Antimicrobial Activity of Cobalt (II)-Citrate against Common Foodborne Pathogens and Its Potential for Incorporation into Food Packaging Material
Abstract
1. Introduction
2. Materials and Methods
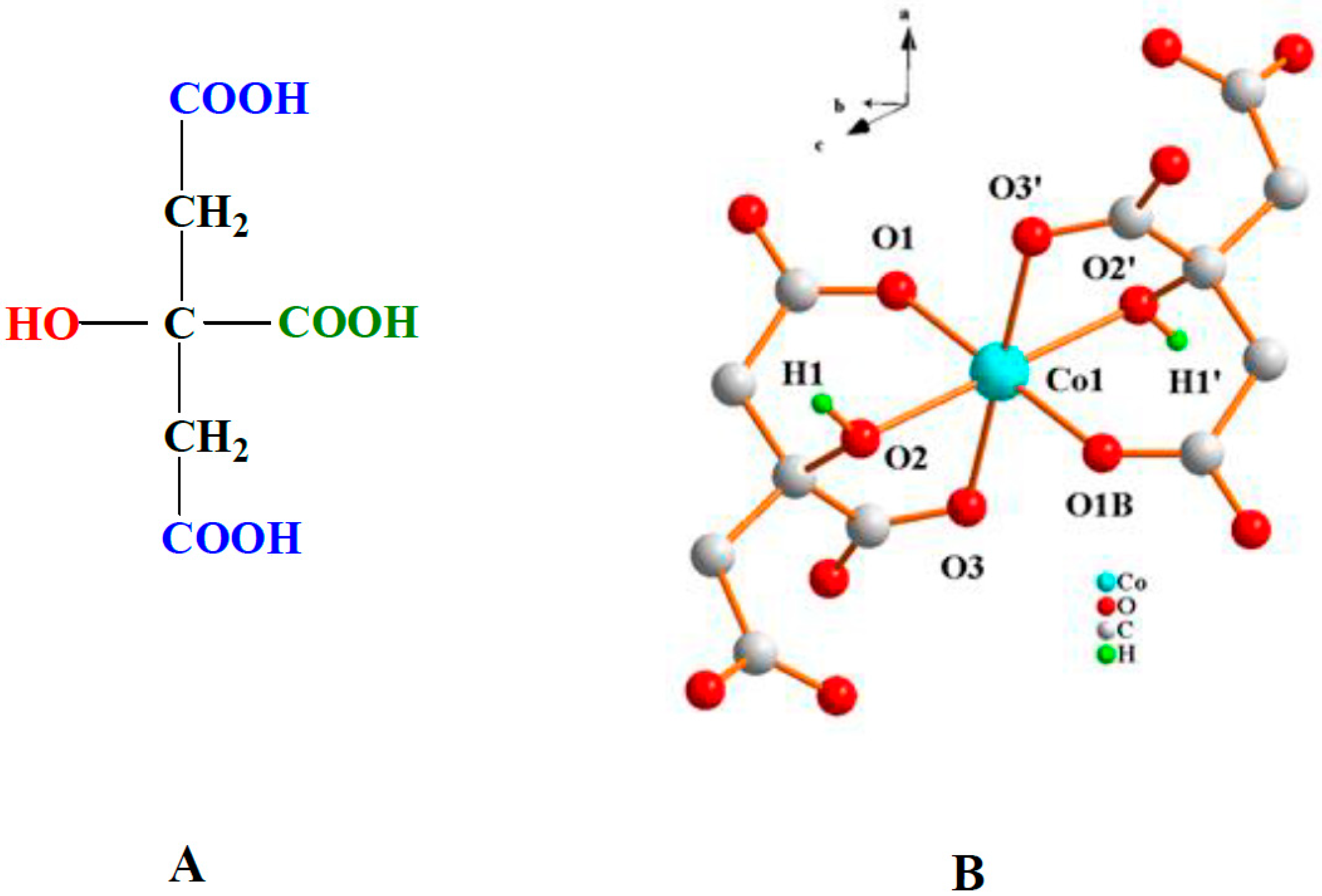
2.1. Preparation of (NH4)4[Co(C6H5O7)2]
2.2. Preparation of Polylactic Acid-Cobalt Citrate Films
2.3. Bacterial Cultures
2.4. Preparation of Substances and Acids for Use in the Bioscreen Instrument
2.5. Photometric Growth Inhibition Experiments
2.6. Antimicrobial Activity of PLA-(Co-cit) Films against L. monocytogenes in Culture Medium
2.7. Antimicrobial Activity of PLA-Cobalt Citrate Films in Foods
- −
- Slices inoculated with L. monocytogenes and covered with a PLA film containing 23.3% w/w Co-cit
- −
- Slices inoculated with L. monocytogenes and covered with a PLA film without Co-cit
- −
- Slices inoculated with L. monocytogenes and left uncovered
- −
- Uninoculated slices left uncovered.
3. Results and Discussion
3.1. Photometric Growth Inhibition Experiments
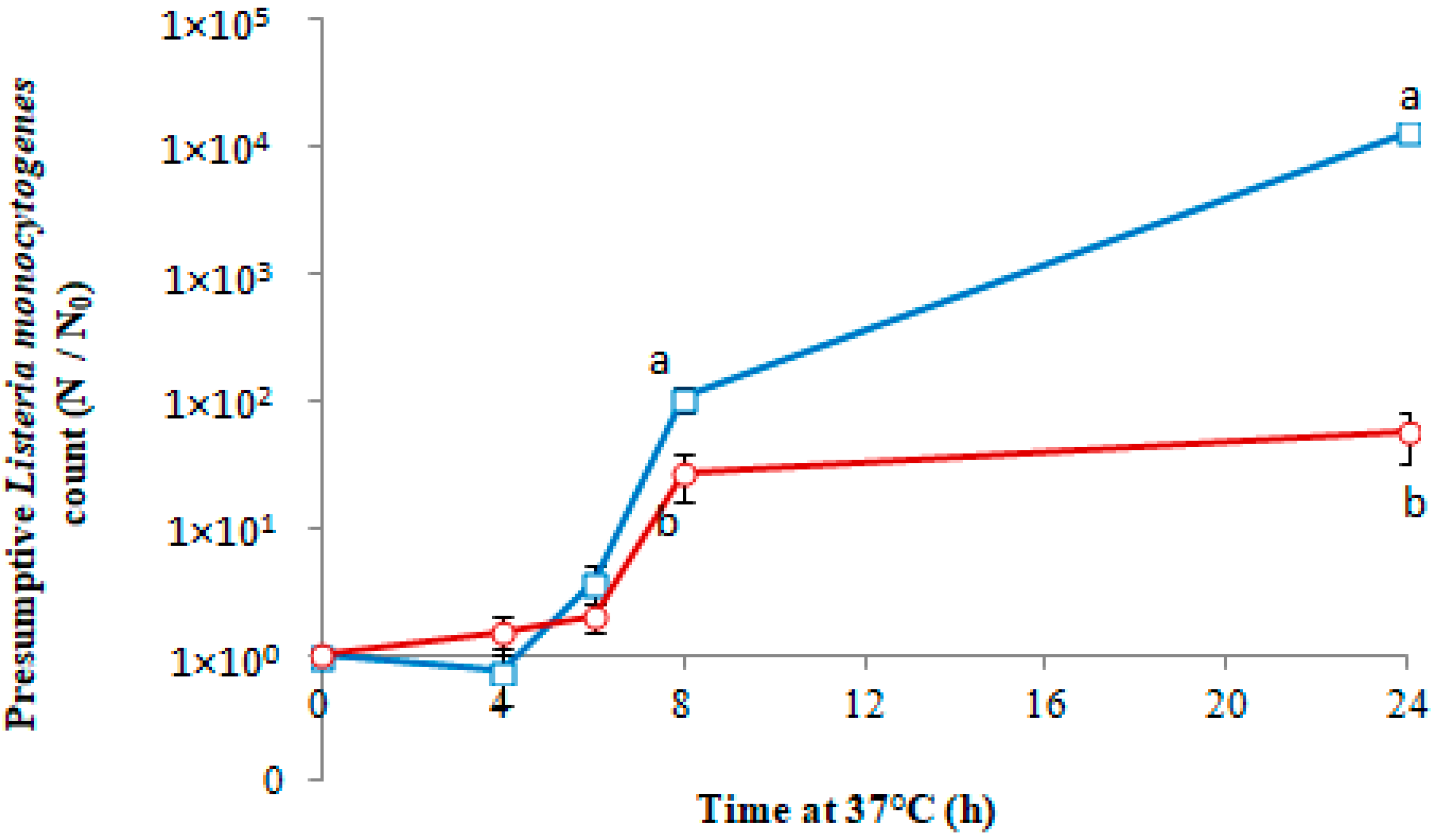
3.2. Antimicrobial Activity of PLA- Co-cit Films against L. monocytogenes in Culture Medium
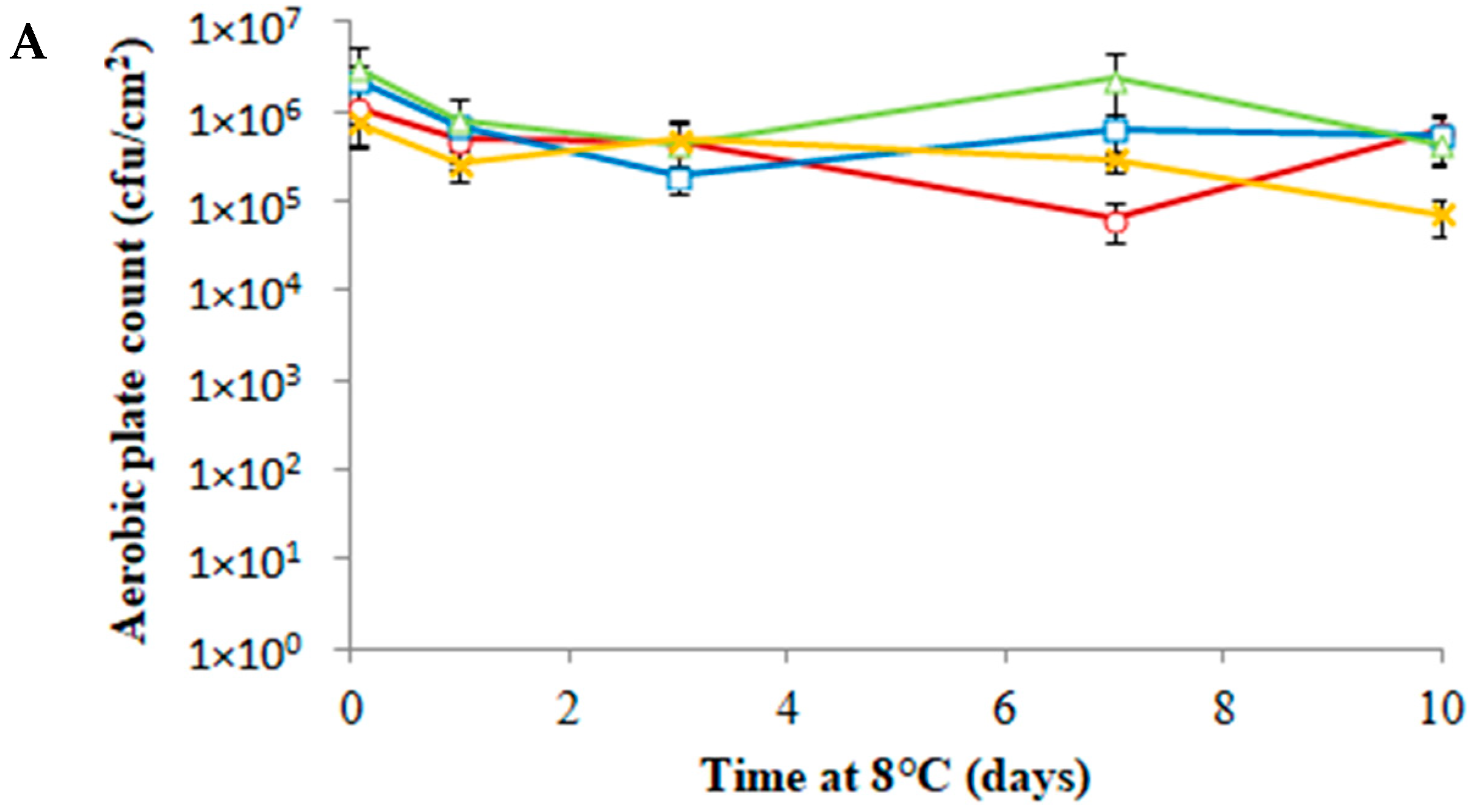
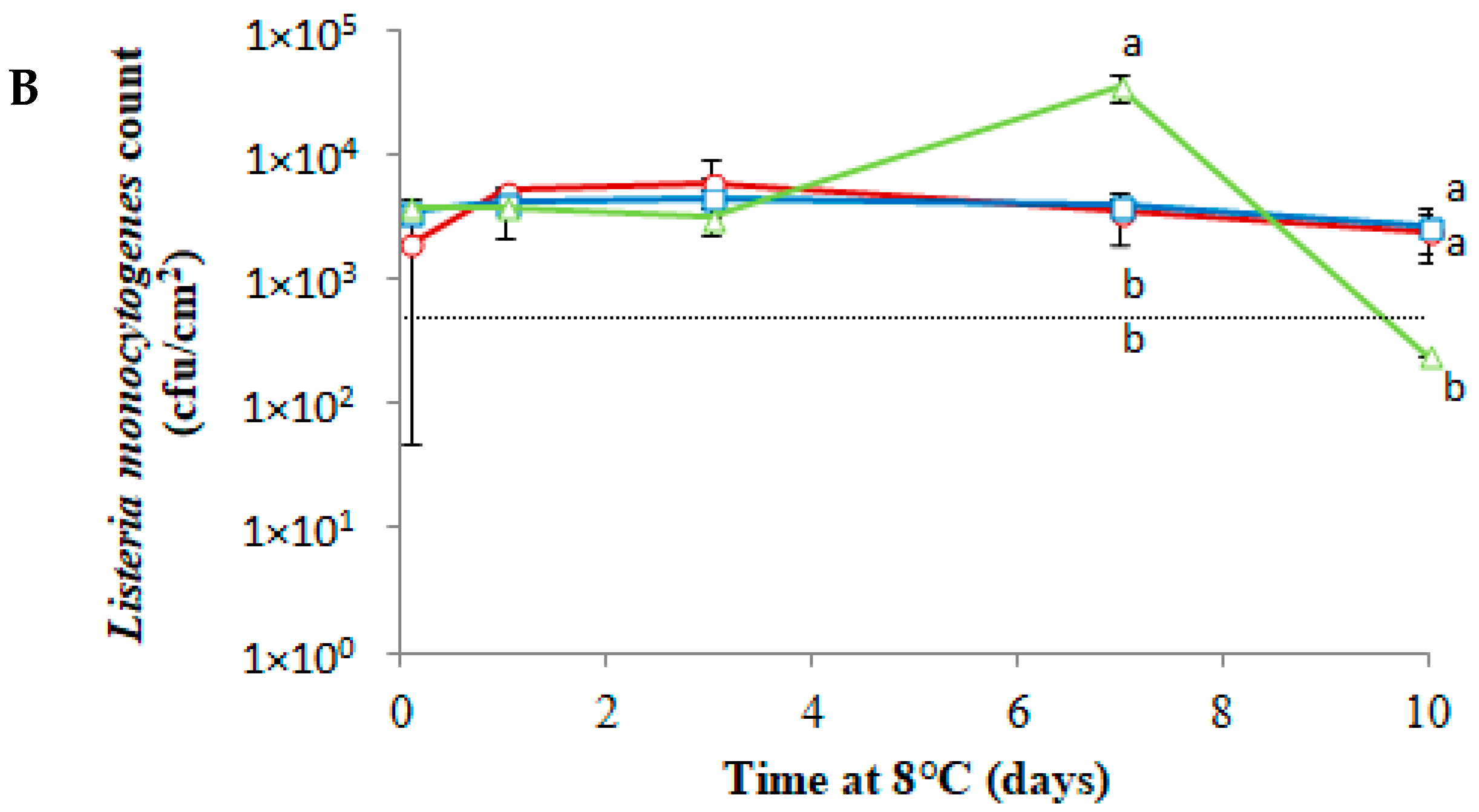
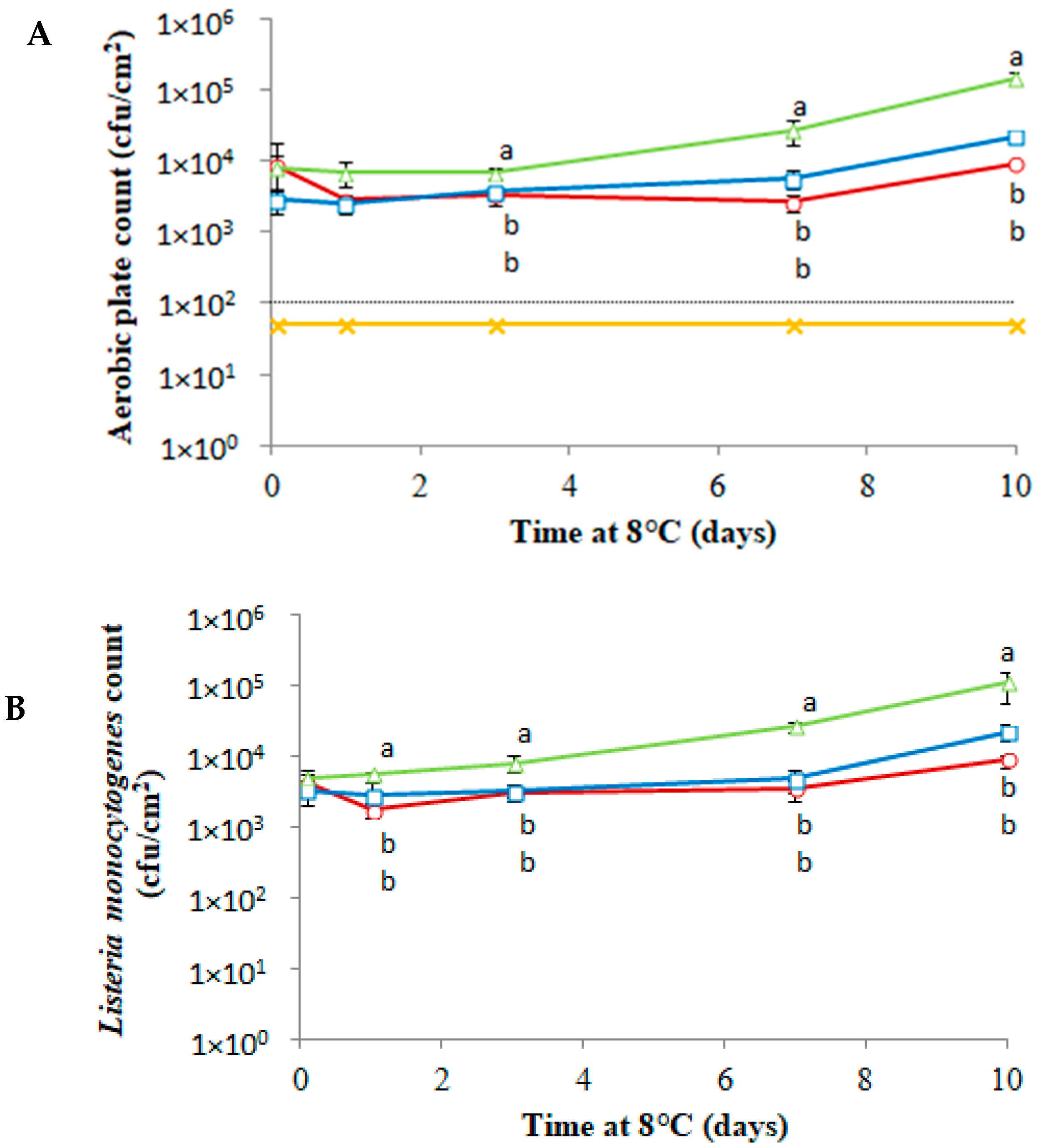
3.3. Antimicrobial Activity of PLA-(Co-cit) Films against L. monocytogenes on Food Samples
3.4. General Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, P.; Kochhar, A. Active Packaging in Food Industry: A Review. J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Padmanabhan, S.C.; Cruz-Romero, M.C.; Kerry, J.P.; Morris, M.A. Chapter 11—Food packaging: Surface engineering and commercialization. In Nanomaterials for Food Packaging: Materials, Processing Technologies, and Safety Issues; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 301–328. [Google Scholar] [CrossRef]
- García Ibarra, V.; Sendón, R.; Rodríguez-Bernaldo De Quirós, A. Chapter 29—Antimicrobial food packaging based on biodegradable materials. In Antimicrobial Food Packaging; Academic Press: Cambridge, MA, USA, 2016; pp. 363–384. [Google Scholar] [CrossRef]
- Yildirim, S.; Röcker, B. Chapter 7—Active packaging. In Nanomaterials for Food Packaging: Materials, Processing Technologies, and Safety Issues; Elsevier: Amsterdam, The Netherlands, 2018; pp. 173–202. [Google Scholar] [CrossRef]
- Moustafa, H.; El-Sayed, S.M.; Youssef, A.M. Synergistic impact of cumin essential oil on enhancing of UV-blocking and antibacterial activity of biodegradable poly(butylene adipate-co-terephthalate)/clay platelets nanocomposites. J. Thermoplast. Compos. Mater. 2021, 1–22. [Google Scholar] [CrossRef]
- Moustafa, H.; Darwish, N.A.; Youssef, A.M. Rational formulations of sustainable polyurethane/chitin/rosin composites reinforced with ZnO-doped-SiO2 nanoparticles for green packaging applications. Food Chem. 2022, 371, 131193. [Google Scholar] [CrossRef]
- Akhidime, I.D.; Saubade, F.; Benson, P.S.; Butler, J.A.; Oliver, S.; Kelly, P.; Verran, J.; Whitehead, K.A. The antimicrobial effect of metal substrates on food pathogens. Food Bioprod. Processing 2019, 113, 68–76. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Processing 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-hindi, R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric Antimicrobial food packaging and its applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Gan, I.; Chow, W.S. Antimicrobial poly(lactic acid)/cellulose bionanocomposite for food packaging application: A review. Food Packag. Shelf Life 2018, 17, 150–161. [Google Scholar] [CrossRef]
- Matzapetakis, M.; Dakanali, M.; Raptopoulou, C.P.; Tangoulis, V.; Terzis, A.; Moon, N.; Giapintzakis, J.; Salifoglou, A. Synthesis, spectroscopic, and structural characterization of the first aqueous cobalt(II)-citrate complex: Toward a potentially bioavailable form of cobalt in biologically relevant fluids. J. Biol. Inorg. Chem. 2000, 5, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Meneguzzo, F.; Delisi, R.; Pagliaro, M. Citric acid: Emerging applications of key biotechnology industrial product. Chem. Cent. J. 2017, 11, 22. [Google Scholar] [CrossRef]
- Goldberg, I.; Stefan Rokem, J. Organic and fatty acid production, microbial. Encycl. Microbiol. 2019, 358–382. [Google Scholar] [CrossRef]
- Lison, D. Chapter 25—Cobalt. In Handbook on the Toxicology of Metals; Academic Press: Cambridge, MA, USA, 2007; pp. 511–528. [Google Scholar] [CrossRef]
- Kouris, E.; Kalogiannis, S.; Perdih, F.; Turel, I.; Psomas, G. Cobalt(II) complexes of sparfloxacin: Characterization, structure, antimicrobial activity and interaction with DNA and albumins. J. Inorg. Biochem. 2016, 163, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Renfrew, A.K.; O’Neill, E.S.; Hambley, T.W.; New, E.J. Harnessing the properties of cobalt coordination complexes for biological application. Coord. Chem. Rev. 2018, 375, 221–233. [Google Scholar] [CrossRef]
- Hokin, B.; Adams, M.; Ashton, J.; Louie, H. Comparison of the dietary cobalt intake in three different Australian diets. Asia Pac. J. Clin. Nutr. 2004, 13, 289–291. [Google Scholar] [PubMed]
- Chang, E.L.; Simmers, C.; Knight, D.A. Cobalt complexes as antiviral and antibacterial agents. Pharmaceuticals 2010, 3, 1711–1728. [Google Scholar] [CrossRef] [PubMed]
- Jeon, M.J.; Ha, J.W. Synergistic bactericidal effect and mechanism of X-ray irradiation and citric acid combination against food-borne pathogens on spinach leaves. Food Microbiol. 2020, 91, 103543. [Google Scholar] [CrossRef] [PubMed]
- Saliu, O.D.; Olatunji, G.A.; Olosho, A.I.; Adeniyi, A.G.; Azeh, Y.; Samo, F.T.; Adebayo, D.O.; Ajetomobi, O.O. Barrier property enhancement of starch citrate bioplastic film by an ammonium-thiourea complex modification. J. Saudi Chem. Soc. 2019, 23, 141–149. [Google Scholar] [CrossRef]
- Seligra, P.G.; Medina Jaramillo, C.; Famá, L.; Goyanes, S. Biodegradable and non-retrogradable eco-films based on starch-glycerol with citric acid as crosslinking agent. Carbohydr. Polym. 2016, 138, 66–74. [Google Scholar] [CrossRef]
- Silveira, V.A.I.; Marim, B.M.; Hipólito, A.; Gonçalves, M.C.; Mali, S.; Kobayashi, R.K.T.; Celligoi, M.A.P.C. Characterization and antimicrobial properties of bioactive packaging films based on polylactic acid-sophorolipid for the control of foodborne pathogens. Food Packag. Shelf Life 2020, 26, 100591. [Google Scholar] [CrossRef]
- Bjarnsholt, T.; Alhede, M.; Jensen, P.Ø.; Nielsen, A.K.; Johansen, H.K.; Homøe, P.; Høiby, N.; Givskov, M.; Kirketerp-Møller, K. Antibiofilm Properties of Acetic Acid. Adv. Wound Care 2015, 4, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Dolan, S.K.; Kohlstedt, M.; Trigg, S.; Ramirez, P.V.; Kaminski, C.F.; Wittmann, C.; Welch, M. Contextual flexibility in Pseudomonas aeruginosa central carbon metabolism during growth in single carbon sources. mBio 2020, 11, e02684-19. [Google Scholar] [CrossRef]
- Kirkpatrick, C.; Maurer, L.M.; Oyelakin, N.E.; Yoncheva, Y.N.; Maurer, R.; Slonczewski, J.L. Acetate and formate stress: Opposite responses in the proteome of Escherichia coli. J. Bacteriol. 2001, 183, 6466–6477. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, N.; Raptopoulou, C.P.; Tangoulis, V.; Terzis, A.; Giapintzakis, J.; Jakusch, T.; Kiss, T.; Salifoglou, A. Correlations of Synthetic, Spectroscopic, Structural, and Speciation Studies in the Biologically Relevant Cobalt(II)-Citrate System: The Tale of the First Aqueous Dinuclear Cobalt(II)-Citrate Complex. Inorg. Chem. 2003, 42, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, S.; Murata, S.; Kimura, K.; Mori, T.; Hojo, K. Antimicrobial activity of sodium citrate against Streptococcus pneumoniae and several oral bacteria. Lett. Appl. Microbiol. 2010, 51, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Burel, C.; Kala, A.; Purevdorj-Gage, L. Impact of pH on citric acid antimicrobial activity against Gram-negative bacteria. Lett. Appl. Microbiol. 2020, 72, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Andritsos, N.D.; Stasinou, V.; Tserolas, D.; Giaouris, E. Temperature distribution and hygienic status of domestic refrigerators in Lemnos island, Greece. Food Control 2021, 127, 108121. [Google Scholar] [CrossRef]
- Wang, S.; Orsi, R.H. Chapter 11—Listeria. In Foodborne Infections and Intoxications, 4th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 199–216. [Google Scholar] [CrossRef]
- Borch, E.; Kant-Muermans, M.L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Yang, R. Antimicrobial Polylactic Acid Packaging Films against Listeria and Salmonella in Culture Medium and on Ready-to-Eat Meat. Food Bioprocess Technol. 2014, 7, 3293–3307. [Google Scholar] [CrossRef]
- Qi, Y.; Ma, H.L.; Du, Z.H.; Yang, B.; Wu, J.; Wang, R.; Zhang, X.Q. Hydrophilic and Antibacterial Modification of Poly(lactic acid) Films by γ-ray Irradiation. ACS Omega 2019, 4, 21439–21445. [Google Scholar] [CrossRef] [PubMed]
- Atria, A.M.; Cortés-Cortés, P.; Garland, M.T.; Baggio, R.; Morales, K.; Soto, M.; Corsini, G. X-ray studies and antibacterial activity in copper and cobalt complexes with imidazole derivative ligands. J. Chil. Chem. Soc. 2011, 56, 786–792. [Google Scholar] [CrossRef]
- Gad, S.C. Cobalt. In Encyclopedia of Toxicology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 996–998. [Google Scholar] [CrossRef]
- Sirsat, S.A.; Hecht, O.; Mirabal, C.; Pepe, D.A.; Yang, W.; Mohammad, Z.; Hadjiev, V.G.; Neal, J.A.; Robles Hernandez, F.C. Bacteriostatic effect of CoO-TiO2 on Listeria monocytogenes by the presence of the co-catalytic CoO nanoparticles. J. Environ. Chem. Eng. 2020, 8, 104259. [Google Scholar] [CrossRef]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763. [Google Scholar] [CrossRef]
- Regulation (EC) No 1935/2004 of the European Parliament and of the Council of 27 October 2004 on Materials and Articles Intended to Come into Contact with Food and Repealing Directives 80/590/EEC and 89/109/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32004R1935 (accessed on 23 September 2022).
- Commission Regulation (EU) 2020/1245 of 2 September 2020 Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32020R1245 (accessed on 23 September 2022).

| Ratio of Growth Parameters of Listeria monocytogenes in: | ||||
|---|---|---|---|---|
| Modification of Nutrient Broth | Modified NB with Co-cit/Modified NB without Co-cit | Modified NB/Standard NB | ||
| Max. Growth Rate | Lag Phase | Max. Growth Rate | Lag Phase | |
| None (standard NB) | 0.61 * | 1.0 | - | - |
| Sorbate (10 g/L) | 0.57 * | 1.0 | 0.69 * | 2.1 |
| Benzoate (2 g/L) | 0.89 † | 0.8 | 0.67 | 1.8 |
| Propionate (20 g/L) | 0.56 * | 0.9 | 1.03 | 1.0 |
| HCl (pH 5.5) | 0.37 *† | 2.0 | 0.95 | 1.2 |
| Acetic acid (pH 5.5) | 0.20 *† | >2.6 ‡ | 0.57 * | 3.6 |
| NaNO2 (0.15 g/L) | 0.52 * | 1.1 | 0.48 * | 1.2 |
| NaCl (50 g/L) | 0.51 * | 1.8 | 0.41 * | 0.6 |
| Ratio of Growth Parameters of Staphylococcus aureus in: | ||||
|---|---|---|---|---|
| Modification of Nutrient Broth | Modified NB with Co-cit/Modified NB without Co-cit | Modified NB/Standard NB | ||
| Max. Growth Rate | Lag Phase | Max. Growth Rate | Lag Phase | |
| None (standard NB) | 0.74 * | 1.5 | - | - |
| Sorbate (10 g/L) | 0.52 *† | 1.4 | 0.72 * | 2.0 |
| Benzoate (2 g/L) | 0.76 * | 1.2 | 0.77 * | 1.6 |
| Propionate (20 g/L) | 0.61 * | 1.2 | 0.80 * | 1.4 |
| HCl (pH 5.5) | 0.75 * | 1.2 | 1.05 | 2.2 |
| Acetic acid (pH 5.5) | 0.65 * | 1.4 | 1.03 | 1.7 |
| NaNO2 (0.15 g/L) | 0.55 * | 0.9 | 1.05 | 1.5 |
| NaCl (50 g/L) | 0.56 * | 1.0 | 0.65 * | 1.0 |
| Ratio of Growth Parameters of Escherichia coli in: | ||||
|---|---|---|---|---|
| Modification of Nutrient Broth | Modified NB with Co-cit/Modified NB without Co-cit | Modified NB/Standard NB | ||
| Max. Growth Rate | Lag Phase | Max. Growth Rate | Lag Phase | |
| None (standard NB) | 0.70 * | 1.2 | - | - |
| Sorbate (10 g/L) | 1.04 † | 1.0 | 0.36 * | 3.7 |
| Benzoate (2 g/L) | 0.76 * | 0.9 | 0.68 * | 2.0 |
| Propionate (20 g/L) | 0.55 *† | 1.3 | 1.02 | 1.2 |
| HCl (pH 5.5) | 0.79 * | 1.8 | 0.99 | 1.2 |
| Acetic acid (pH 5.5) | 1.05 † | 1.2 | 0.78 * | 3.8 |
| NaNO2 (0.15 g/L) | 0.62 * | 0.8 | 0.84 * | 1.0 |
| NaCl (50 g/L) | 0.43 *† | 1.2 | 0.21 * | 2.8 |
| Ratio of Growth Parameters of Pseudomonas aeruginosa in: | ||||
|---|---|---|---|---|
| Modification of Nutrient Broth | Modified NB with Co-cit/Modified NB without Co-cit | Modified NB/Standard NB | ||
| Max. Growth Rate | Lag Phase | Max. Growth Rate | Lag Phase | |
| None (standard NB) | 0.51 * | 6.9 | - | - |
| Sorbate (10 g/L) | 0.69 *† | 4.1 | 0.91 * | 1.8 |
| Benzoate (2 g/L) | 0.61 * | 6.9 | 0.84 * | 1.3 |
| Propionate (20 g/L) | 0.00 *† | >15.0 ‡ | 1.07 | 1.6 |
| HCl (pH 5.5) | 0.00 *† | >7.8 ‡ | 1.01 | 1.9 |
| Acetic acid (pH 5.5) | 0.04 *† | >2.2 ‡ | 1.32 * | 6.6 |
| NaNO2 (0.15 g/L) | 0.24 *† | >5.7 ‡ | 1.06 | 1.1 |
| NaCl (50 g/L) | 0.00 *† | >3.4 ‡ | 0.49 * | 1.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhoades, J.; Katsouda, V.; Matsia, S.; Rogkotis, K.; Taousani, S.; Kiriazidi, N.; Salifoglou, A.; Likotrafiti, E. Antimicrobial Activity of Cobalt (II)-Citrate against Common Foodborne Pathogens and Its Potential for Incorporation into Food Packaging Material. Appl. Sci. 2022, 12, 10855. https://doi.org/10.3390/app122110855
Rhoades J, Katsouda V, Matsia S, Rogkotis K, Taousani S, Kiriazidi N, Salifoglou A, Likotrafiti E. Antimicrobial Activity of Cobalt (II)-Citrate against Common Foodborne Pathogens and Its Potential for Incorporation into Food Packaging Material. Applied Sciences. 2022; 12(21):10855. https://doi.org/10.3390/app122110855
Chicago/Turabian StyleRhoades, Jonathan, Vilelmini Katsouda, Sevasti Matsia, Konstantinos Rogkotis, Stella Taousani, Nonna Kiriazidi, Athanasios Salifoglou, and Eleni Likotrafiti. 2022. "Antimicrobial Activity of Cobalt (II)-Citrate against Common Foodborne Pathogens and Its Potential for Incorporation into Food Packaging Material" Applied Sciences 12, no. 21: 10855. https://doi.org/10.3390/app122110855
APA StyleRhoades, J., Katsouda, V., Matsia, S., Rogkotis, K., Taousani, S., Kiriazidi, N., Salifoglou, A., & Likotrafiti, E. (2022). Antimicrobial Activity of Cobalt (II)-Citrate against Common Foodborne Pathogens and Its Potential for Incorporation into Food Packaging Material. Applied Sciences, 12(21), 10855. https://doi.org/10.3390/app122110855






