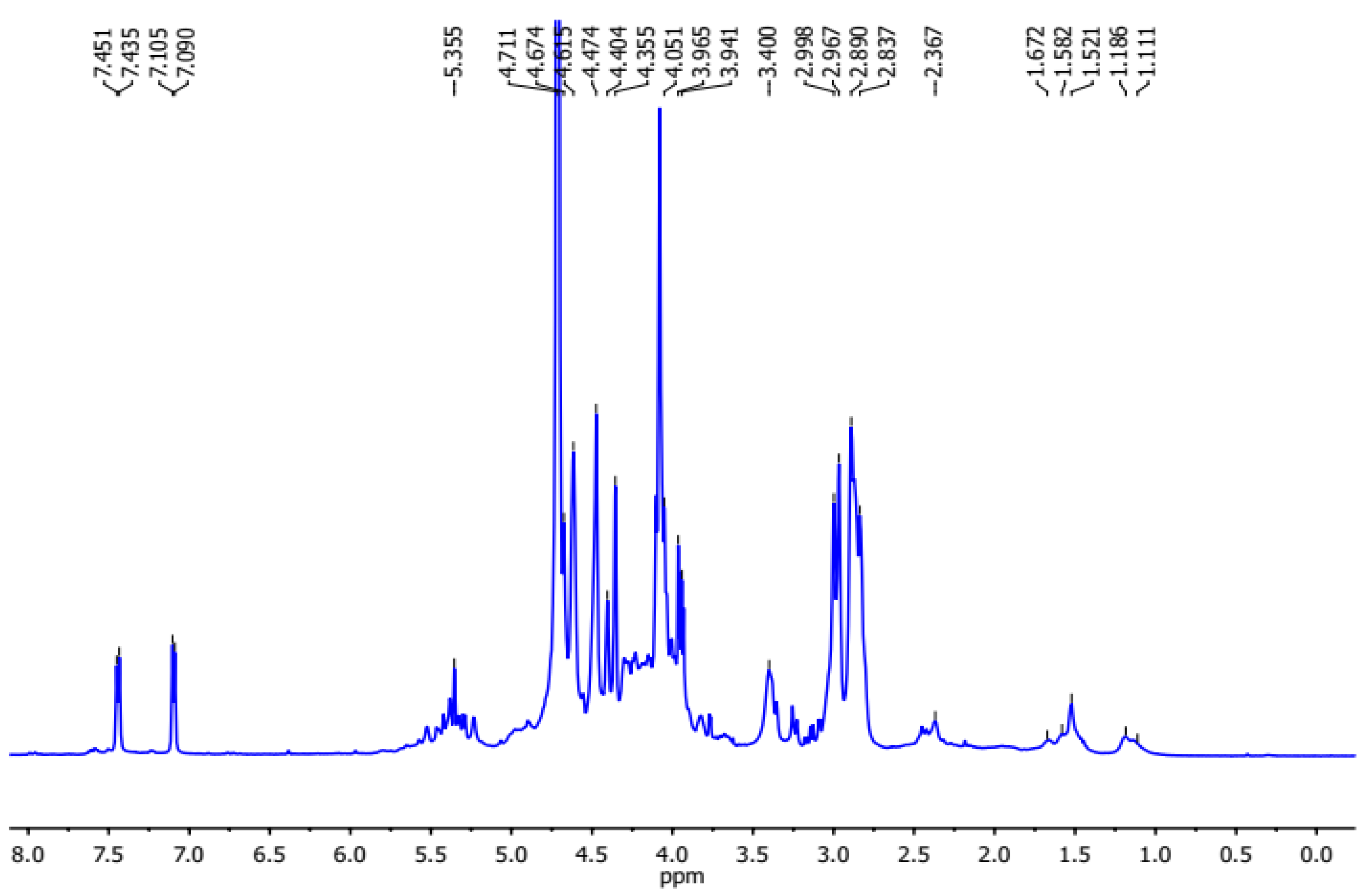
2.3.5. NMR Spectrometric Analysis
2.4. In Vivo Study
2.4.1. Animals
2.4.2. Experimental Induction in Rats
2.4.3. Acute Toxicity Study
2.4.4. Experimental Protocols
- Group I: vehicle control rats, received standard laboratory diet and saline water ad libitum for 15 days.
- Group II: ISO-treated rats, received saline water for 15 days, and subcutaneously injected with ISO (85 mg/kg body weight) on the 14th day and 24 h later.
- Group III (ISO + CFPS): rats were given CFPS at a dose of 100 mg/kg daily for 15 days by gastric gavage and subcutaneously received ISO (85 mg/kg body weight) for two consecutive days (14th and 15th days)
- Group IV (ISO + Pid): Rats were treated with pidogrel (Pid) 150 µg/kg body weight orally) for 15 days and were subcutaneously injected with ISO (85 mg/kg body weight) on the 14th and 15th day.
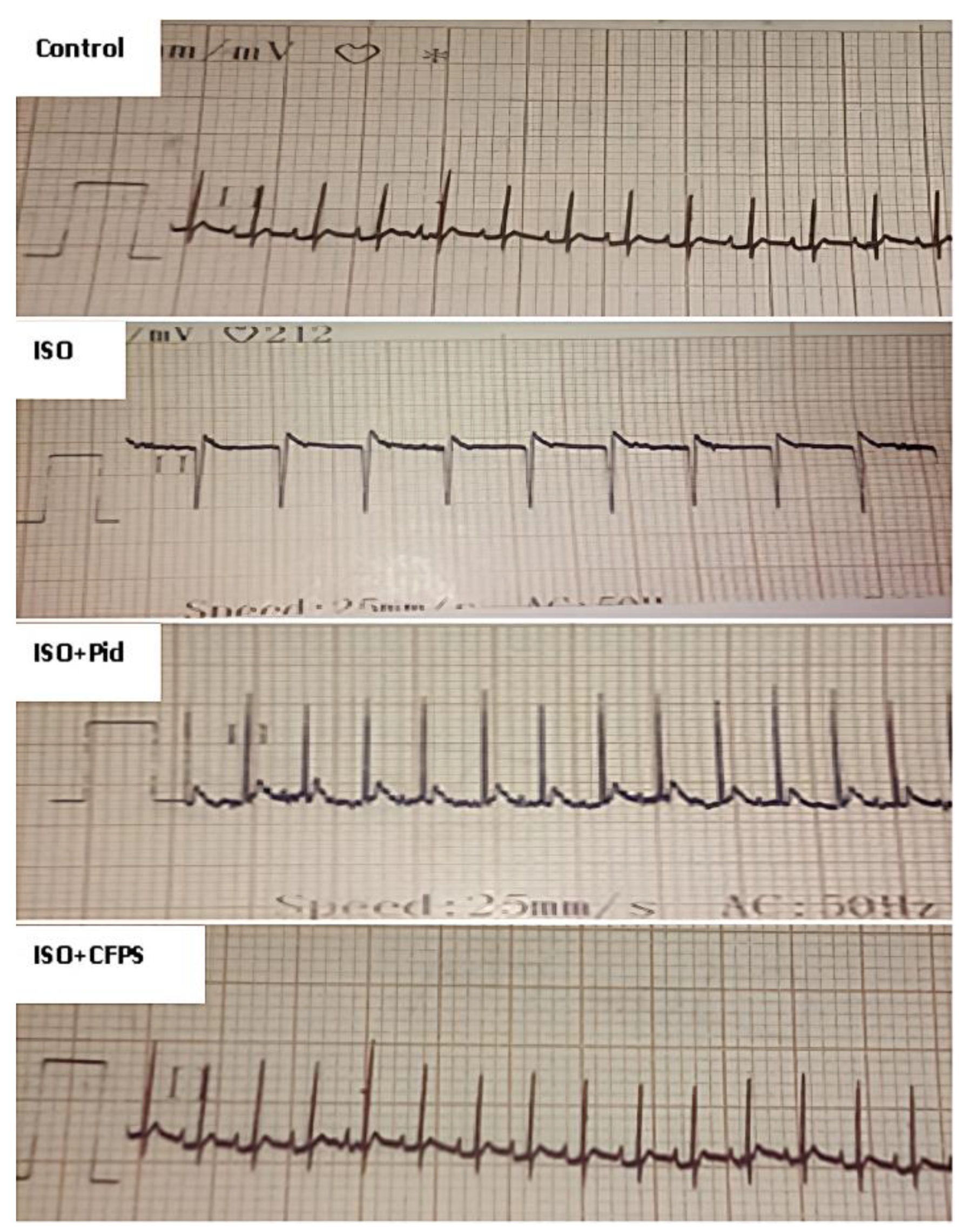
2.4.5. Electrocardiography
2.4.6. Biochemical Assay
2.4.7. Determination of Lipid Peroxidation
2.4.8. DNA Fragmentation Assay
2.4.9. Measurement of Myocardial Infarction Area
2.4.10. Histopathological Studies
2.5. Statistical Tests
3. Results
3.1. Chemical Composition
3.2. Preliminary Structural Features of CFPS
3.2.1. FT-IR Spectra
3.2.2. Monosaccharide Composition
3.2.3. Macromolecular Characteristics of CFPS
3.2.4. NMR Investigation
3.3. In Vivo Activities
3.3.1. Heart Weight and Body Weight
3.3.2. Effect of CFPS on ECG Findings
3.3.3. Cardiac Injury Biomarkers
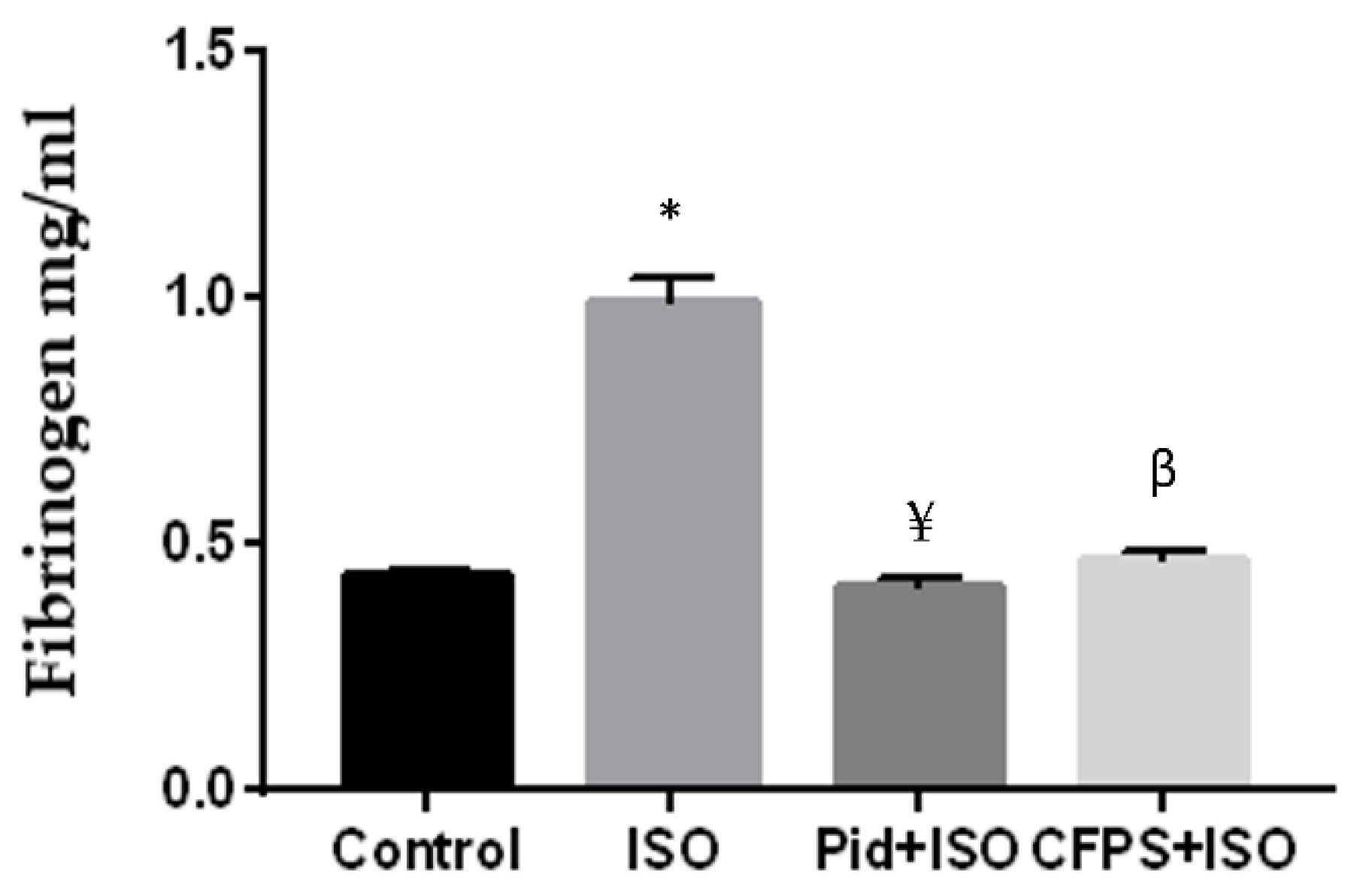
3.3.4. Effect of CFPS on Fibrinogen Level
3.3.5. Lipid Profile
3.3.6. Effects of CFPS on the Lipid Peroxidation
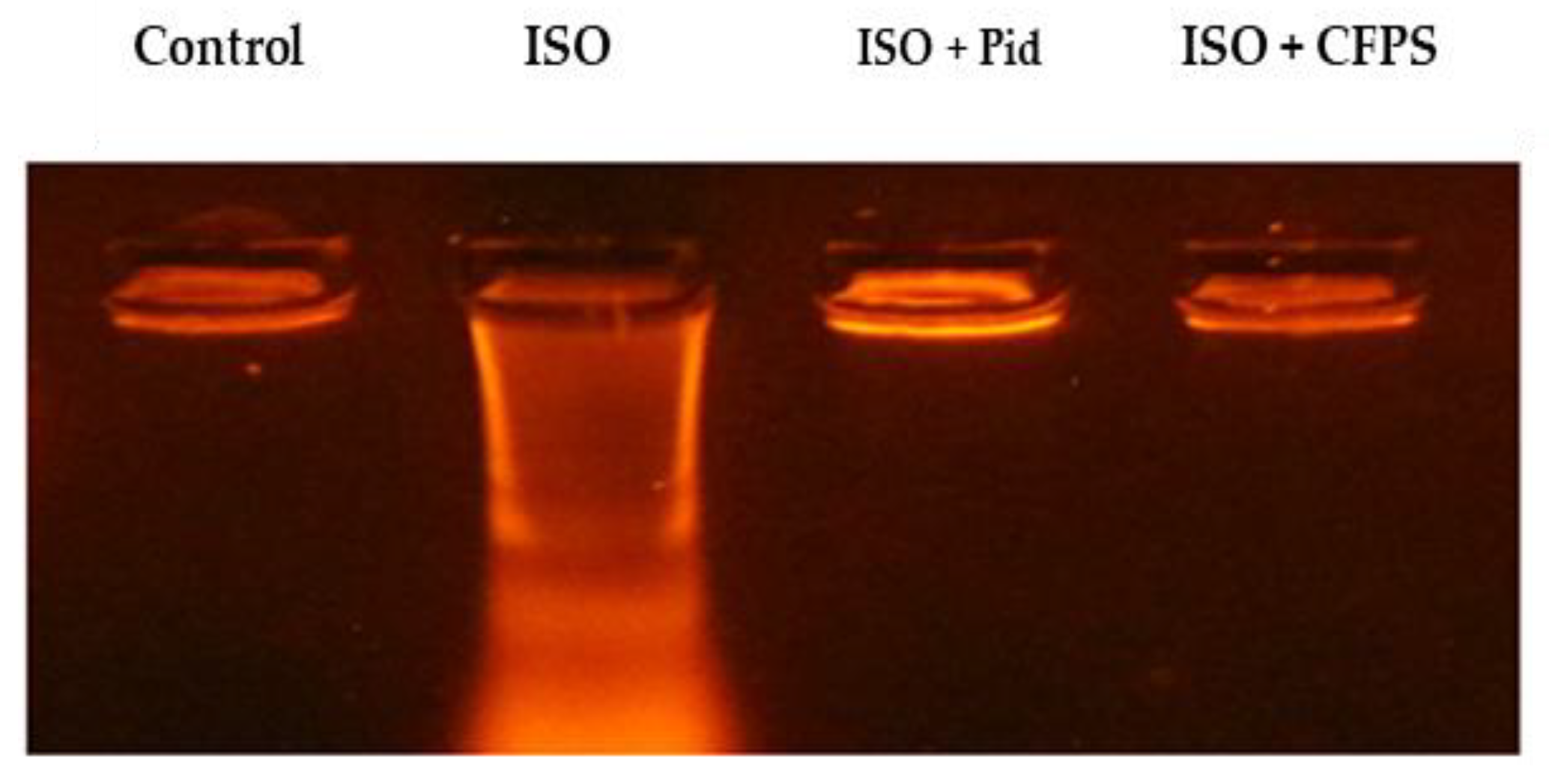
3.3.7. DNA Fragmentation Analysis
3.3.8. TTC Staining
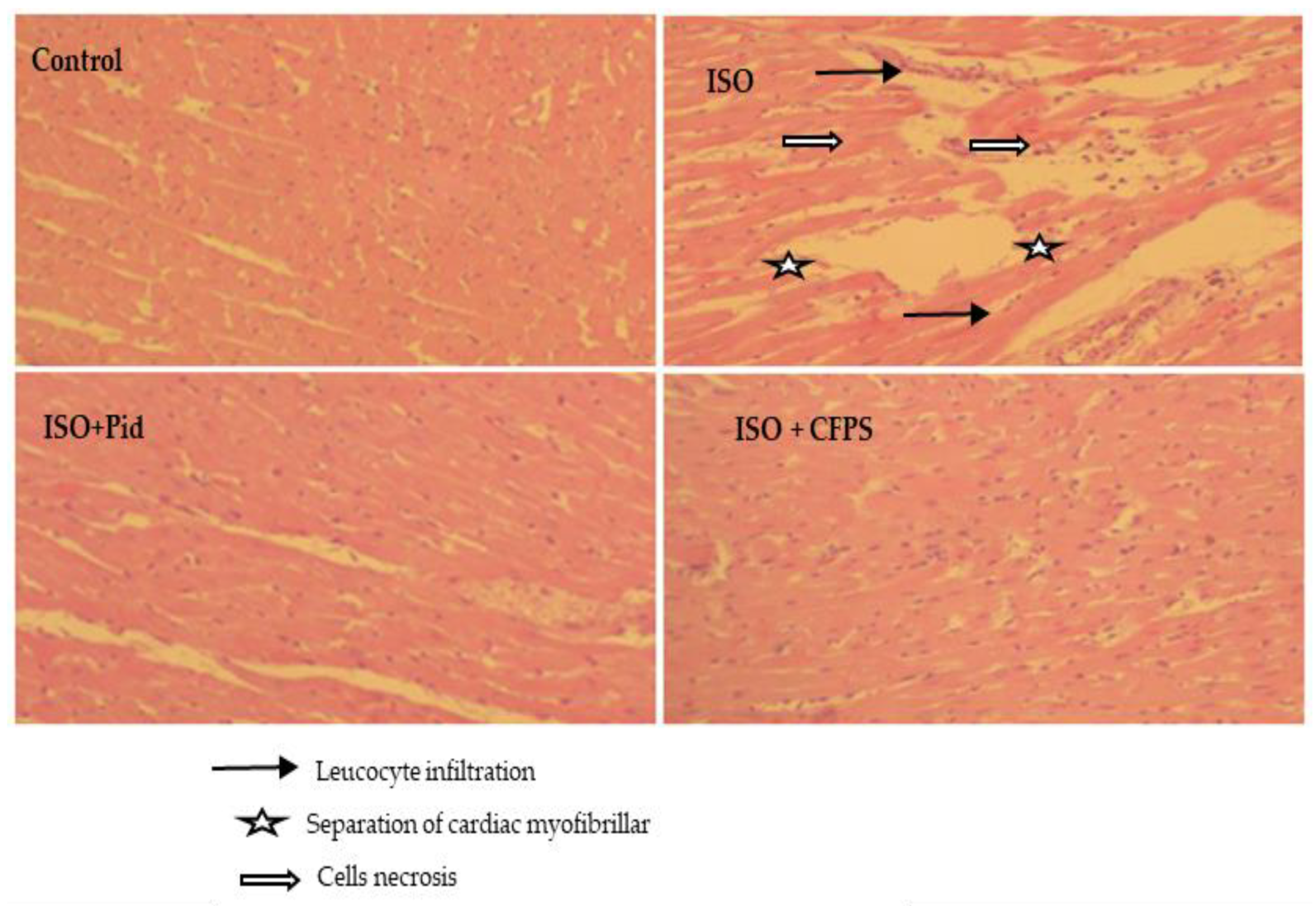
3.3.9. Histopathological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.B.; Tian, S.; Yang, F.; Yang, H.G.; Yang, X.Y.; Du, G.H. Cardioprotective effect of salvianolic acid A on isoproterenol-induced myocardial infarction in rats. Eur. J. Pharmacol. 2009, 615, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Radhiga, T.; Rajamanickam, C.; Senthil, S.; Pugalendi, K.V. Effect of ursolic acid on cardiac marker enzymes. lipid profile and macroscopic enzyme mapping assay in isoproterenolinduced myocardial ischemic rats. Food Chem. Toxicol. 2012, 50, 3971–3977. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Cui, Q.; Su, G.; Guo, X.; Liu, X.; Zhang, G. MicroRNA-208b Alleviates post-infarction myocardial fibrosis in a rat model by inhibiting GATA4. Med. Sci. Monit. 2016, 22, 1808–1816. [Google Scholar] [CrossRef] [Green Version]
- Buja, L.M.; Entman, M.L. Modes of Myocardial Cell Injury and Cell Death in Ischemic Heart Disease. Circulation 1998, 98, 1355–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef]
- Rona, G.; Chappel, C.I.; Balazs, T.; Gaudry, R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. Arch. Pathol. 1959, 67, 443–455. Available online: https://cir.nii.ac.jp/crid/1573105974856922496 (accessed on 28 November 2021).
- de Sànchez, V.C.; Hernández-Muñoz, R.; López-Barrera, F.; Yañez, L.; Vidrio, S.; Suárez, J.; Cota-Garza, M.; Aranda-Fraustro, A.; Cruz, D. Sequential changes of energy metabolism and mitochondrial function in myocardial infarction induced by isoproterenol in rats: A long-term and integrative study. Can. J. Physiol. Pharmacol. 1997, 75, 1300–1311. [Google Scholar] [CrossRef]
- Rona, G. Catecholamine cardiotoxicity. J. Mol. Cell. Cardiol. 1985, 17, 291–306. [Google Scholar] [CrossRef]
- Tasatargil, A.; Kuscu, N.; Dalaklioglu, S.; Adiguzel, C.; Celik-Ozenci, S.; Ozdem, A.; Barutcigil, S.; Ozdem, S. Cardioprotective effect of nesfatin-1 against isoproterenol-induced myocardial infarction in rats: Role of the Akt/GSK-3β pathway. Peptides 2017, 95, 1–9. [Google Scholar] [CrossRef]
- Al-Olayan, E.M.; El-Khadragy, M.F.; Metwally, D.M.; Abdel Moneim, A.E. Protective effects of pomegranate (Punica granatum) juice on testes against carbon tetrachloride intoxication in rats. BMC Complement. Altern. Med. 2014, 14, 164. [Google Scholar] [CrossRef] [Green Version]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-Y.; Chen, J.-J.; Li, H.-Y.; Ma, Z.-Q.; Ma, S.-P.; Fu, Q. Cardioprotective effects of timosaponin B II from Anemarrhenae asphodeloides Bge on isoproterenol-induced myocardial infarction in rats. Chem.-Biol. Interactions 2015, 240, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Vellosa, J.; Khalil, N.; Formenton, V.; Ximenes, V.; Fonseca, L.; Furlan, M.; Brunetti, I.; Oliveira, O. Antioxidant activity of Maytenus ilicifolia root bark. Fitoterapia 2006, 77, 243–244. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, A.; Karunakaran, R.J. Activity-guided isolation and identification of free radical-scavenging components from an aqueous extract of Coleus aromaticus. Food Chem. 2007, 100, 356–361. [Google Scholar] [CrossRef]
- Song, M.; Huang, L.; Zhao, G.; Song, Y. Beneficial effects of a polysaccharide from Salvia miltiorrhiza on myocardial ischemia–reperfusion injury in rats. Carbohydr. Polym. 2013, 98, 1631–1636. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Keys, D.J. Chinese Herbs, Botany, Chemistry and Pharmacodynamics; Charles E and Tutle Company: Tokyo, Japan, 1985. [Google Scholar]
- Gruenwald, J.; Brendler, T.; Jaenicke, C. PDR for Herbal Medicines, 2nd ed.; Medical Economics Company: Montvale, NJ, USA, 2000. [Google Scholar]
- Chawla, R.; Kumar, S.; Sharma, A. The genus Clematis (Ranunculaceae): Chemical and pharmacological perspectives. J. Ethnopharmacol. 2012, 143, 116–150. [Google Scholar] [CrossRef]
- Saidi, R.; Khanous, L.; Allah, S.K.; Hamdi, B.; Ayadi, A.; Damak, M.; Hammami, H.; Mezghani-Jarraya, R. Antifungal, molluscicidal and larvicidal assessment of anemonin and Clematis flammula L. extracts against mollusc Galba truncatula, intermediate host of Fasciola hepatica in Tunisia. Asian Pac. J. Trop. Med. 2017, 10, 967–973. [Google Scholar] [CrossRef]
- Atmani, D.; Larrea, M.R.; Sanz, J.R.; Lizcano, L.; Bakkali, F. Antioxidant potential, cytotoxic activity and phenolic content of Clematis flammula leaf extracts. J. Med. Plants Res. 2011, 5, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, X.; Zhao, M.; Qian, K. Optimization of polysaccharides extraction from Clematis huchouensis Tamura and its antioxidant activity. Carbohydr. Polym. 2014, 111, 762–767. [Google Scholar] [CrossRef]
- Zhu, Y.; Di, S.; Hu, W.; Feng, Y.; Zhou, Q.; Gong, B.; Tang, X.; Liu, J.; Zhang, W.; Xi, M.; et al. A new flavonoid glycoside (APG) isolated from Clematis tangutica attenuates myocardial ischemia/reperfusion injury via activating PKCε signaling. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.R.; Feng, J.T.; Ma, Z.Q.; Xie, N.; Zhang, J.; Zhang, X.; Tang, H.F. Three new glycosides from the whole plant of Clematis lasiandra Maxim and their cytotoxicity. Phytochem. Lett. 2014, 10, 168–172. [Google Scholar] [CrossRef]
- Saidi, R.; Ghrab, F.; Kallel, R.; Feki, A.E.; Boudawara, T.; Chesné, C. Tunisian Clematis flammula Essential Oil Enhances Wound Healing: GC-MS Analysis. Biochemical and Histological Assessment. J. Oleo Sci. 2018, 67, 1483–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rjeibi, I.; Feriani, A.; Hentati, F.; Hfaiedh, N.; Michaud, P.; Pierre, G. Structural characterization of water-soluble polysaccharides from Nitraria retusa fruits and their antioxidant and hypolipidemic activities. Int. J. Biol. Macromol. 2019, 129, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pierre, G.; Graber, M.; Rafiliposon, B.A.; Dupuy, C.; Orvain, F.; De Crignis, M.; Maugard, T. Biochemical Composition and Changes of Extracellular Polysaccharides (ECPS) Produced during Microphytobenthic Biofilm Development (Marennes-Oléron, France). Microb. Ecol. 2011, 63, 157–169. [Google Scholar] [CrossRef] [Green Version]
- Pierre, G.; Zhao, J.M.; Orvain, F.; Dupuy, C.; Klein, G.L.; Graber, M.; Maugard, T. Seasonal dynamics of extracellular polymeric substances (EPS) in surface sediments of a diatom-dominated intertidal mudflat (Marennes–Oléron, France). J. Sea Res. 2014, 92, 26–35. [Google Scholar] [CrossRef]
- Mnafgui, K.; Khlif, I.; Hajji, R.; Derbali, F.; Kraiem, F.; Ellefi, F.; Gharsallah, N.; Allouche, N. Preventive effects of oleuropein against cardiac remodeling after myocardial infarction in Wistar rat through inhibiting angiotensin-converting enzyme activity. oxicol. Mech. Methods 2015, 25, 538–546. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Chtourou, Y.; Aouey, B.; Kebieche, M.; Fetoui, H. Protective role of naringin against cisplatin induced oxidative stress, inflammatory response and apoptosis in rat striatum via suppressing ROS-mediated NF-κB and P53 signaling pathways. Chem. Interactions 2015, 239, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Sellins, K.S.; Cohen, J.J. Nuclear Changes in the Cytotoxic T Lymphocyte-induced Model of Apoptosis. Immunol. Rev. 1995, 146, 266. [Google Scholar] [CrossRef] [PubMed]
- Khalil, P.N.; Siebeck, M.; Huss, R.; Pollhammer, M.; Khalil, M.N.; Neuhof, C.; Fritz, H. Histochemical assessment of early myocardial infarction using 2,3,5-triphenyltetrazolium chloride in blood-perfused porcine hearts. J. Pharmacol. Toxicol. Methods 2006, 54, 307–312. [Google Scholar] [CrossRef]
- RuiDian, K.; ShunFa, L.; Yi, C.; ChuRong, J.; QiaGuang, S. Analysis of chemical composition of polysaccharides from Poria cocos Wolf and its anti-tumor activity by NMR spectroscopy. Carbohydr. Polym. 2010, 80, 31–34. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Marais, M.-F.; Vignon, M.R. An arabinogalactan from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2004, 339, 1201–1205. [Google Scholar] [CrossRef]
- Song, J.; Wu, Y.; Ma, X.; Feng, L.; Wang, Z.; Jiang, G.; Tong, H. Structural characterization and α-glycosidase inhibitory activity of a novel polysaccharide fraction from Aconitum coreanum. Carbohydr. Polym. 2019, 230, 115586. [Google Scholar] [CrossRef]
- Kalegowda, P.; Chauhan, A.S.; Urs, S.M.N. Opuntia dillenii (Ker-Gawl) Haw cladode mucilage: Physico-chemical, rheological and functional behavior. Carbohydr. Polym. 2017, 157, 1057–1064. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.S.; Navid, M.H.; Ghosh, T.; Schnitzler, P.; Ray, B. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry 2011, 72, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Sengkhamparn, N.; Verhoef, R.; Schols, H.A.; Sajjaanantakul, T.; Voragen, A.G.J. Characterisation of cell wall polysaccharides from okra (Abelmoschus esculentus (L.) Moench). Carbohydr. Polym. 2009, 344, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Petera, B.; Delattre, C.; Pierre, G.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from Cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Feriani, A.; Khdhiri, E.; Tir, M.; Elmufti, A.; Tlili, N.; Hajji, R.; Mnafgui, K. (E)-N′-(1-(7-Hydroxy-2-Oxo-2H-Chromen-3-Yl) Ethylidene) benzohydrazide, a novel synthesized coumarin, ameliorates isoproterenol-induced myocardial infarction in rats through attenuating oxidative stress, inflammation, and apoptosis. Oxid. Med. Cell. Longev. 2020, 2020, 2432918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, R.; Tang, F.; Lu, C.; Xu, J.; Hu, J.; Mei, M.; Wang, H. Protective effects of Astragalus polysaccharides against endothelial dysfunction in hypertrophic rats induced by isoproterenol. Int. Immunopharmacol. 2016, 38, 306–312. [Google Scholar] [CrossRef]
- Prince, P.C.M.; Hemalatha, K.L. A molecular mechanism on the antiapoptotic effects of zingerone in isoproterenol induced myocardial infarcted rats. Eur. J. Pharmacol. 2018, 821, 105–111. [Google Scholar] [CrossRef]
- Mnafgui, K.; Hajji, R.; Derbali, F.; Khlif, I.; Kraiem, F.; Ellefi, H.; Allouche, N.; Gharsallah, N. Protective effect of hydroxytyrosol against cardiac remodeling after isoproterenol-induced myocardial infarction in Rat. Cardiovasc. Toxicol. 2016, 16, 147–155. [Google Scholar] [CrossRef]
- Hamed, M.; Bougatef, H.; Karoud, W.; Krichen, F.; Haddar, A.; Bougatef, A.; Silaa, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity and potential application on meat as preservative. Ind. Crop. Prod. 2020, 148, 112315. [Google Scholar] [CrossRef]
- Fan, R.; Xie, Y.; Zhu, C.; Qiu, D.; Zeng, J.; Liu, Z. Structural elucidation of an acidic polysaccharide from Citrus grandis “Tomentosa” and its anti-proliferative effects on LOVO and SW620 cells. Int. J. Biol. Macromol. 2019, 138, 511–518. [Google Scholar] [CrossRef]
- Hammami, N.; Ben Gara, A.; Bargougui, K.; Ayedi, H.; Ben Abdalleh, F.; Belghith, K. Improved in vitro antioxidant and antimicrobial capacities of polysaccharides isolated from Salicornia arabica. Int. J. Biol. Macromol. 2018, 120, 2123–2130. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Han, R.; Classen, B.; Quetin-Leclerq, J.; Mahy, G.; Ruan, C.-J.; Qin, P.; Alfocea, F.P.; Lutts, S. Mucilage and polysaccharides in the halophyte plant species Kosteletzkya virginica: Localization and composition in relation to salt stress. J. Plant Physiol. 2010, 167, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Golbargi, F.; Gharibzahedi, S.M.T.; Zoghi, A.; Mohammadi, M.; Hashemifesharaki, R. Microwave-assisted extraction of arabinan-rich pectic polysaccharides from melon peels: Optimization, purification, bioactivity, and techno-functionality. Carbohydr. Polym. 2021, 256, 117522. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Y.; Wu, Q.; John, A.; Jiang, Y.; Yang, J.; Liu, H.; Yang, B. Structure characterisation of polysaccharides in vegetable “okra” and evaluation of hypoglycemic activity. Food Chem. 2018, 242, 211–216. [Google Scholar] [CrossRef]
- Zhang, S.; Hea, B.; Ge, J.; Li, H.; Luo, X.; Zhang, H.; Li, Y.; Zhai, C.; Liu, P.; Liu, X.; et al. Extraction, chemical analysis of Angelica sinensis polysaccharides and antioxidant activity of the polysaccharides in ischemia–reperfusion rats. Int. J. Biol. Macromol. 2010, 47, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Rozi, P.; Abuduwaili, A.; Ma, S.; Bao, X.; Xu, H.; Zhu, J.; Yadikar, N.; Wang, J.; Yang, X.; Yili, A. Isolations, characterizations and bioactivities of polysaccharides from the seeds of three species Glycyrrhiza. Int. J. Biol. Macromol. 2020, 145, 364–371. [Google Scholar] [CrossRef]
- El-Naggar, N.E.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, extraction and characterization of Chlorella vulgaris soluble polysaccharides and their applications in AgNPs biosynthesis and biostimulation of plant growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Li, M.; Luo, Y.; Wu, W. Isolation and structural characterization of an immunostimulating polysaccharide from fuzi, Aconitum carmichaeli. Carbohydr. Res. 2006, 341, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Derbali, A.; Mnafgui, K.; Affes, M.; Derbali, F.; Hajji, R.; Gharsallah, N.; Allouche, N.; El Feki, A. Cardioprotective effect of linseed oil against isoproterenol-induced myocardial infarction in Wistar rats: A biochemical and electrocardiographic study. J. Physiol. Biochem. 2015, 71, 281–288. [Google Scholar] [CrossRef]
- Ghazouani, L.; Khdhiri, E.; Elmufti, A.; Feriani, A.; Tir, M.; Baaziz, I.; Mnafgui, K. Cardioprotective effects of (E)-4-hydroxy-N′-(1-(3-oxo-3H-benzo[f]chromen-2-yl)ethylidene) benzohydrazide: A newly synthesized coumarin hydrazone against isoproterenol-induced myocardial infarction in a rat model. Can. J. Physiol. Pharmacol. 2019, 97, 989–998. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A.; Ramamurthy, V. Cardioprotective effect of vincristine on isoproterenol-induced myocardial necrosis in rats. Eur. J. Pharmacol. 2014, 723, 451–458. [Google Scholar] [CrossRef]
- Khalil, M.I.; Ahmmed, I.; Ahmed, R.; Tanvir, E.M.; Afroz, R.; Paul, S.; Hua Gan, S.; Alam, N. Amelioration of isoproterenol-induced oxidative damage in rat myocardium by with aniasomnifera leaf extract. BioMed Res. Int. 2015, 2015, 624159. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kasala, E.R.; Bodduluru, L.N.; Dahiya, V.; Lahkar, M. Baicalein protects isoproterenol induced myocardial ischemic injury in male Wistar rats by mitigating oxidative stress and inflammation. Inflamm. Res. 2016, 65, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Cardioprotective effects of a Fructus Aurantii polysaccharide in isoproterenol-induced myocardial ischemic rats. Int. J. Biol. Macromol. 2020, 155, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Shojaie, M.; Pourahmad, M.; Eshraghian, A.; Izadi, H.R.; Naghshvar, F. Fibrinogen as a risk factor for premature myocardial infarction in Iranian patients: A case control study. Vasc. Health Risk Manag. 2009, 5, 673–676. [Google Scholar] [PubMed] [Green Version]
- Machlus, K.R.; Cardenas, J.C.; Church, F.C.; Wolberg, A.S. Causal relationship between hyperfibrinogenemia, thrombosis, and resistance to thrombolysis in mice. Blood 2011, 117, 4953–4963. [Google Scholar] [CrossRef] [Green Version]
- Murugesan, S.; Pandiyan, A.; Saravanakumar, L.; Moodley, K.; Mackraj, I. Protective role of wild garlic on isoproterenol-induced myocardial necrosis in wistar rats. J. Ethnopharmacol. 2019, 237, 108–115. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X. Hypoglycemic activity in vitro of polysaccharides from Camellia oleifera Abel, seed cake. Int. J. Biol. Macromol. 2018, 115, 811–819. [Google Scholar] [CrossRef]
- Walton, B.L.; Getz, T.M.; Bergmeier, W.; Lin, F.-C.; de Willige, S.U.; Wolberg, A.S. The fibrinogen γA/γ′ isoform does not promote acute arterial thrombosis in mice. J. Thromb. Haemost. 2014, 12, 680–689. [Google Scholar] [CrossRef] [Green Version]
- Vijayakumar, R.; Nachiappan, V. Cassia auriculata flower extract attenuates hyperlipidemia in male Wistar rats by regulating the hepatic cholesterol metabolism. Biomed. Pharmacother. 2017, 95, 394–401. [Google Scholar] [CrossRef]
- Anandan, R.; Mathew, S.; Sankar, T.V.; Viswanathan, P.G. Protective effect of n-3 polyunsaturated fatty acids concentrate on isoproterenol-induced myocardial infarction in rats. Prostaglandins Leukot. Essent. Fat. Acids 2007, 76, 153–158. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Li, J.; Xiong, L.; Chen, H.; Liu, X.; Wang, W. Antihyperlipidemic and hepatoprotective activities of polysaccharide fraction from Cyclocaryapaliurus in high-fat emulsion-induced hyperlipidaemic mice. Carbohydr. Polym. 2018, 183, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Li, J.; Xu, N.; Zhang, J.; Song, X.; Wang, X.; Gao, Z.; Jing, H.; Li, S.; Zhang, C.; et al. Anti-hyperlipidemic and antioxidant effects of alkali-extractable mycelia polysaccharides by Pleurotus eryngii var. tuolensis. Carbohydr. Polym. 2017, 175, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.B.; Zhao, J.; Chen, H.; Xiong, L.; Wang, W.J. Polysaccharides from Cyclocaryapaliurus: Chemical composition and lipid-lowering effect on rats challenged with high-fat diet. J. Funct. Foods 2017, 36, 262–273. [Google Scholar] [CrossRef]
- Liu, D.; Chen, L.; Zhao, J.; Cui, K. Cardioprotection activity and mechanism of Astragalus polysaccharide in vivo and in vitro. Int. J. Biol. Macromol. 2018, 111, 947–952. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Gómez-Caravaca, A.M.; Contreras, M.D.M.; Talhaoui, N.; Taamalli, A.; Segura-Carretero, A.; Ghazouani, L.; Mufti, A.; Tlili, N.; et al. HPLC-DAD-ESI-QTOF-MS/MS profiling of Zygophyllum album roots extract and assessment of its cardioprotective effect against deltamethrin-induced myocardial injuries in rat, by suppression of oxidative stress-related inflammation and apoptosis via NF-κB signaling pathway. J. Ethnopharmacol. 2019, 247, 112266. [Google Scholar] [CrossRef]


| Extraction Yield (%, w/w) | Total Carbohydrates (%, w/w) | Uronic Acids (%, w/w) | Neutral Sugars (%, w/w) | Proteins (%, w/w) | Phenolic Compounds (%, w/w) | (NaCl) eq. (%) | Conductivity (µs/cm) |
|---|---|---|---|---|---|---|---|
| 6.5 | 34.5 a ± 0.35 | 17.81 ± 0.25 | 16.11 ± 0.12 | 2.5 ± 0.45 | 1.6 ± 0.12 | 8.26 | 110 |
| 38.1 b ± 1.1 |
| Mw a (g/mol) | Mn b (g/mol) | Đ c | Rh d (nm) | [η] e (mL/g) | Monosaccharides f (mol%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ara | Rha | Gal | Glc | GalA | Glucuronide | Glucoside | Me-Hexp | |||||
| 1.82 × 105 | 4.68 × 104 | 3.89 | 21 | 165 | 16.1 | 3.55 | 6.15 | 5.49 | 19.1 | 41.5 | 5.08 | 3.02 |
| Control | ISO | Pid + ISO | CFPS + ISO | |
|---|---|---|---|---|
| Body weight (g) | 293.5 ± 2.31 | 294.4 ± 2.55 | 295.3 ± 1.76 | 294.6 ± 2.79 |
| Heart weight (g) | 0.75 ± 0.01 | 1. 32 ± 0.08 * | 1.02 ± 0.09 ¥ | 1.01 ± 0.12 ¥ |
| CWI | 0.25 ± 0.01 | 0.44 ± 0.01 * | 0.35 ± 0.01 ¥ | 0.34 ± 0.02 ¥ |
| Control | ISO | ISO + Pid | Iso + CFPS | |
|---|---|---|---|---|
| cTn-I (ng/mL) | 0.44 ± 0.05 | 2.26 ± 0.13 * | 1.32 ± 0.03 ¥ | 1.45 ± 0.12 ¥,β |
| CK-MB (U/L) | 57.43 ± 1.30 | 128.9 ± 1.50 * | 95.35 ± 1.11 ¥ | 102.3 ± 1.00 ¥,β |
| LDH (U/L) | 58.03 ± 1.66 | 166.7 ± 1.32 * | 129.9 ± 1.45 ¥ | 134.4 ± 0.72 ¥,β |
| Control | Iso | ISO + Pid | Iso + CFPS | |
|---|---|---|---|---|
| TC (mg/dL) | 86.19 ± 1.18 | 118.6 ± 2.11 * | 93.48 ± 0.66 ¥ | 100.1 ± 1.26 ¥,β |
| TG (mg/dL) | 40.26 ± 0.72 | 56.69 ± 1.44 * | 48.58 ± 0.90 ¥ | 51.04 ± 0.91 ¥,β |
| LDL-c (mg/dL) | 29.25 ± 0.63 | 64.99 ± 0.87 * | 35.82 ± 1.04 ¥ | 40.76 ± 0.46 ¥,β |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baaziz, I.; Ghazouani, L.; Rjeibi, I.; Feriani, A.; Mnafgui, K.; Mufti, A.; Traikia, M.; Le Cerf, D.; Michaud, P.; Pierre, G.; et al. Structural Characterization and Cardioprotective Effect of Water-Soluble Polysaccharides Extracted from Clematis flammula. Appl. Sci. 2022, 12, 10818. https://doi.org/10.3390/app122110818
Baaziz I, Ghazouani L, Rjeibi I, Feriani A, Mnafgui K, Mufti A, Traikia M, Le Cerf D, Michaud P, Pierre G, et al. Structural Characterization and Cardioprotective Effect of Water-Soluble Polysaccharides Extracted from Clematis flammula. Applied Sciences. 2022; 12(21):10818. https://doi.org/10.3390/app122110818
Chicago/Turabian StyleBaaziz, Intissar, Lakhdar Ghazouani, Ilhem Rjeibi, Anouar Feriani, Kais Mnafgui, Afoua Mufti, Mounir Traikia, Didier Le Cerf, Philippe Michaud, Guillaume Pierre, and et al. 2022. "Structural Characterization and Cardioprotective Effect of Water-Soluble Polysaccharides Extracted from Clematis flammula" Applied Sciences 12, no. 21: 10818. https://doi.org/10.3390/app122110818





