The Synergic Effect of Primary and Secondary Flame Retardants on the Improvement in the Flame Retardant and Mechanical Properties of Thermoplastic Polyurethane Nanocomposites
Abstract
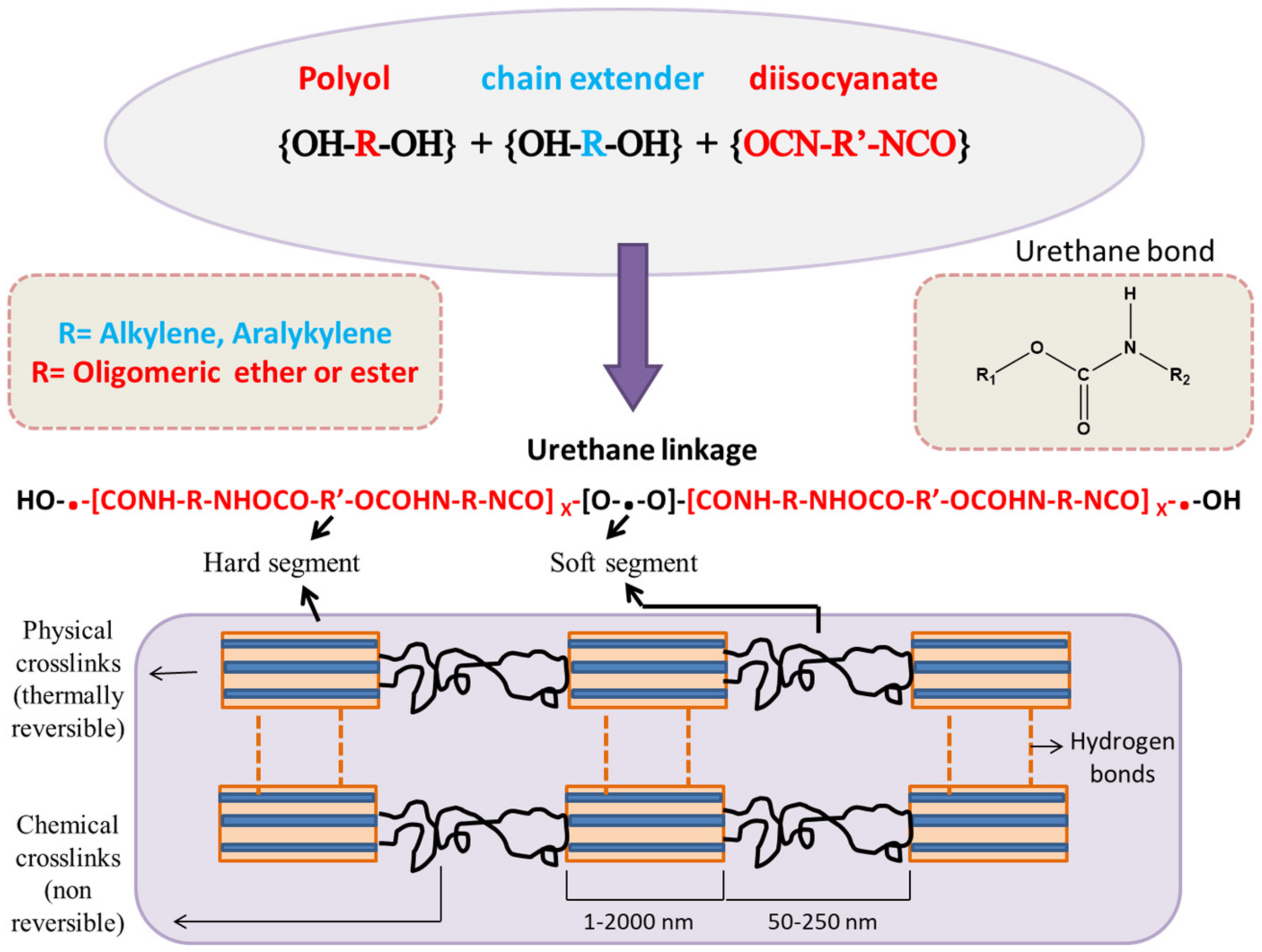
:1. Introduction
2. Experiments
2.1. Materials
2.2. Synthesis of Nanocomposites
2.3. Characterization
3. Results and Discussion
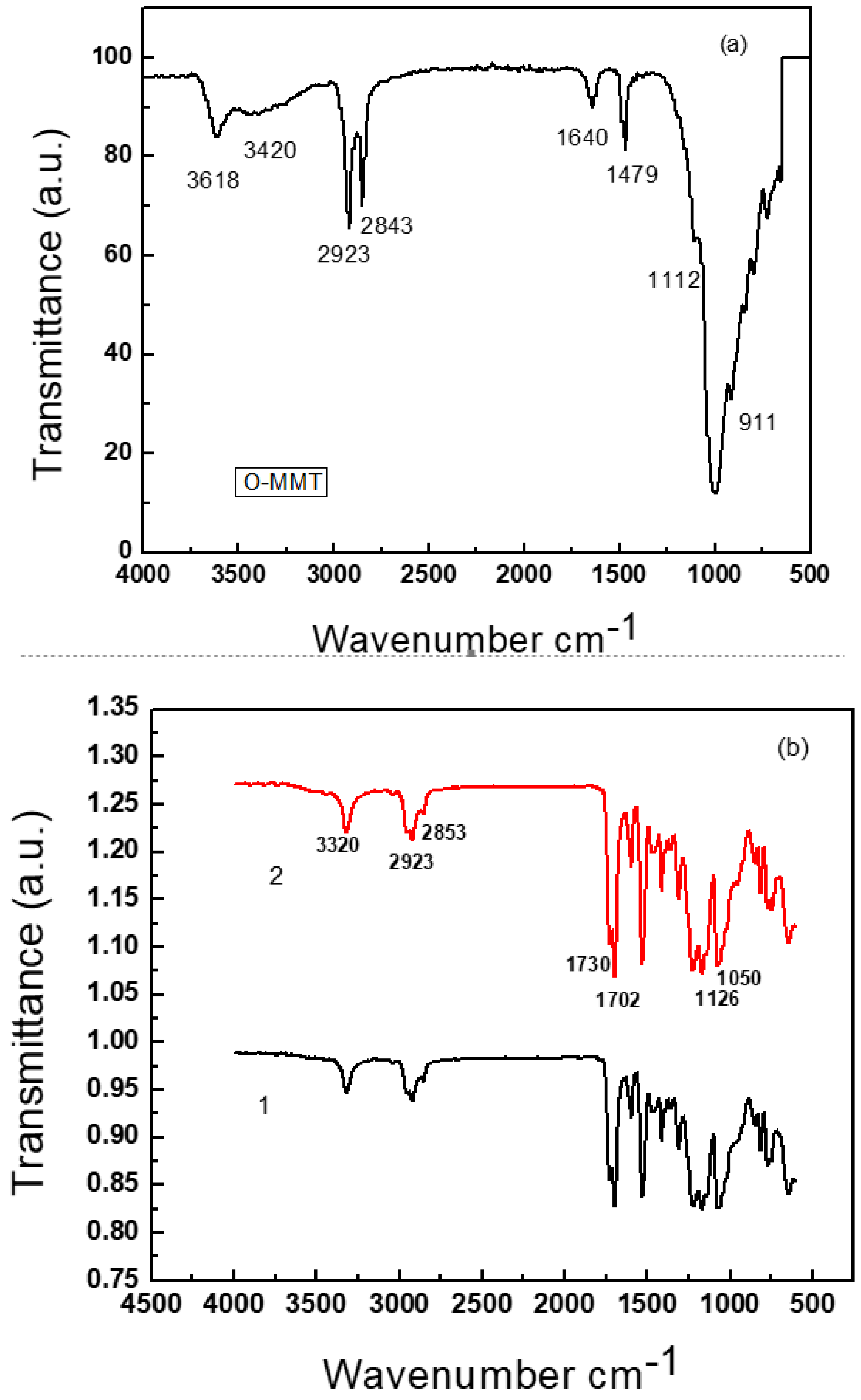
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
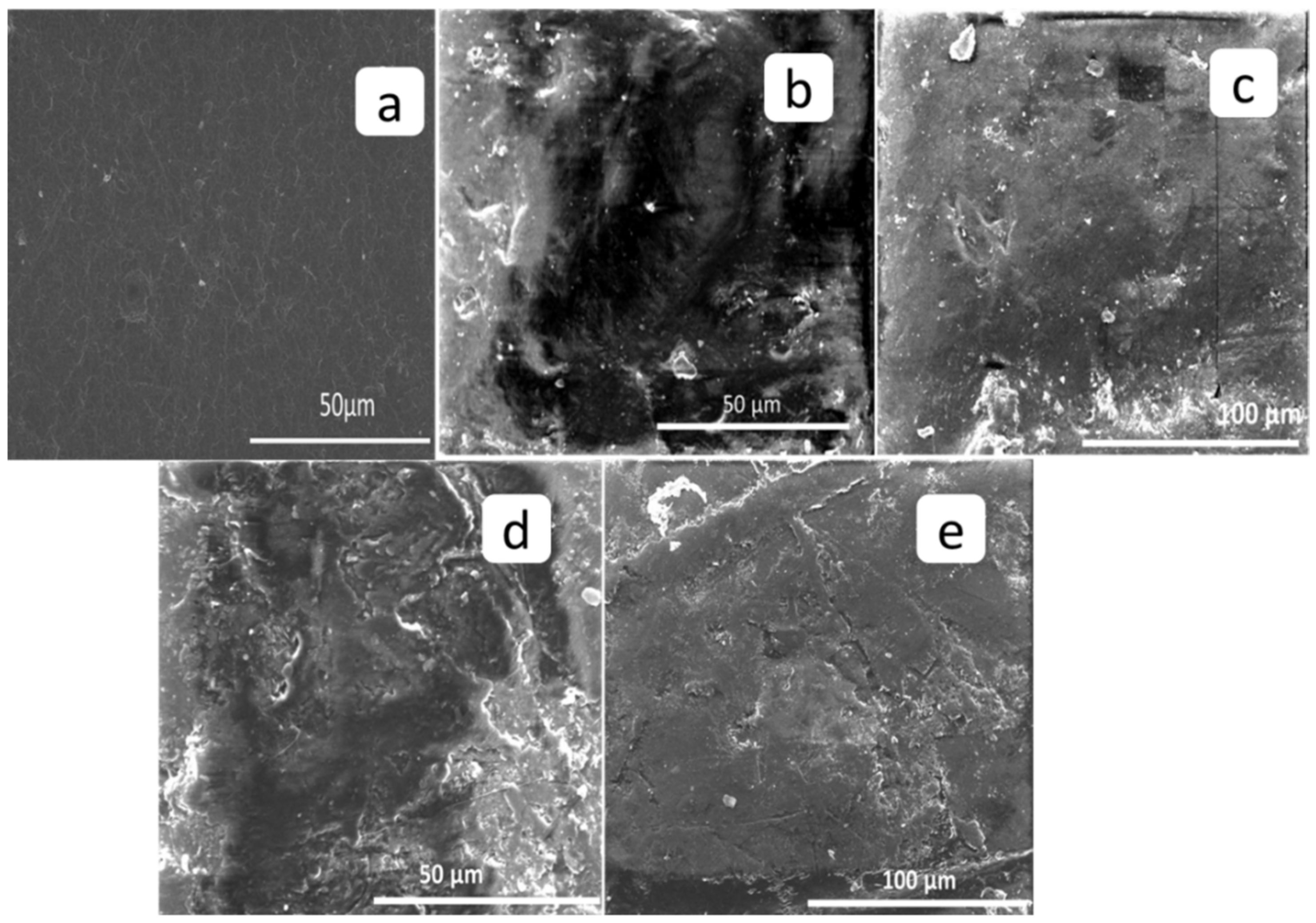
3.2. Scanning Electron Microscopy (SEM)
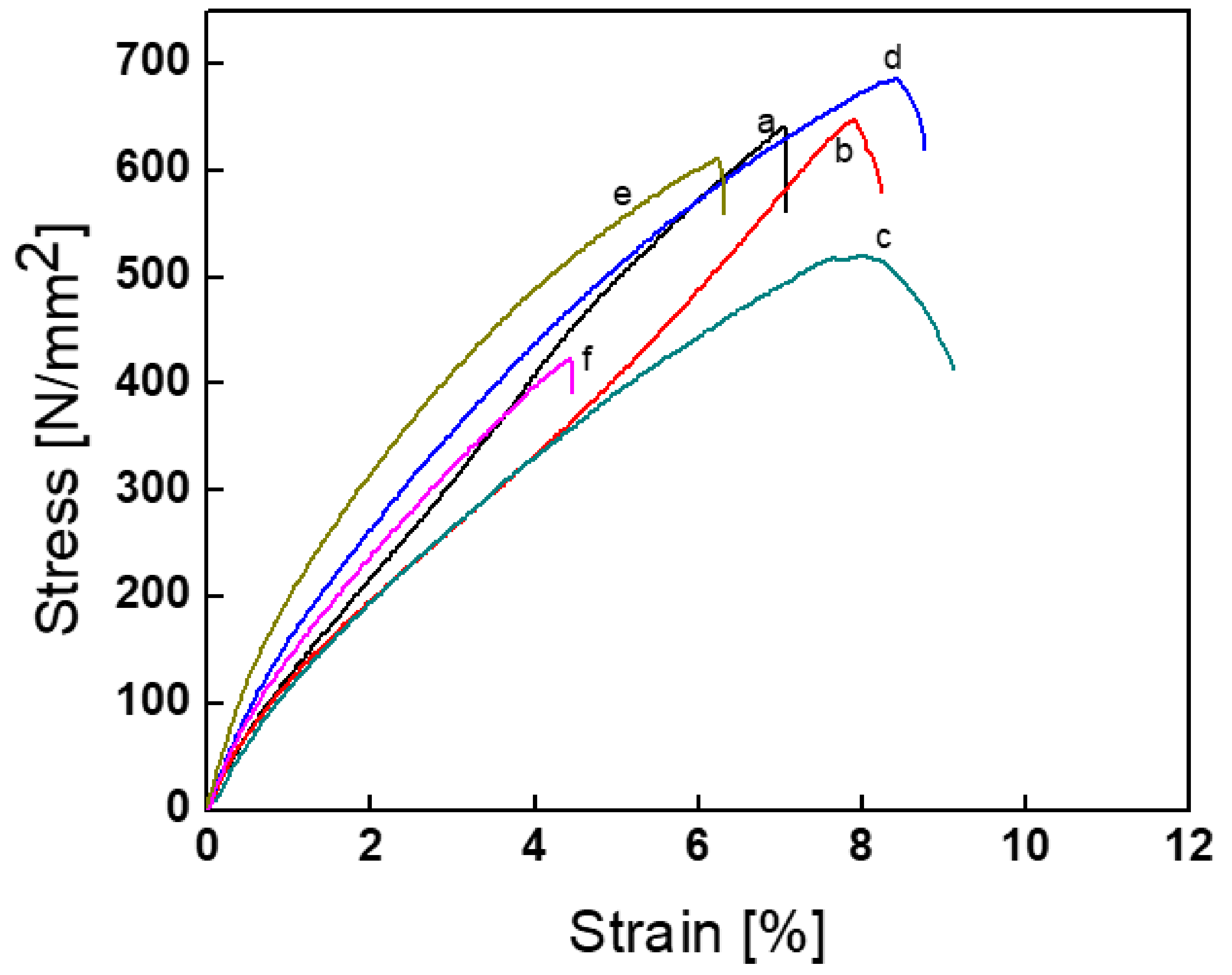
3.3. Tensile Test
3.4. Limiting Oxygen Index (LOI)
3.5. UL-94 Flammability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ates, B.; Koytepe, S.; Ulu, A.; Gürses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020, 120, 9304–9362. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Qian, X.; Yang, R. Thermal conductivity of polymers and polymer nanocomposites. Mater. Sci. Eng. R. Rep. 2018, 132, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Guo, J.; Tang, W.; Li, H.; Gu, X.; Sun, J.; Zhang, S. Enhancing the flame retardancy of thermoplastic polyurethane by introducing montmorillonite nanosheets modified with phosphorylated chitosan. Compos. Part A Appl. Sci. Manuf. 2019, 119, 291–298. [Google Scholar] [CrossRef]
- Amin, K.N.M.; Amiralian, N.; Annamalai, P.K.; Edwards, G.; Chaleat, C.; Martin, D.J. Scalable processing of thermoplastic polyurethane nanocomposites toughened with nanocellulose. Chem. Eng. J. 2016, 302, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.; Osman, A.; Andriani, Y.; Edwards, G. Thermoplastic Polyurethane (TPU)-Based Polymer Nanocomposites. In Advances in Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2012; pp. 321–350. [Google Scholar]
- Shi, Y.; Fu, L.; Chen, X.; Guo, J.; Yang, F.; Wang, J.; Zheng, Y.; Hu, Y. Hypophosphite/graphitic carbon nitride hybrids: Preparation and flame-retardant application in thermoplastic polyurethane. Nanomaterials 2017, 7, 259. [Google Scholar] [CrossRef] [Green Version]
- Mi, H.-Y.; Jing, X.; Peng, J.; Turng, L.-S.; Peng, X.-F. Influence and prediction of processing parameters on the properties of microcellular injection molded thermoplastic polyurethane based on an orthogonal array test. J. Cell. Plast. 2013, 49, 439–458. [Google Scholar] [CrossRef]
- Huang, A.; Wang, H.; Ellingham, T.; Peng, X.; Turng, L.-S. An improved technique for dispersion of natural graphite particles in thermoplastic polyurethane by sub-critical gas-assisted processing. Compos. Sci. Technol. 2019, 182, 107783. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Wang, S.; Gao, Z.; Li, C.; Hu, G.-H.; Xiong, C. Enhanced dielectric property and energy storage density of PVDF-HFP based dielectric composites by incorporation of silver nanoparticles-decorated exfoliated montmorillonite nanoplatelets. Compos. Part A Appl. Sci. Manuf. 2018, 108, 62–68. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Tabuani, D.; Bellucci, F.; Terenzi, A.; Camino, G. Flame retarded Thermoplastic Polyurethane (TPU) for cable jacketing application. Polym. Degrad. Stab. 2012, 97, 2594–2601. [Google Scholar] [CrossRef]
- Dheyaa, B.M.; Jassim, W.H.; A Hameed, N. Evaluation of the epoxy/antimony trioxide nanocomposites as flame retardant. J. Physics Conf. Ser. 2018, 1003, 012078. [Google Scholar] [CrossRef]
- Jadhav, S.D. A review of non-halogenated flame retardant. Pharma Innov. J. 2018, 7, 380. [Google Scholar]
- Riyazuddin; Bano, S.; Husain, F.M.; Khan, R.A.; Alsalme, A.; Siddique, J.A. Influence of Antimony Oxide on Epoxy Based Intumescent Flame Retardation Coating System. Polymers 2020, 12, 2721. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.-S.; Deng, C.-L.; Wang, D.-Y.; Song, Y.-P.; Wang, Y.-Z. The synergistic flame-retardant effect of O-MMT on the intumescent flame-retardant PP/CA/APP systems. Polym. Adv. Technol. 2010, 21, 789–796. [Google Scholar] [CrossRef]
- Li, J.; Wu, W.; Hu, H.; Rui, Z.; Zhao, T.; Zhang, X. The synergism effect of montmorillonite on the intumescent flame retardant thermoplastic polyurethane composites prepared by selective laser sintering. Polym. Compos. 2022, 43, 5863–5876. [Google Scholar] [CrossRef]
- Yousefi, N.; Gudarzi, M.M.; Zheng, Q.; Lin, X.; Shen, X.; Jia, J.; Sharif, F.; Kim, J.-K. Highly aligned, ultralarge-size reduced graphene oxide/polyurethane nanocomposites: Mechanical properties and moisture permeability. Compos. Part A Appl. Sci. Manuf. 2013, 49, 42–50. [Google Scholar] [CrossRef]
- Šupová, M.; Martynková, G.S.; Barabaszová, K. Effect of Nanofillers Dispersion in Polymer Matrices: A Review. Sci. Adv. Mater. 2010, 3, 1–25. [Google Scholar] [CrossRef]
- Friedrich, K. Chapter 1—Routes for Achieving Multifunctionality in Reinforced Polymers and Composite Structures. In Multifunctionality of Polymer Composites; Friedrich, K., Breuer, U., Eds.; William Andrew Publishing: Oxford, UK, 2015; pp. 3–41. [Google Scholar]
- Pras, M.; Gérard, J.-F.; Golanski, L.; Quintard, G.; Duchet-Rumeau, J. Key Role of the Dispersion of Carbon Nanotubes (CNTs) within Epoxy Networks on their Ability to Release. Polymers 2020, 12, 2530. [Google Scholar] [CrossRef]
- Martins, M.G.; Martins, D.O.; de Carvalho, B.L.; Mercante, L.A.; Soriano, S.; Andruh, M.; Vieira, M.D.; Vaz, M.G. Synthesis and characterization of montmorillonite clay intercalated with molecular magnetic compounds. J. Solid State Chem. 2015, 228, 99–104. [Google Scholar] [CrossRef]
- Jia, Q.; Zheng, M.; Chen, H.; Shen, R. Synthesis and characterization of polyurethane/epoxy interpenetrating network nanocomposites with organoclays. Polym. Bull. 2005, 54, 65–73. [Google Scholar] [CrossRef]
- Alshabanat, M.; Al-Arrash, A.; Mekhamer, W. Polystyrene/montmorillonite nanocomposites: Study of the morphology and effects of sonication time on thermal stability. J. Nanomater. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Adak, B.; Joshi, M.; Butola, B.S. Polyurethane/clay nanocomposites with improved helium gas barrier and mechanical properties: Direct versus master-batch melt mixing route. J. Appl. Polym. Sci. 2018, 135, 46422. [Google Scholar] [CrossRef]
- Lan, B.; Li, P.; Luo, X.; Luo, H.; Yang, Q.; Gong, P. Hydrogen bonding and topological network effects on optimizing thermoplastic polyurethane/organic montmorillonite nanocomposite foam. Polymer 2021, 212, 123159. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, Y.; Song, L.; Liu, L.; Wang, Z.; Chen, Z.; Fan, W. Synthesis and characterization of thermoplastic polyurethane/montmorillonite nanocomposites produced by reactive extrusion. J. Mater. Sci. 2007, 42, 5785–5790. [Google Scholar] [CrossRef]
- Li, Y.; Lv, L.; Wang, W.; Zhang, J.; Lin, J.; Zhou, J.; Dong, M.; Gan, Y.; Seok, I.; Guo, Z. Effects of chlorinated polyethylene and antimony trioxide on recycled polyvinyl chloride/acryl-butadiene-styrene blends: Flame retardancy and mechanical properties. Polymer 2020, 190, 122198. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M. Thermoplastic polymer nanocomposites based on inorganic fullerene-like nanoparticles and inorganic nanotubes. Inorganics 2014, 2, 291–312. [Google Scholar] [CrossRef] [Green Version]
- Barick, A.K.; Tripathy, D.K. Tripathy, and Science, Effect of organoclay on the morphology, mechanical, thermal, and rheological properties of organophilic montmorillonite nanoclay based thermoplastic polyurethane nanocomposites prepared by melt blending. Polym. Eng. Sci. 2010, 50, 484–498. [Google Scholar] [CrossRef]
- Sattar, R.; Kausar, A.; Siddiq, M. Advances in thermoplastic polyurethane composites reinforced with carbon nanotubes and carbon nanofibers: A review. J. Plast. Film Sheeting 2015, 31, 186–224. [Google Scholar] [CrossRef]
- Wang, L.-C.; Sun, Q.; Zhang, C.-C. The charring effect and flame retardant properties of thermoplastic elastomers composites applied for cable. Fibers Polym. 2020, 21, 2599–2606. [Google Scholar] [CrossRef]
- de Oliveira, S.V.; dos Santos Filho, E.A.; Araújo, E.M.; Pereira, C.M.; Passador, F.R. Preparation and Flammability Properties of Polyethylene/Organoclay Nanocomposites. In Diffusion Foundations; Trans Tech Publications Ltd.: Switzerland, 2019. [Google Scholar]
| Raw Material (wt. %) | TPU-1 | TPU-2 | TPU-3 | TPU-4 | TPU-5 | TPU-6 |
|---|---|---|---|---|---|---|
| TPU | 100.00 | 97.00 | 97.00 | 94.00 | 93.80 | 93.50 |
| Benzoflex | 3.00 | 3.00 | 3.00 | 3.00 | ||
| Sb2O3 | 3.00 | 3.00 | 3.00 | 3.00 | ||
| MMT nanoclay | 0.20 | 0.50 | ||||
| Total wt. % | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Sample | Young’s Modulus (MPa) | Tensile Strength (MPa) | Stress at Break (MPa) | Strain at Break (%) |
|---|---|---|---|---|
| TPU-1 | 147.348 | 4.234 | 422.04 | 4.40 |
| TPU-2 | 161.811 | 6.105 | 612.56 | 6.99 |
| TPU-3 | 147.641 | 5.194 | 517.56 | 6.24 |
| TPU-4 | 195.053 | 6.848 | 647.86 | 8.12 |
| TPU-5 | 244.568 | 6.488 | 684.72 | 8.35 |
| TPU-6 | 180.989 | 6.408 | 638.52 | 7.86 |
| Sample | LOI | UL-Ranking | UL-Dripping | t1/t2 (s) a | N b |
|---|---|---|---|---|---|
| TPU-1 | 19 | V-2 | yes | >50 | >28 |
| TPU-2 | 19 | V-2 | yes | 42/15 | >18 |
| TPU-3 | 21 | V-1 | yes | 20/10 | >12 |
| TPU-4 | 21 | V-1 | yes | 18/8 | >10 |
| TPU-5 | 26 | V-1 | yes | 9/7 | 0/3 |
| TPU-6 | 29 | V-0 | no | 0/2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faryal, S.; Zafar, M.; Nazir, M.S.; Ali, Z.; Hussain, M.; Muhammad Imran, S. The Synergic Effect of Primary and Secondary Flame Retardants on the Improvement in the Flame Retardant and Mechanical Properties of Thermoplastic Polyurethane Nanocomposites. Appl. Sci. 2022, 12, 10866. https://doi.org/10.3390/app122110866
Faryal S, Zafar M, Nazir MS, Ali Z, Hussain M, Muhammad Imran S. The Synergic Effect of Primary and Secondary Flame Retardants on the Improvement in the Flame Retardant and Mechanical Properties of Thermoplastic Polyurethane Nanocomposites. Applied Sciences. 2022; 12(21):10866. https://doi.org/10.3390/app122110866
Chicago/Turabian StyleFaryal, Sidra, Muhammad Zafar, M. Shahid Nazir, Zulfiqar Ali, Manwar Hussain, and Syed Muhammad Imran. 2022. "The Synergic Effect of Primary and Secondary Flame Retardants on the Improvement in the Flame Retardant and Mechanical Properties of Thermoplastic Polyurethane Nanocomposites" Applied Sciences 12, no. 21: 10866. https://doi.org/10.3390/app122110866
APA StyleFaryal, S., Zafar, M., Nazir, M. S., Ali, Z., Hussain, M., & Muhammad Imran, S. (2022). The Synergic Effect of Primary and Secondary Flame Retardants on the Improvement in the Flame Retardant and Mechanical Properties of Thermoplastic Polyurethane Nanocomposites. Applied Sciences, 12(21), 10866. https://doi.org/10.3390/app122110866







