Estimation of Physicochemical Parameters and Vertical Migration of Atmospheric Radionuclides in a Raised Peat Bog in the Arctic Zone of Russia
Abstract
:1. Introduction
2. Materials and Methods
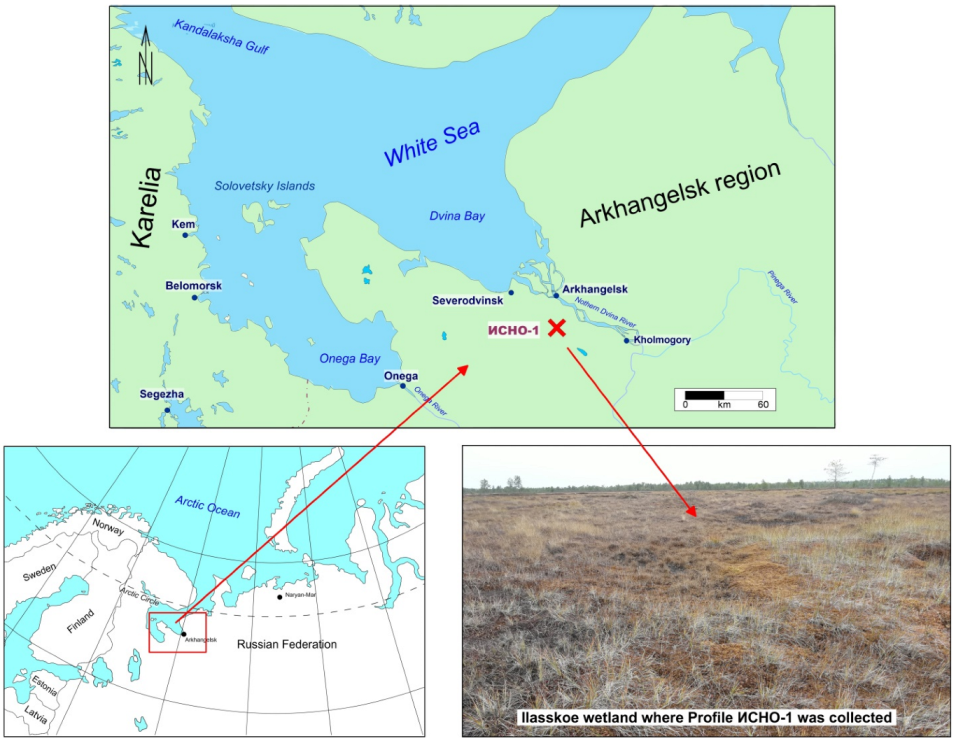
2.1. Study Area and Sample Collection
2.2. Study of Physicochemical Parameters of Peat Samples
2.2.1. Determination of Active and Exchangeable Acidity
2.2.2. Determination of Water-Soluble Salts Content
2.2.3. Determination of the Mass Fraction of Organic Matter and Carbonates
2.2.4. Assessment of the Degree of Decomposition, Natural Moisture Content and Bulk Density of Peat
2.2.5. In Situ Determination of the Oxidation–Reduction Potential and pH of the Peat Profile
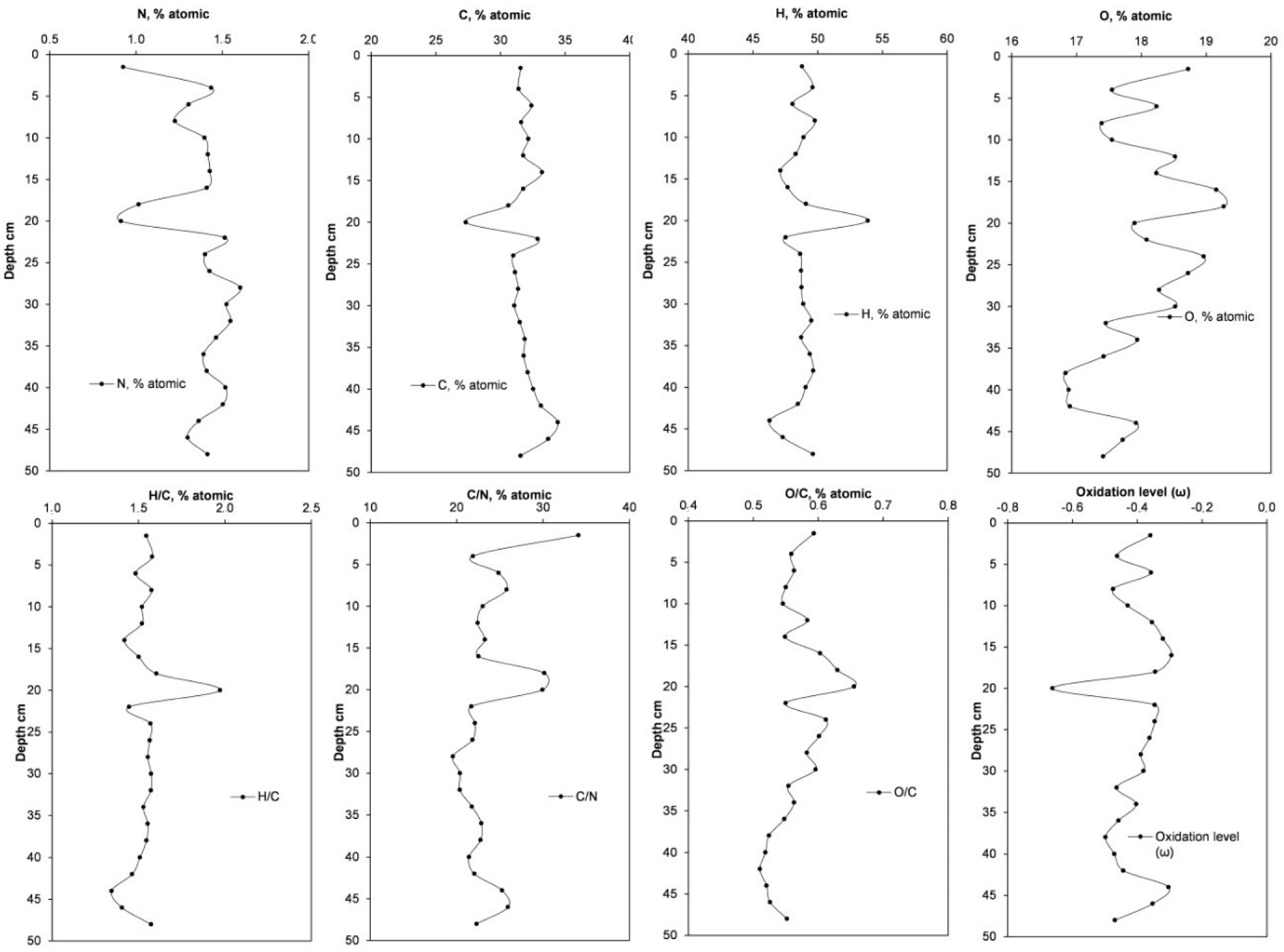
2.2.6. Determination of C, H, N and O in the Peat Profile
2.3. Gamma Spectrometry Measurements
2.4. Determination of 210Pb and U Isotopes
2.5. 210Pb Dating of the Peat Core
3. Results and Discussion
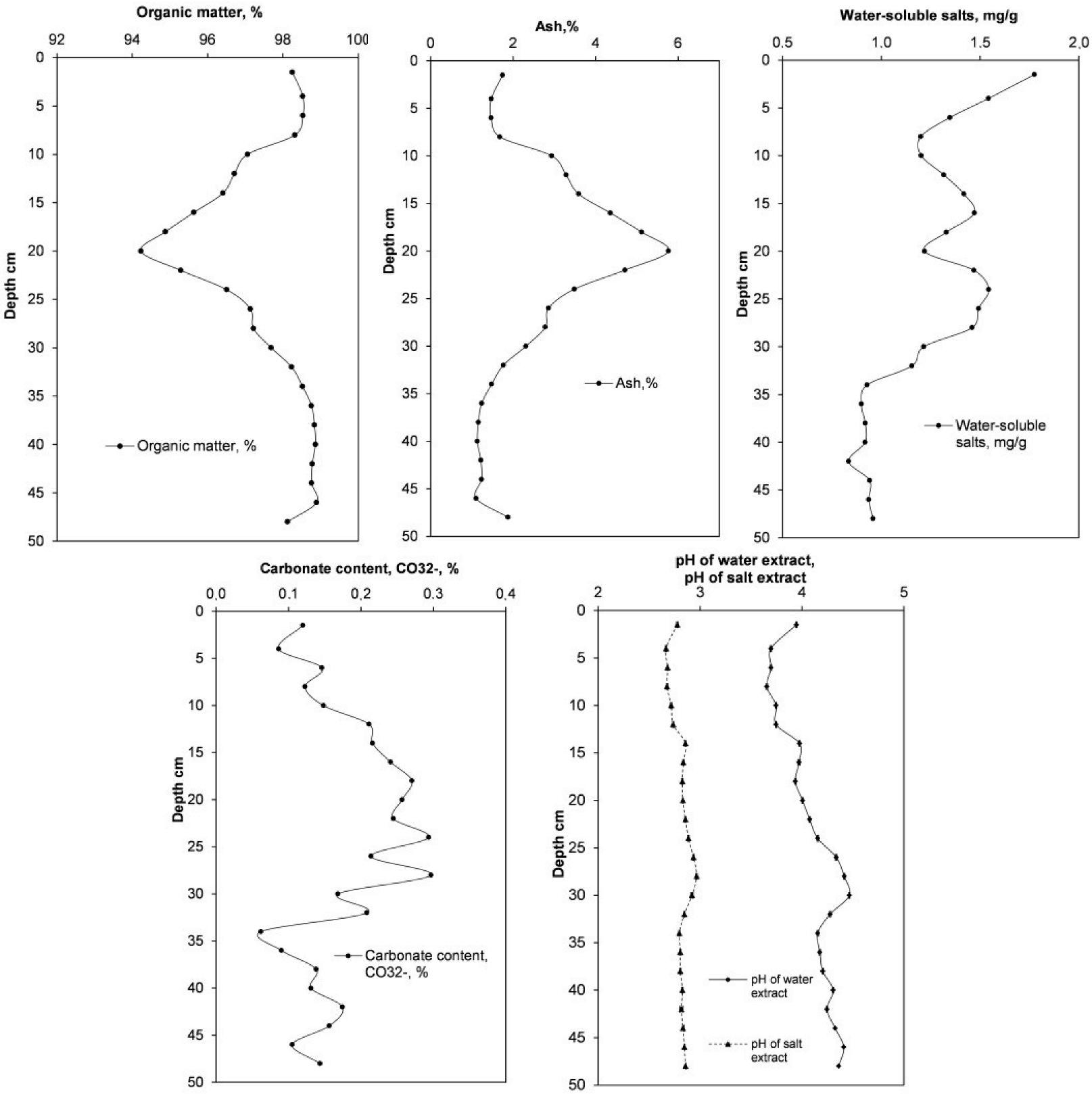
3.1. Physicochemical Characteristics of the Peat Profile
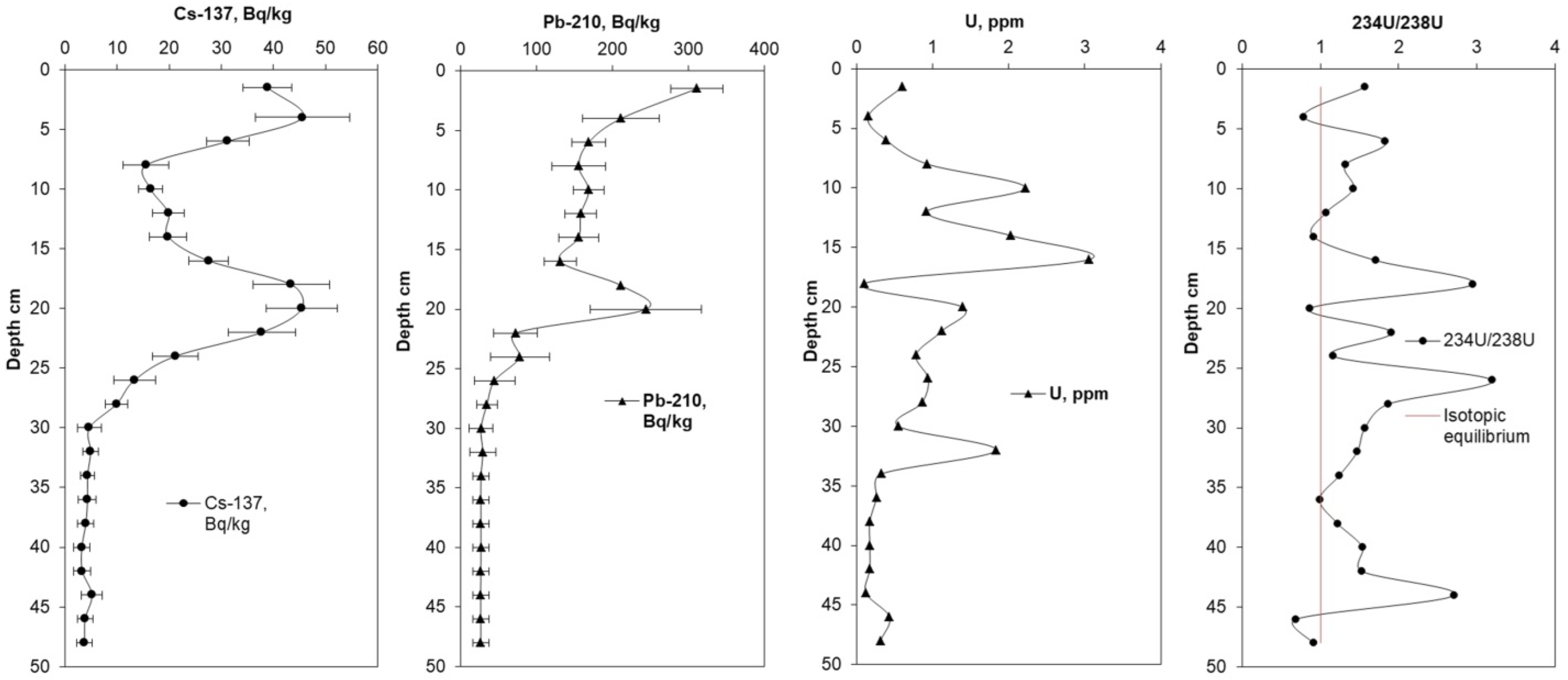
3.2. Vertical Distribution of Radionuclides in the Peat Profile
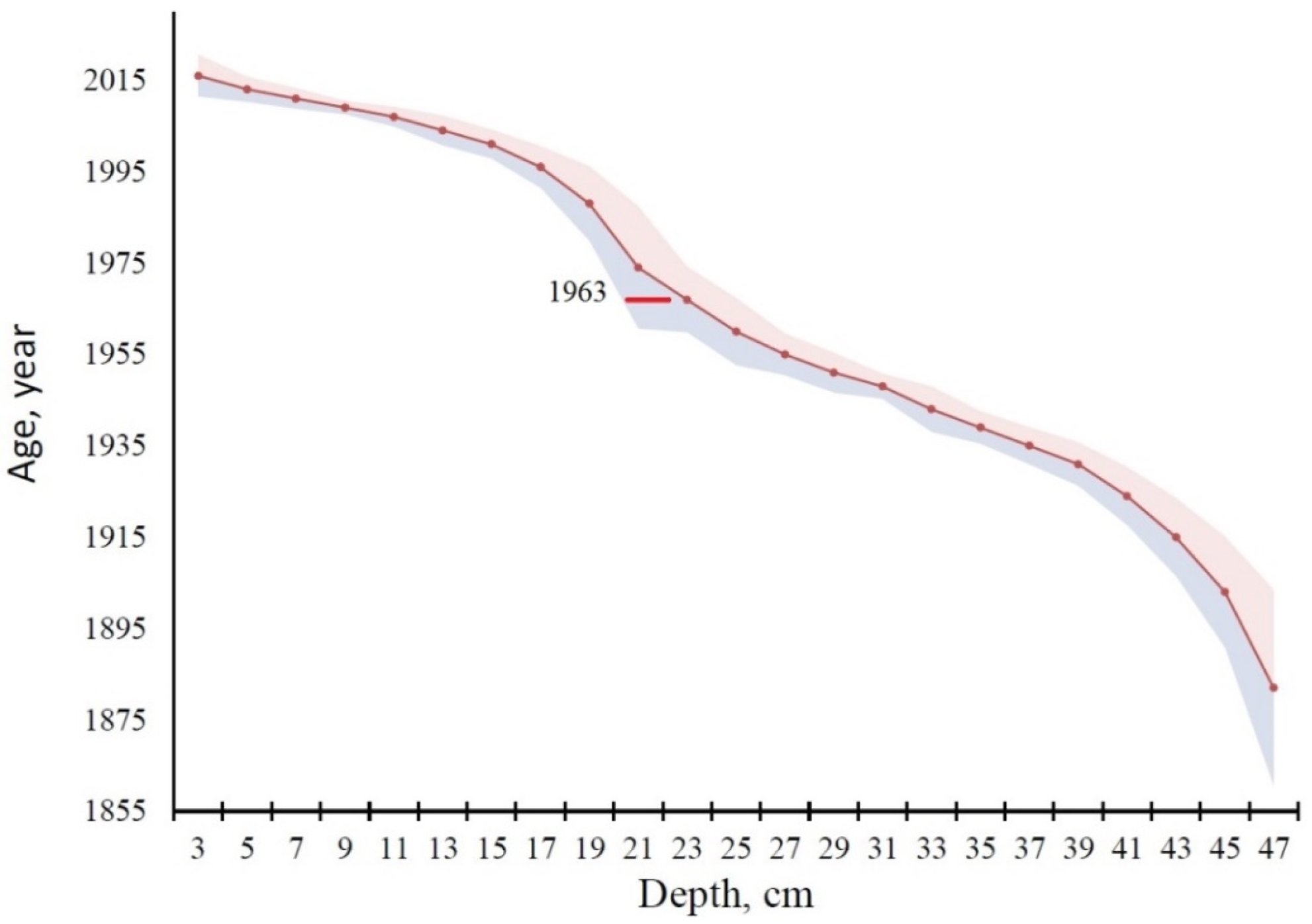
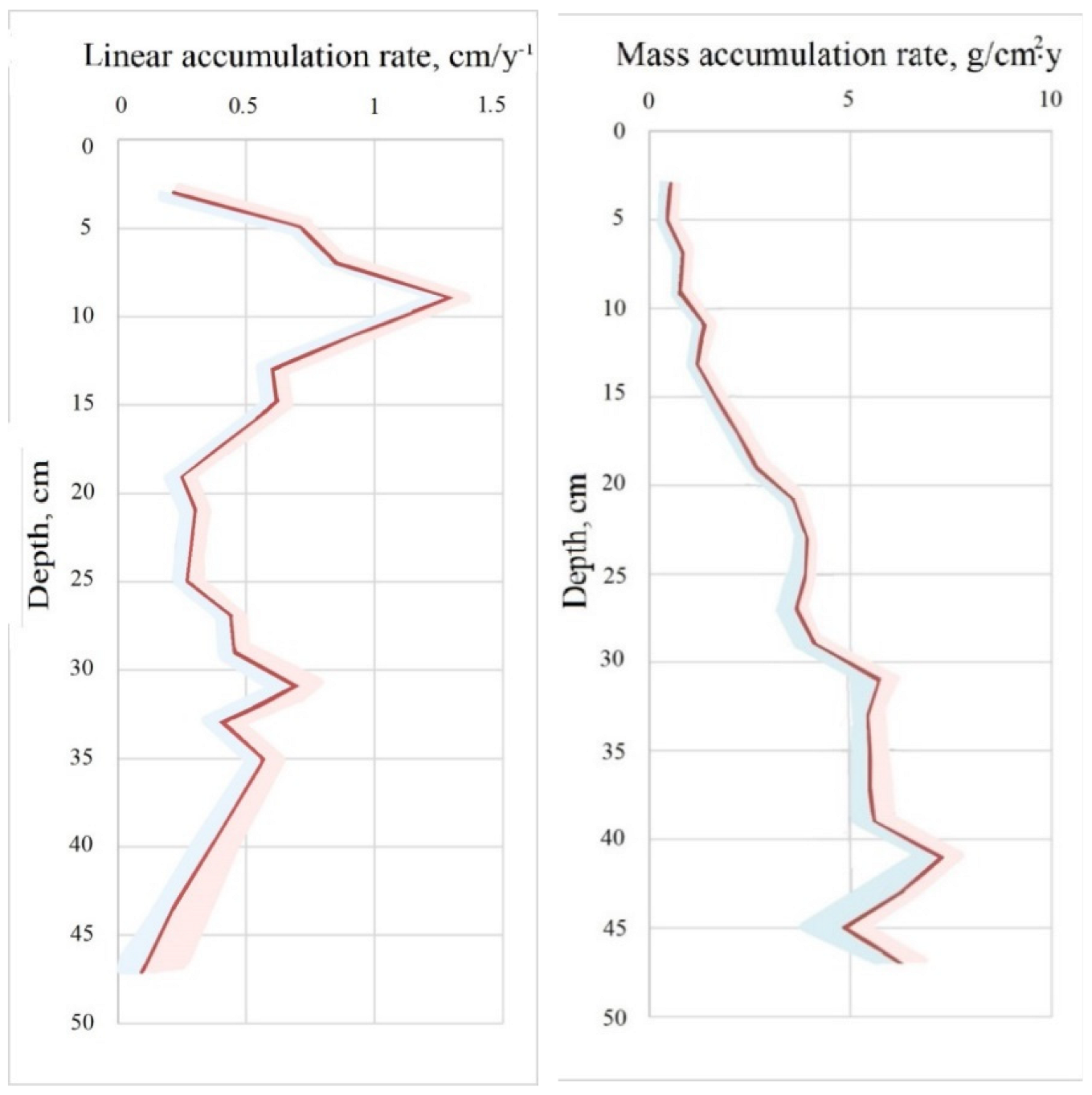
3.3. Peat Chronology and Accumulation Rate
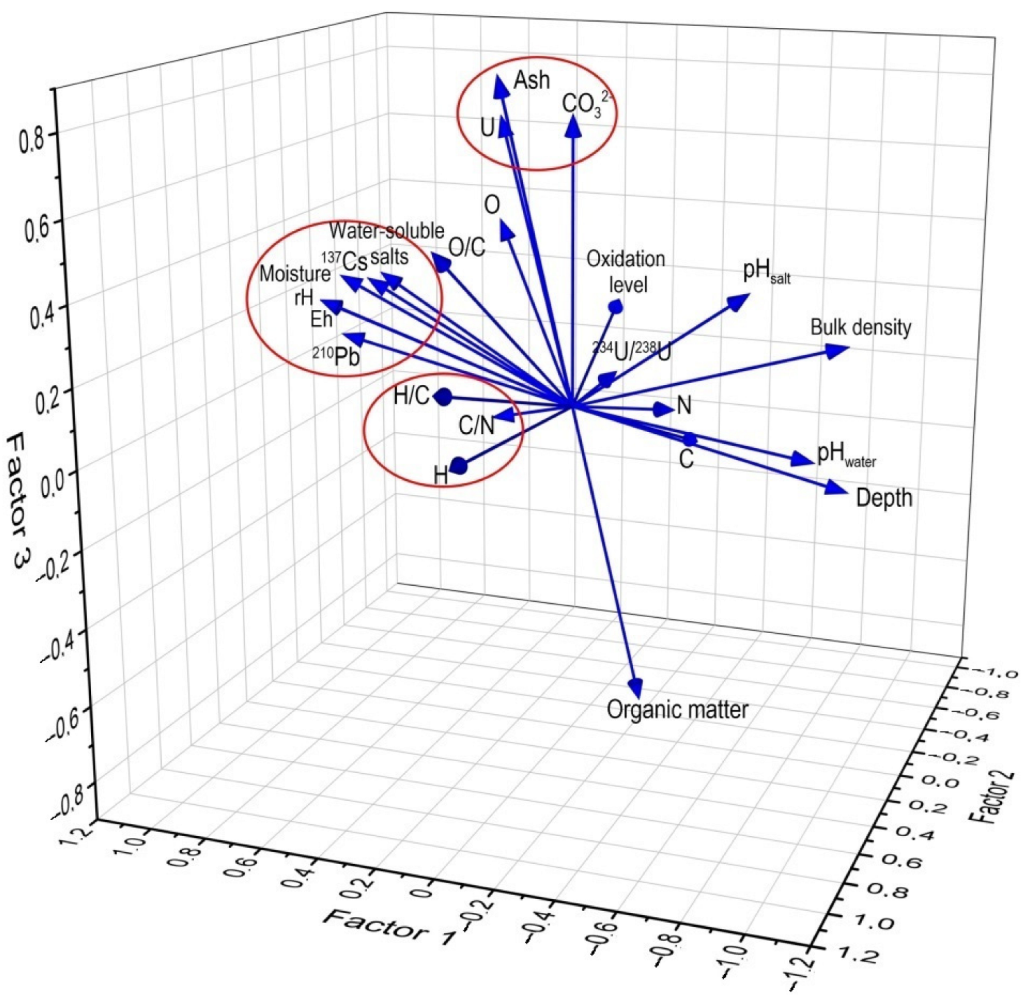
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Layer (cm) | LOI (%) | Carbonate Content (CO32− %) | Ash (%) | pH of Water Extract | pH of Salt Extract | Water-Soluble Salts (mg/g) | Organic Matter (%) |
|---|---|---|---|---|---|---|---|
| 0–3 | 0.09 ± 0.018 | 0.12 ± 0.024 | 1.75 | 3.95 ± 0.005 | 2.78 ± 0.005 | 1.78 | 98.25 ± 0.005 |
| 3–5 | 0.06 ± 0.003 | 0.09 ± 0.004 | 1.47 | 3.70 ± 0.005 | 2.67 ± 0.005 | 1.54 | 98.53 ± 0.035 |
| 5–7 | 0.11 ± 0.054 | 0.15 ± 0.074 | 1.46 | 3.70 ± 0.005 | 2.68 ± 0.005 | 1.35 | 98.53 ± 0.067 |
| 7–9 | 0.09 ± 0.029 | 0.12 ± 0.039 | 1.68 | 3.66 ± 0.005 | 2.68 ± 0.005 | 1.20 | 98.32 ± 0.012 |
| 9–11 | 0.11 ± 0.045 | 0.15 ± 0.061 | 2.94 | 3.75 ± 0.005 | 2.72 ± 0.005 | 1.20 | 97.06 ± 0.029 |
| 11–13 | 0.16 ± 0.010 | 0.21 ± 0.013 | 3.29 | 3.75 ± 0.005 | 2.74 ± 0.005 | 1.32 | 96.71 ± 0.039 |
| 13–15 | 0.16 ± 0.020 | 0.22 ± 0.028 | 3.59 | 3.98 ± 0.005 | 2.86 ± 0.005 | 1.42 | 96.41 ± 0.008 |
| 15–17 | 0.18 ± 0.015 | 0.24 ± 0.020 | 4.36 | 3.97 ± 0.010 | 2.84 ± 0.005 | 1.47 | 95.64 ± 0.028 |
| 17–19 | 0.20 ± 0.004 | 0.27 ± 0.001 | 5.12 | 3.94 ± 0.005 | 2.83 ± 0.005 | 1.33 | 94.88 ± 0.135 |
| 19–21 | 0.19 ± 0.010 | 0.26 ± 0.014 | 5.77 | 4.01 ± 0.005 | 2.83 ± 0.005 | 1.22 | 94.23 ± 0.251 |
| 21–23 | 0.18 ± 0.004 | 0.24 ± 0.006 | 4.72 | 4.08 ± 0.005 | 2.86 ± 0.005 | 1.47 | 95.28 ± 0.247 |
| 23–25 | 0.22 ± 0.015 | 0.29 ± 0.020 | 3.49 | 4.16 ± 0.005 | 2.89 ± 0.005 | 1.54 | 96.51 ± 0.115 |
| 25–27 | 0.16 ± 0.026 | 0.21 ± 0.036 | 2.86 | 4.34 ± 0.005 | 2.94 ± 0.005 | 1.49 | 97.14 ± 0.061 |
| 27–29 | 0.22 ± 0.041 | 0.30 ± 0.056 | 2.78 | 4.42 ± 0.005 | 2.97 ± 0.005 | 1.46 | 97.22 ± 0.023 |
| 29–31 | 0.12 ± 0.015 | 0.17 ± 0.021 | 2.31 | 4.47 ± 0.005 | 2.92 ± 0.010 | 1.22 | 97.69 ± 0.001 |
| 31–33 | 0.15 ± 0.008 | 0.21 ± 0.010 | 1.77 | 4.28 ± 0.005 | 2.85 ± 0.005 | 1.16 | 98.23 ± 0.001 |
| 33–35 | 0.05 ± 0.023 | 0.06 ± 0.031 | 1.48 | 4.16 ± 0.005 | 2.80 ± 0.005 | 0.93 | 98.52 ± 0.020 |
| 35–37 | 0.07 ± 0.029 | 0.09 ± 0.039 | 1.24 | 4.18 ± 0.005 | 2.81 ± 0.005 | 0.90 | 98.76 ± 0.004 |
| 37–39 | 0.10 ± 0.019 | 0.14 ± 0.025 | 1.16 | 4.21 ± 0.005 | 2.81 ± 0.005 | 0.92 | 98.84 ± 0.020 |
| 39–41 | 0.10 ± 0.013 | 0.13 ± 0.017 | 1.13 | 4.31 ± 0.005 | 2.83 ± 0.005 | 0.92 | 98.87 ± 0.011 |
| 41–43 | 0.13 ± 0.023 | 0.17 ± 0.023 | 1.22 | 4.25 ± 0.005 | 2.82 ± 0.015 | 0.84 | 98.78 ± 0.017 |
| 43–45 | 0.11 ± 0.008 | 0.16 ± 0.011 | 1.24 | 4.33 ± 0.005 | 2.83 ± 0.010 | 0.94 | 98.76 ± 0.019 |
| 45–47 | 0.08 ± 0.017 | 0.11 ± 0.023 | 1.10 | 4.41 ± 0.010 | 2.85 ± 0.005 | 0.94 | 98.90 ± 0.013 |
| 47–50 | 0.11 ± 0.013 | 0.14 ± 0.018 | 1.88 | 4.36 ± 0.010 | 2.86 ± 0.005 | 0.96 | 98.12 ± 0.010 |
| Layer (cm) | 137Cs (Bq·kg−1) | 210Pb (Bq·kg−1) | 234U (Bq·kg−1) | 238U (Bq·kg−1) | U (ppm) | 234U/238U |
|---|---|---|---|---|---|---|
| 0–3 | 38.8 ± 4.66 | 310.76 ± 34.18 | 1.29 ± 0.80 | 0.82 ± 0.59 | 0.60 ± 0.010 | 1.57 ± 0.10 |
| 3–5 | 45.6 ± 9.11 | 211.16 ± 50.68 | 0.17 ± 0.06 | 0.21 ± 0.07 | 0.15 ± 0.100 | 0.79 ± 0.07 |
| 5–7 | 31.2 ± 4.06 | 168.39 ± 21.89 | 0.98 ± 0.36 | 0.54 ± 0.17 | 0.39 ± 0.070 | 1.83 ± 0.11 |
| 7–9 | 15.5 ± 4.35 | 155.36 ± 35.73 | 1.69 ± 0.53 | 1.28 ± 0.38 | 0.93 ± 0.010 | 1.32 ± 0.06 |
| 9–11 | 16.4 ± 2.30 | 168.49 ± 20.22 | 4.29 ± 2.51 | 3.03 ± 2.05 | 2.22 ± 0.090 | 1.42 ± 0.08 |
| 11–13 | 19.8 ± 2.97 | 158.02 ± 20.54 | 1.34 ± 0.46 | 1.26 ± 0.37 | 0.92 ± 0.020 | 1.07 ± 0.05 |
| 13–15 | 19.8 ± 3.56 | 155.02 ± 26.35 | 2.52 ± 0.87 | 2.76 ± 0.72 | 2.03 ± 0.010 | 0.91 ± 0.08 |
| 15–17 | 27.5 ± 3.85 | 131.06 ± 20.97 | 7.12 ± 2.77 | 4.17 ± 1.27 | 3.06 ± 0.060 | 1.71 ± 0.09 |
| 17–19 | 43.4 ± 7.38 | 180.80 ± 20.32 | 0.42 ± 0.25 | 0.14 ± 0.05 | 0.10 ± 0.020 | 2.95 ± 0.08 |
| 19–21 | 45.5 ± 6.82 | 243.94 ± 73.18 | 1.62 ± 0.55 | 1.85 ± 0.49 | 1.39 ± 0.020 | 0.87 ± 0.06 |
| 21–23 | 37.8 ± 6.42 | 72.21 ± 28.88 | 2.95 ± 0.90 | 1.55 ± 0.49 | 1.12 ± 0.050 | 1.91 ± 0.09 |
| 23–25 | 21.1 ± 4.44 | 77.90 ± 38.95 | 1.25 ± 0.40 | 1.08 ± 0.35 | 0.78 ± 0.020 | 1.16 ± 0.34 |
| 25–27 | 13.3 ± 4.00 | 44.42 ± 26.65 | 4.10 ± 1.25 | 1.29 ± 0.37 | 0.94 ± 0.010 | 3.19 ± 0.07 |
| 27–29 | 9.9 ± 2.17 | 34.35 ± 13.74 | 2.22 ± 0.75 | 1.19 ± 0.43 | 0.86 ± 0.020 | 1.87 ± 0.09 |
| 29–31 | 4.6 ± 2.32 | 26.55 ± 15.93 | 1.19 ± 0.38 | 0.76 ± 0.28 | 0.55 ± 0.010 | 1.57 ± 0.16 |
| 31–33 | 4.9 ± 1.46 | 28.80 ± 17.28 | 3.69 ± 1.09 | 2.50 ± 0.88 | 1.83 ± 0.010 | 1.47 ± 0.09 |
| 33–35 | 4.3 ± 1.29 | 26.52 ± 10.61 | 0.54 ± 0.29 | 0.44 ± 0.26 | 0.32 ± 0.003 | 1.24 ± 0.11 |
| 35–37 | 4.3 ± 1.71 | 26.34 ± 10.53 | 0.35 ± 0.13 | 0.36 ± 0.09 | 0.26 ± 0.010 | 0.99 ± 0.10 |
| 37–39 | 4.0 ± 1.58 | 26.41 ± 10.57 | 0.28 ± 0.09 | 0.23 ± 0.08 | 0.17 ± 0.003 | 1.22 ± 0.11 |
| 39–41 | 3.2 ± 1.60 | 26.50 ± 10.60 | 0.35 ± 0.21 | 0.23 ± 0.16 | 0.17 ± 0.002 | 1.54 ± 0.09 |
| 41–43 | 3.3 ± 1.64 | 26.30 ± 10.52 | 0.36 ± 0.20 | 0.24 ± 0.14 | 0.17 ± 0.010 | 1.53 ± 0.05 |
| 43–45 | 5.1 ± 2.05 | 26.19 ± 10.47 | 0.44 ± 0.24 | 0.16 ± 0.14 | 0.12 ± 0.020 | 2.71 ± 0.12 |
| 45–47 | 3.8 ± 1.53 | 26.24 ± 10.49 | 0.41 ± 0.14 | 0.58 ± 0.19 | 0.43 ± 0.060 | 0.69 ± 0.04 |
| 47–50 | 3.7 ± 1.50 | 26.32 ± 10.53 | 0.38 ± 0.12 | 0.42 ± 0.16 | 0.31 ± 0.020 | 0.91 ± 0.08 |
| Layer (cm) | Elemental Composition of Organic Matter of Peat (% Atomic) | H/C | C/N | Oxidation Level (ω) | |||
|---|---|---|---|---|---|---|---|
| N | C | H | O | ||||
| 0–3 | 0.93 ± 0.11 | 31.57 ± 0.48 | 48.79 ± 0.89 | 18.72 | 1.55 | 34.1 | −0.36 |
| 3–5 | 1.44 ± 0.14 | 31.41 ± 0.03 | 49.60 ± 0.60 | 17.55 | 1.58 | 21.9 | −0.46 |
| 5–7 | 1.30 ± 0.04 | 32.41 ± 0.01 | 48.06 ± 0.97 | 18.23 | 1.48 | 24.9 | −0.36 |
| 7–9 | 1.23 ± 0.03 | 31.61 ± 0.06 | 49.78 ± 0.30 | 17.39 | 1.57 | 25.8 | −0.47 |
| 9–11 | 1.40 ± 0.02 | 32.16 ± 0.05 | 48.90 ± 0.50 | 17.55 | 1.52 | 23.0 | −0.43 |
| 11–13 | 1.42 ± 0.01 | 31.76 ± 0.06 | 48.30 ± 0.21 | 18.52 | 1.52 | 22.4 | −0.35 |
| 13–15 | 1.43 ± 0.03 | 33.22 ± 0.14 | 47.12 ± 0.59 | 18.23 | 1.42 | 23.3 | −0.32 |
| 15–17 | 1.41 ± 0.16 | 31.77 ± 0.02 | 47.67 ± 0.42 | 19.16 | 1.50 | 22.5 | −0.29 |
| 17–19 | 1.02 ± 0.06 | 30.62 ± 0.01 | 49.10 ± 4.21 | 19.27 | 1.60 | 30.2 | −0.34 |
| 19–21 | 0.91 ± 0.05 | 27.32 ± 0.79 | 53.87 ± 1.79 | 17.90 | 1.97 | 29.9 | −0.66 |
| 21–23 | 1.51 ± 0.01 | 32.88 ± 0.05 | 47.53 ± 0.43 | 18.08 | 1.45 | 21.7 | −0.35 |
| 23–25 | 1.40 ± 0.01 | 30.99 ± 0.18 | 48.64 ± 1.72 | 18.96 | 1.57 | 22.1 | −0.35 |
| 25–27 | 1.43 ± 0.05 | 31.13 ± 0.25 | 48.72 ± 0.28 | 18.72 | 1.56 | 21.8 | −0.36 |
| 27–29 | 1.60 ± 0.02 | 31.38 ± 0.13 | 48.75 ± 0.06 | 18.27 | 1.55 | 19.6 | −0.39 |
| 29–31 | 1.52 ± 0.03 | 31.08 ± 0.06 | 48.88 ± 0.80 | 18.52 | 1.57 | 20.4 | −0.38 |
| 31–33 | 1.55 ± 0.01 | 31.50 ± 0.12 | 49.50 ± 1.43 | 17.45 | 1.57 | 20.4 | −0.46 |
| 33–35 | 1.46 ± 0.06 | 31.88 ± 0.02 | 48.72 ± 0.78 | 17.94 | 1.53 | 21.8 | −0.40 |
| 35–37 | 1.39 ± 0.05 | 31.80 ± 0.08 | 49.39 ± 0.28 | 17.42 | 1.55 | 22.9 | −0.46 |
| 37–39 | 1.41 ± 0.12 | 32.11 ± 0.26 | 49.65 ± 0.29 | 16.83 | 1.55 | 22.8 | −0.50 |
| 39–41 | 1.52 ± 0.10 | 32.54 ± 0.15 | 49.07 ± 0.41 | 16.88 | 1.51 | 21.4 | −0.47 |
| 41–43 | 1.50 ± 0.01 | 33.13 ± 0.03 | 48.47 ± 0.36 | 16.90 | 1.46 | 22.0 | −0.44 |
| 43–45 | 1.36 ± 0.09 | 34.43 ± 0.23 | 46.28 ± 1.64 | 17.92 | 1.34 | 25.3 | −0.30 |
| 45–47 | 1.30 ± 0.05 | 33.69 ± 0.03 | 47.30 ± 0.68 | 17.71 | 1.40 | 25.9 | −0.35 |
| 47–50 | 1.41 ± 0.17 | 31.55 ± 0.46 | 49.62 ± 0.36 | 17.41 | 1.57 | 22.3 | −0.47 |
Appendix B



References
- Bindler, R.; Klaminder, J. Reply to comment by William Shotyk on “Does within-bog spatial variability of mercury and lead constrain reconstructions of absolute deposition rates from single peat records? The example of Store Mosse, Sweden”. Glob. Biogeochem. Cycles 2006, 20, GB2005. [Google Scholar] [CrossRef]
- van der Linden, M.; Barke, J.; Vickery, E.; Charman, D.; van Geel, B. Late Holocene human impact and climate change recorded in a North Swedish peat deposit. Palaeogeogr. Palaeoclim. Palaeoecol. 2008, 258, 1–27. [Google Scholar] [CrossRef]
- Le Roux, G.; Marshall, W.A. Constructing recent peat accumulation chronologies using atmospheric fall-out radionuclides. Mires Peat 2011, 7, 1–14. [Google Scholar]
- Ndiaye, A.; Bentaleb, I.; Favier, C.; Fourel, F.; Sebag, D.; Fall, M.; Giresse, P.; Diouf, B. Reconstruction of the holocene climate and environmental changes of Niayes peat bog in northern coast of Senegal (NW Africa) based on stable isotopes and charcoals analysis. Quat. Sci. Rev. 2022, 289, 107609. [Google Scholar] [CrossRef]
- Li, H.-C.; Wang, J.; Sun, J.-J.; Chou, C.-Y.; Xia, Y.-Y.; Zhao, H.-Y.; Yang, Q.-N.; Kashyap, S. Study of Jinchuan Mire in NE China I: AMS 14C, 210Pb and 137Cs dating on peat cores. Quat. Int. 2019, 528, 9–17. [Google Scholar] [CrossRef]
- Sun, A.; Yang, Y.; Wu, H.; Ran, M. Climate change on the northeastern Tibetan Plateau during the past~600 years inferred from peat pollen records. Rev. Palaeobot. Palynol. 2020, 276, 104194. [Google Scholar] [CrossRef]
- De Vleeschouwer, F.; Gérard, L.; Goormaghtigh, C.; Mattielli, N.; Le Roux, G.; Fagel, N. Atmospheric lead and heavy metal pollution records from a Belgian peat bog spanning the last two millenia: Human impact on a regional to global scale. Sci. Total Environ. 2007, 377, 282–295. [Google Scholar] [CrossRef] [Green Version]
- Ul’Yanovskii, N.V.; Lakhmanov, D.E.; Pikovskoi, I.I.; Falev, D.I.; Popov, M.S.; Kozhevnikov, A.Y.; Kosyakov, D.S. Migration and transformation of 1,1-dimethylhydrazine in peat bog soil of rocket stage fall site in Russian North. Sci. Total Environ. 2020, 726, 138483. [Google Scholar] [CrossRef]
- Magiera, T.; Szuszkiewicz, M.M.; Michczyński, A.; Chróst, L.; Szuszkiewicz, M. Peat bogs as archives of local ore mining and smelting activities over the centuries: A case study of Miasteczko Śląskie (Upper Silesia, Poland). CATENA 2021, 198, 105063. [Google Scholar] [CrossRef]
- Gwynn, J.P.; Dowdall, M.; Lind, B. The Radiological Environment of Svalbard; Strålevern Rapport 2004:2; Norwegian Radiation Protection Authority: Østerås, Norway, 2004; p. 55. [Google Scholar]
- Dowdall, M.; Gwynn, J.P.; Moran, C.; Davids, C.; O’Dea, J.; Lind, B. Organic soil as a radionuclide sink in a High Arctic environment. J. Radioanal. Nucl. Chem. 2005, 266, 217–223. [Google Scholar] [CrossRef]
- Fialkiewicz-Koziel, B.; Kolaczek, P.; Piotrowska, N.; Michczynski, A.; Lokas, E.; Wachniew, P.; Woszczyk, M.; Sensula, B. High-Resolution Age-Depth Model of a Peat Bog in Poland as an Important Basis for Paleoenvironmental Studies. Radiocarbon 2014, 56, 109–125. [Google Scholar] [CrossRef]
- Lokas, E.; Bartminski, P.; Wachniew, P.; Mietelski, J.W.; Kawiak, T.; Srodon, J. Sources and pathways of artificial radionuclides to soils at a High Arctic site. Environ. Sci. Pollut. Res. 2014, 21, 12479–12493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mroz, T.; Lokas, E.; Kocurek, J.; Gasiorek, M. Atmospheric fallout radionuclides in peatland from Southern Poland. J. Environ. Radioact. 2017, 175–176, 25–33. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, X.; Zhang, D.; Yang, Y.; Huang, Z.; Liu, Q. Records of iodine isotopes (129I, 127I) in the Barkol peat bog from northwest China and their sources, transport and preservation. Chemosphere 2021, 279, 130531. [Google Scholar] [CrossRef]
- Strack, M. (Ed.) Peatlands and Climate Change; IPS, International Peat Society: Quebec, QC, Canada, 2008. [Google Scholar]
- Freeman, C.; Fenner, N.; Ostle, N.; Kang, H.; Dowrick, D.J.; Reynolds, B.; Lock, M.A.; Sleep, D.; Hughes, S.; Hudson, J. Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 2004, 430, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Antala, M.; Juszczak, R.; van der Tol, C.; Rastogi, A. Impact of climate change-induced alterations in peatland vegetation phenology and composition on carbon balance. Sci. Total Environ. 2022, 827, 154294. [Google Scholar] [CrossRef]
- Kudelsky, A.; Smith, J.; Ovsiannikova, S.; Hilton, J. Mobility of Chernobyl-derived 137Cs in a peatbog system within the catchment of the Pripyat River, Belarus. Sci. Total Environ. 1996, 188, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Tositti, L.; Mingozzi, M.; Sandrini, S.; Forlani, L.; Buoso, M.; De Poli, M.; Ceccato, D.; Zafiropoulos, D. A multitracer study of peat profiles from Tunguska, Siberia. Glob. Planet. Chang. 2006, 53, 278–289. [Google Scholar] [CrossRef]
- Parry, L.E.; Charman, D.J.; Blake, W.H. Comparative dating of recent peat deposits using natural and anthropogenic fallout radionuclides and Spheroidal Carbonaceous Particles (SCPs) at a local and landscape scale. Quat. Geochronol. 2013, 15, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Begy, R.; Kovacs, T.; Veres, D.; Simon, H. Atmospheric flux, transport and mass balance of 210Pb and 137Cs radiotracers in different regions of Romania. Appl. Radiat. Isot. 2016, 111, 31–39. [Google Scholar] [CrossRef]
- Martinsson, J.; Pédehontaa-Hiaa, G.; Malmborg, V.; Madsen, D.; Rääf, C. Experimental wildfire induced mobility of radiocesium in a boreal forest environment. Sci. Total Environ. 2021, 792, 148310. [Google Scholar] [CrossRef] [PubMed]
- Mihalik, J.; Bartuskova, M.; Holgye, Z.; Jezkova, T.; Henych, O. Fractionation of 137Cs and Pu in natural peatland. J. Environ. Radioact. 2014, 134, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Komatsuzaki, M. Vertical distribution of radiocesium affects soil-to-crop transfer coefficient in various tillage systems after the Fukushima Daiichi Nuclear Power Plant accident. Soil Tillage Res. 2018, 178, 179–188. [Google Scholar] [CrossRef]
- Orlov, O.O.; Irklienko, S.P.; Turko, V.M. The 137Cs content in biogeocenosis components of mesooligotrophic bogs of Ukrainian Polessie and the role of sphagous cover in redistribution of potassium and 137Cs streams in their ecosystems. In Problemy Ekologii Lesov I Lesoplozovania Na Ukrainskom Polessie; Volyn: Zhytomir, Ukraine, 1999. (In Ukraine) [Google Scholar]
- Mietelski, J.W.; Kubica, B.; Gaca, P.; Tomankiewicz, E.; Błażej, S.; Tuleja-Krysa, M.; Stobiński, M. 238Pu, 239+240Pu, 241Am, 90Sr and 137Cs in mountains soil samples from the Tatra National Park (Poland). J. Radioanal. Nucl. Chem. 2007, 275, 523–533. [Google Scholar] [CrossRef]
- Rosen, K.; Vinichuk, M.; Johanson, K. 137Cs in a raised bog in central Sweden. J. Environ. Radioact. 2009, 100, 534–539. [Google Scholar] [CrossRef]
- Appleby, P. Three decades of dating recent sediments by fallout radionuclides: A review. Holocene 2008, 18, 83–93. [Google Scholar] [CrossRef]
- Piotrowska, N.; De Vleeschouwer, F.; Sikorski, J.; Pawlyta, J.; Fagel, N.; Le Roux, G.; Pazdur, A. Intercomparison of radiocarbon bomb pulse and 210Pb age models. A study in a peat bog core from North Poland. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1163–1166. [Google Scholar] [CrossRef] [Green Version]
- Fialkiewicz-Koziel, B.; Lokas, E.; Galka, M.; Kolaczek, P.; De Vleeschouwer, F.; Le Roux, G.; Smieja-Krol, B. Influence of transboundary transport of trace elements on mountain peat geochemistry (Sudetes, Central Europe). Quat. Sci. Rev. 2020, 230, 106162. [Google Scholar] [CrossRef]
- Vile, M.A.; Wieder, R.K.; Novák, M. Mobility of Pb in Sphagnum-derived peat. Biogeochemistry 1999, 45, 35–52. [Google Scholar] [CrossRef]
- MacKenzie, A.; Farmer, J.; Sugden, C. Isotopic evidence of the relative retention and mobility of lead and radiocaesium in Scottish ombrotrophic peats. Sci. Total Environ. 1997, 203, 115–127. [Google Scholar] [CrossRef]
- Ali, A.A.; Ghaleb, B.; Garneau, M.; Asnong, H.; Loisel, J. Recent peat accumulation rates in minerotrophic peatlands of the Bay James region, Eastern Canada, inferred by 210Pb and 137Cs radiometric techniques. Appl. Radiat. Isot. 2008, 66, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Cwanek, A.; Lokas, E.; Edward, A.D.M.; Mazei, Y.; Gaca, P.; Milton, J.A. Temporal variability of Pu signatures in a 210Pb-dated Sphagnum peat profile from the Northern Ural, Russian Federation. Chemosphere 2021, 281, 130962. [Google Scholar] [CrossRef] [PubMed]
- Shotyk, W.; Kempter, H.; Krachler, M.; Zaccone, C. Stable (206Pb, 207Pb, 208Pb) and radioactive (210Pb) lead isotopes in 1year of growth of Sphagnum moss from four ombrotrophic bogs in southern Germany: Geochemical significance and environmental implications. Geochim. Cosmochim. Acta 2015, 163, 101–125. [Google Scholar] [CrossRef]
- Selyanina, S.B.; Parfyonova, L.N.; Trufanova, M.V.; Bogolicyn, K.G.; Mal’ceva, E.V.; Bogdanov, M.V.; YArygina, O.N. Extraction of Bitumen from High-Moor Peat; Bulletin of NArFU; Series Chemistry; Publisher of NArFU: Arkhangelsk, Russia, 2013; pp. 43–50. [Google Scholar]
- Yakovlev, E.; Spirov, R.; Druzhinin, S.; Ocheretenko, A.; Druzhinina, A.; Mishchenko, E.; Zhukovskaya, E. Atmospheric fallout of radionuclides in peat bogs in the Western Segment of the Russian Arctic. Environ. Sci. Pollut. Res. 2021, 28, 25460–25478. [Google Scholar] [CrossRef] [PubMed]
- Batuyev, I.K.V. Analysis of the factors determining the long-term change in runoff from oligotrophic bogs. Water Manag. Russia 2020, 6, 28–46. [Google Scholar] [CrossRef]
- Byzova, N.M.; Preminina, Y.K. Geography of the Arkhangelsk Region; Sport Akadem Press: Moscow, Russia, 2001. [Google Scholar]
- GOST 11623-89; Peat and Products of Its Processing for Agriculture. Methods for the Determination of Exchange and Active Acidity. 1993. Standards Publishing House: Moscow, Russia, 1992.
- GOST 26423-85; Soils. Methods for Determination of Specific Electric Conductivity, pH and Solid Residue of Water Extract. 1985. Standartinform: Moscow, Russia, 2011.
- GOST 28268-89; Soils. Methods of Determination of Moisture, Maximum Hygroscopic Moisture and Moisture of Steady Plant Fading. 1990. Standartinform: Moscow, Russia, 2006.
- GOST 26213-91; Soils. Methods for Determination of Organic Matter. 1993. Standards Publishing House: Moscow, Russia, 1992.
- GOST 27784-88; Soils. Methods for Determination of Ash Content in Peat and Peat-Containing Soil Horizons. 1988. Standards Publishing House: Moscow, Russia, 1988.
- Noskova, M.G. Field Atlas of Sphagnum Mosses in the Taiga Zone of European Russia; Publisher Aquarius: Tula City, Russia, 2016. [Google Scholar]
- Lishtvan, I.I.; Bazin, Y.T.; Gamayunov, N.I. Peat Physics and Chemistry; Nedra: Moscow, Russia, 1989. (In Russian) [Google Scholar]
- Urquhart, C.; Gore, A.J.P. The redox characteristics of four peat profiles. Soil Biol. Biochem. 1973, 5, 659–672. [Google Scholar] [CrossRef]
- Orlov, D.S. Soil Chemistry; MSU Publishing House: Moscow, Russia, 1992; p. 259. [Google Scholar]
- MVI Recommendation: MN 3421-2010; Method for Performing Measurements on a Gamma Spectrometer. Belarusian State Institute of Metrology. BelGIM: Minsk, Russia, 2010.
- MVI Recommendation 2453-2000; Methods of Radiation Control. General Requirements. VNIIFTI: Moscow, Russia, 2000.
- Bahur, A.E.; Manuilova, L.I.; Zueva, D.M.; Ovsyannikova, T.M.; Trukhina, T. The Method for Measuring the Specific Activity of Polonium-210 (210Po) and Lead-210 (210Pb) in Samples of Soils, Soils, Bottom Sediments, Rocks and Building Materials Based on Them by α-β Radiometric Method with Radiochemical Preparation; VIMS: Moscow, Russia, 2013. [Google Scholar]
- Bahur, A.E.; Manuilova, L.I.; Zueva, D.M.; Ovsyannikova, T.M.; Trukhina, T.P. The Method for Measuring the Specific Activity of Uranium Isotopes (238U, 234U, 235U) in Samples of Soils, Soils, Bottom Sediments, Rocks and Building Materials Based on Them Using an α-Spectrometric Method with Radiochemical Preparation; VIMS: Moscow, Russia, 2013. [Google Scholar]
- Appleby, P.; Oldfield, F. The calculation of lead-210 dates assuming a constant rate of supply of unsupported 210Pb to the sediment. CATENA 1978, 5, 1–8. [Google Scholar] [CrossRef]
- Sanchez-Cabeza, J.; Ruiz-Fernández, A. 210Pb sediment radiochronology: An integrated formulation and classification of dating models. Geochim. Cosmochim. Acta 2012, 82, 183–200. [Google Scholar] [CrossRef]
- Sanchez-Cabeza, J.-A.; Ruiz-Fernández, A.C.; Ontiveros-Cuadras, J.F.; Bernal, L.H.P.; Olid, C. Monte Carlo uncertainty calculation of 210Pb chronologies and accumulation rates of sediments and peat bogs. Quat. Geochronol. 2014, 23, 80–93. [Google Scholar] [CrossRef]
- Parfenova, L.; Selyanina, S.; Trufanova, M.; Bogolitsyn, K.; Orlov, A.; Volkova, N.; Ponomareva, T.; Sokolova, T. Influence of climatic and hydrological factors on structure and composition of peat from northern wetland territories with low anthropogenic impact. Sci. Total Environ. 2016, 551–552, 108–115. [Google Scholar] [CrossRef]
- Shishov, L.L.; Tonkonogov, V.D.; Lebedeva, I.I.; Gerasimova, M.I. Classification and detection of soils in Russia. Oykumena Smolensk 2004, 2, 341. (In Russian) [Google Scholar]
- Zubov, I.N.; Orlov, A.S.; Selyanina, S.B.; Zabelina, S.A.; Ponomareva, T.I. Redox potential and acidity of peat are key diagnostic physicochemical properties for the stratigraphic zones of a boreal raised bog. Mires Peat 2022, 28, 16. [Google Scholar]
- Ponomareva, T.; Selyanina, S.; Shtang, A.; Zubov, I.; Yarygina, O. Transformation of an oligotrophic sphagnum bog during the process of rewetting. Land 2021, 10, 670. [Google Scholar] [CrossRef]
- Orlov, D.S. Soil Humic Acids and the General Theory of Humification; MSU Publishing House: Moscow, Russia, 1990; p. 325. [Google Scholar]
- Schulz, C.; Meier-Uhlherr, R.; Luthardt, V.; Joosten, H. Portraits of peatland deposits Germany. Mires Peat 2019, 24, 20. [Google Scholar]
- Shevchenko, V.P.; Kuznecov, O.L.; Politova, N.V.; Zareckaya, N.E.; Kutenkov, S.A.; Lisicyn, A.P.; Pokrovskij, O.S. The entry of trace elements from the atmosphere, registered in the natural archive (on the example of the Ilas raised bog, the catchment area of the White Sea). Rep. Acad. Sci. 2015, 465, 587–592. [Google Scholar]
- Savichev, O.; Soldatova, E.; Rudmin, M.; Mazurov, A. Geochemical barriers in oligotrophic peat bog (Western Siberia). Appl. Geochem. 2020, 113, 104519. [Google Scholar] [CrossRef]
- Olid, C.; Diego, D.; Garcia-Orellana, J.; Cortizas, A.M.; Klaminder, J. Modeling the downward transport of 210 Pb in Peatlands: Initial Penetration-Constant Rate of Supply (IP-CRS) model. Sci. Total Environ. 2016, 541, 1222–1231. [Google Scholar] [CrossRef]
- Schleich, B.N.; Degering, D.; Unterricker, S. Natural and artificial radionuclides in forest and bog soils: Tracers for migration processes and soil development. Radiochim. Acta 2000, 88, 803–808. [Google Scholar] [CrossRef]
- Husson, O. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: A transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 2013, 362, 389–417. [Google Scholar] [CrossRef] [Green Version]
- Vodyanickij, Y.N.; Grebenkin, N.A.; Manahov, D.V.; Sashchenko, A.V.; Tyuleneva, V.M. Positive anomalies of uranium content in peatlands of the humid zone (review). Soil Sci. 2019, 12, 1492–1501. [Google Scholar]
- Rachkova, N.G.; Shuktomova, I.I.; Taskaev, A.I. The state of natural radionuclides of uranium, radium, and thorium in soils. Eurasian Soil Sci. 2010, 43, 651–658. [Google Scholar] [CrossRef]
- Putyrskaya, V.; Klemt, E.; Röllin, S.; Corcho-Alvarado, J.; Sahli, H. Dating of recent sediments from Lago Maggiore and Lago di Lugano (Switzerland/Italy) using 137Cs and 210Pb. J. Environ. Radioact. 2020, 212, 106135. [Google Scholar] [CrossRef] [PubMed]
- Paatero, J.; Jaakkola, T.; Kulmala, S. Lichen (sp. Cladonia) as a deposition indicator for transuranium elements investigated with the Chernobyl fallout. J. Environ. Radioact. 1998, 38, 223–247. [Google Scholar] [CrossRef]
- Rachkova, N.G.; Shuktomova, I.I.; Taskaev, A.I. Sorption of uranium, radium, and thorium from saline solutions on hydrolyzed wood lignin. Russ. J. Appl. Chem. 2006, 79, 715–721. [Google Scholar] [CrossRef]
- Selyanina, S.B.; Trufanova, M.V.; YArygina ONOrlov, A.S.; Ponomareva, T.I.; Titova, K.V.; Zubov, I.N. Features of the biotransformation of organic substances in the conditions of the bog ecosystems of the North (on the example of the Ilas bog massif). Proc. IBVV RAS 2017, 79, 200–206. [Google Scholar]
- Han, C.H.; Park, J.W. Analysis of the natural radioactivity concentrations of the fine dust samples in Jeju Island, Korea and the annual effective radiation dose by inhalation. J. Radioanal. Nucl. Chem. 2018, 316, 1173–1179. [Google Scholar] [CrossRef] [Green Version]
- Chernyshev, I.V.; Golubev, V.N.; Chugaev, A.V.; Mandzhieva, G.V.; Gareev, B.I. Behavior of the 238U, 235U, and 234U isotopes at weathering of volcanic rocks with u mineralization: A case study at the Tulukuevskoe deposit, Eastern Transbaikalia. Petrology 2019, 27, 446–467. [Google Scholar] [CrossRef]
- Rigol, A.; Vidal, M.; Rauret, G. An overview of the effect of organic matter on soil–radiocaesium interaction: Implications in root uptake. J. Environ. Radioact. 2002, 58, 191–216. [Google Scholar] [CrossRef]
- Arbuzov, S.I.; Il’enok, S.S.; Volostnov, A.V.; Maslov, S.G.; Arhipov, V.S. Forms of uranium in the coals and peat of North Asia. Bull. Tomsk. Polytech. Univ. 2011, 319, 109–115. [Google Scholar]
| Layer, cm | Degree of Decomposition R, % | Natural Moisture Content, g/g Absolutely Dry Matter | Bulk Density, g/cm3 | Eh4, mV | rH |
|---|---|---|---|---|---|
| 0–10 | 0 | 23.96 ± 0.23 | 0.075 ± 0.002 | 428 ± 6 | 22.5 |
| 10–20 | 5 | 23.57 ± 0.04 | 0.095 ± 0.001 | 340 ± 7 | 19.5 |
| 20–30 | 5 | 24.49 ± 0.07 | 0.097 ± 0.002 | 200 ± 1 | 14.8 |
| 30–40 | 7 | 20.66 ± 1.20 | 0.099 ± 0.003 | 125 ± 3 | 12.2 |
| 40–50 | 7 | 15.19 ± 0.11 | 0.109 ± 0.004 | 109 ± 7 | 11.7 |
| Parameter | Factor | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Depth | −0.97 | −0.06 | −0.17 | −0.09 | −0.10 |
| 137Cs | 0.67 | 0.21 | 0.34 | 0.17 | 0.43 |
| 210Pb | 0.78 | 0.19 | 0.18 | 0.00 | 0.53 |
| 234U/238U | −0.08 | −0.23 | 0.06 | 0.70 | 0.12 |
| U | 0.30 | −0.08 | 0.74 | −0.19 | −0.24 |
| CO32− | −0.04 | 0.12 | 0.78 | 0.43 | −0.08 |
| Ash | 0.17 | 0.24 | 0.88 | 0.22 | 0.20 |
| pHwater | −0.87 | 0.01 | −0.08 | 0.30 | −0.22 |
| pHsalt | −0.65 | 0.06 | 0.35 | 0.54 | −0.24 |
| Water-soluble salts | 0.71 | 0.02 | 0.32 | 0.52 | −0.02 |
| Organic matter | −0.17 | −0.24 | −0.88 | −0.22 | −0.20 |
| N | −0.27 | −0.31 | −0.05 | −0.07 | −0.89 |
| C | −0.17 | −0.91 | −0.28 | −0.16 | −0.12 |
| H | 0.04 | 0.96 | 0.04 | −0.20 | 0.14 |
| O | 0.32 | −0.12 | 0.47 | 0.72 | 0.17 |
| H/C | 0.10 | 0.95 | 0.21 | −0.01 | 0.18 |
| C/N | 0.25 | 0.12 | −0.02 | 0.10 | 0.92 |
| O/C | 0.28 | 0.54 | 0.48 | 0.53 | 0.21 |
| Oxidation level | 0.12 | −0.82 | 0.14 | 0.49 | −0.05 |
| Moisture content | 0.75 | 0.26 | 0.35 | 0.36 | −0.20 |
| Bulk density | −0.96 | −0.06 | 0.21 | −0.05 | −0.09 |
| Eh | 0.95 | 0.02 | 0.23 | 0.04 | 0.18 |
| rHcp | 0.95 | 0.02 | 0.23 | 0.04 | 0.19 |
| Variance | 9.97 | 4.25 | 3.88 | 1.84 | 1.05 |
| Percentage of Variance (%) | 43.35 | 18.50 | 16.87 | 8.00 | 4.55 |
| Cumulative (%) | 43.35 | 61.84 | 78.72 | 86.72 | 91.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakovlev, E.; Orlov, A.; Kudryavtseva, A.; Zykov, S. Estimation of Physicochemical Parameters and Vertical Migration of Atmospheric Radionuclides in a Raised Peat Bog in the Arctic Zone of Russia. Appl. Sci. 2022, 12, 10870. https://doi.org/10.3390/app122110870
Yakovlev E, Orlov A, Kudryavtseva A, Zykov S. Estimation of Physicochemical Parameters and Vertical Migration of Atmospheric Radionuclides in a Raised Peat Bog in the Arctic Zone of Russia. Applied Sciences. 2022; 12(21):10870. https://doi.org/10.3390/app122110870
Chicago/Turabian StyleYakovlev, Evgeny, Alexander Orlov, Alina Kudryavtseva, and Sergey Zykov. 2022. "Estimation of Physicochemical Parameters and Vertical Migration of Atmospheric Radionuclides in a Raised Peat Bog in the Arctic Zone of Russia" Applied Sciences 12, no. 21: 10870. https://doi.org/10.3390/app122110870






