Experimental Investigation of Crystal Blocking in Drainage Pipes for Tunnels in the Karst Region
Abstract
1. Introduction
2. Experimental Equipment
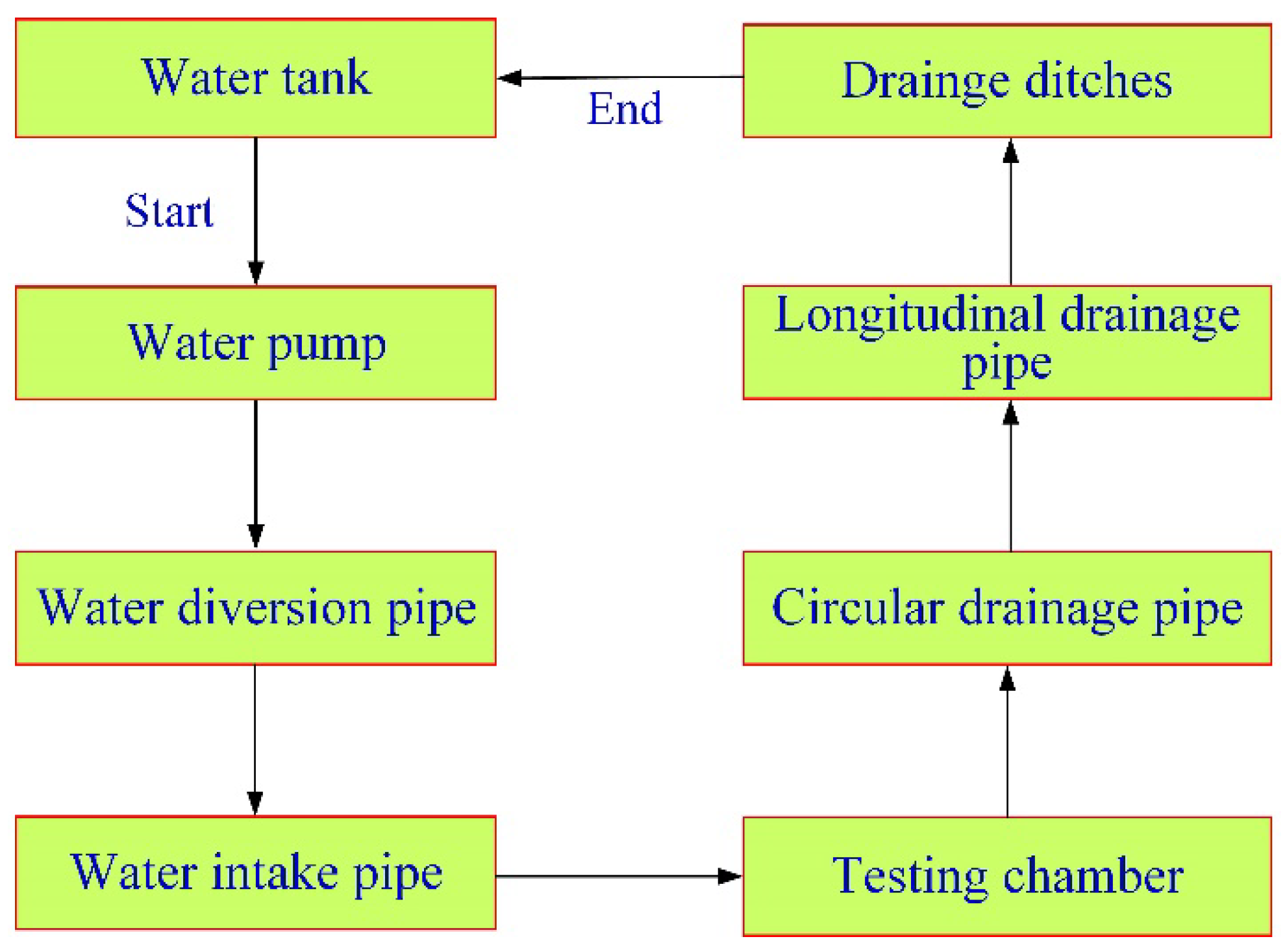
2.1. Water Supply System
2.2. Drainage System
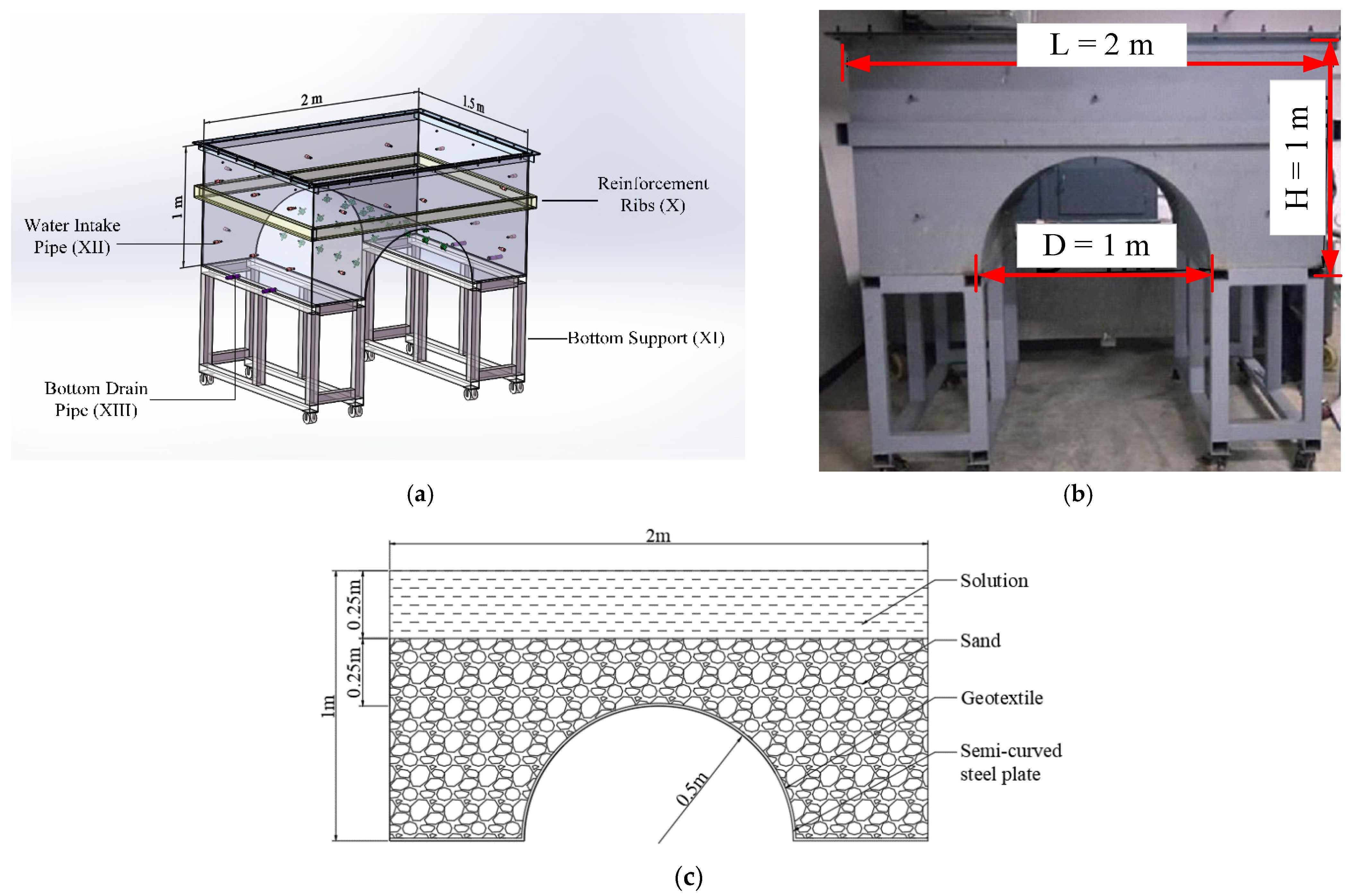
2.3. Testing Chamber
3. Testing Design
3.1. Ionic Solution Preparation
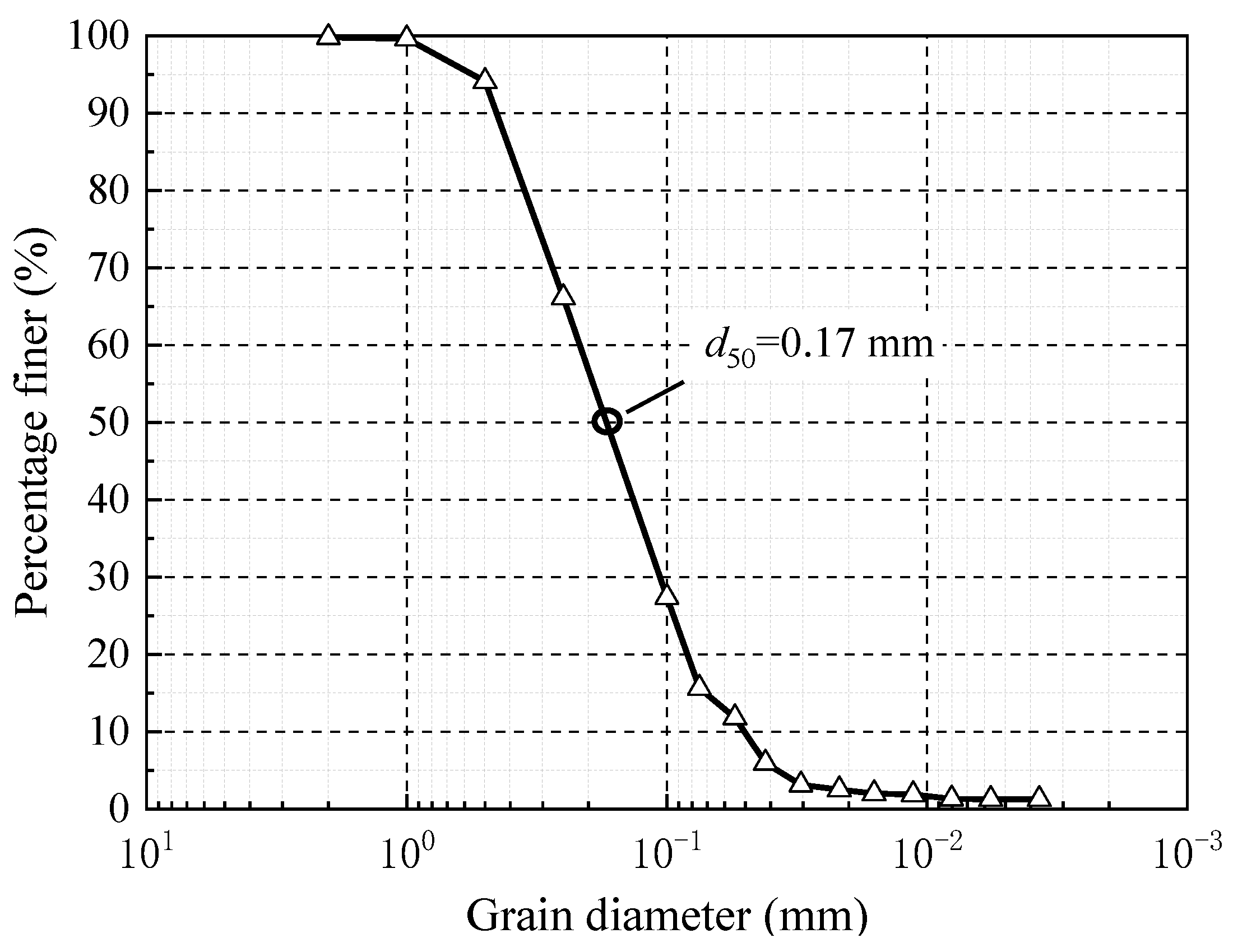
3.2. Formation Parameters
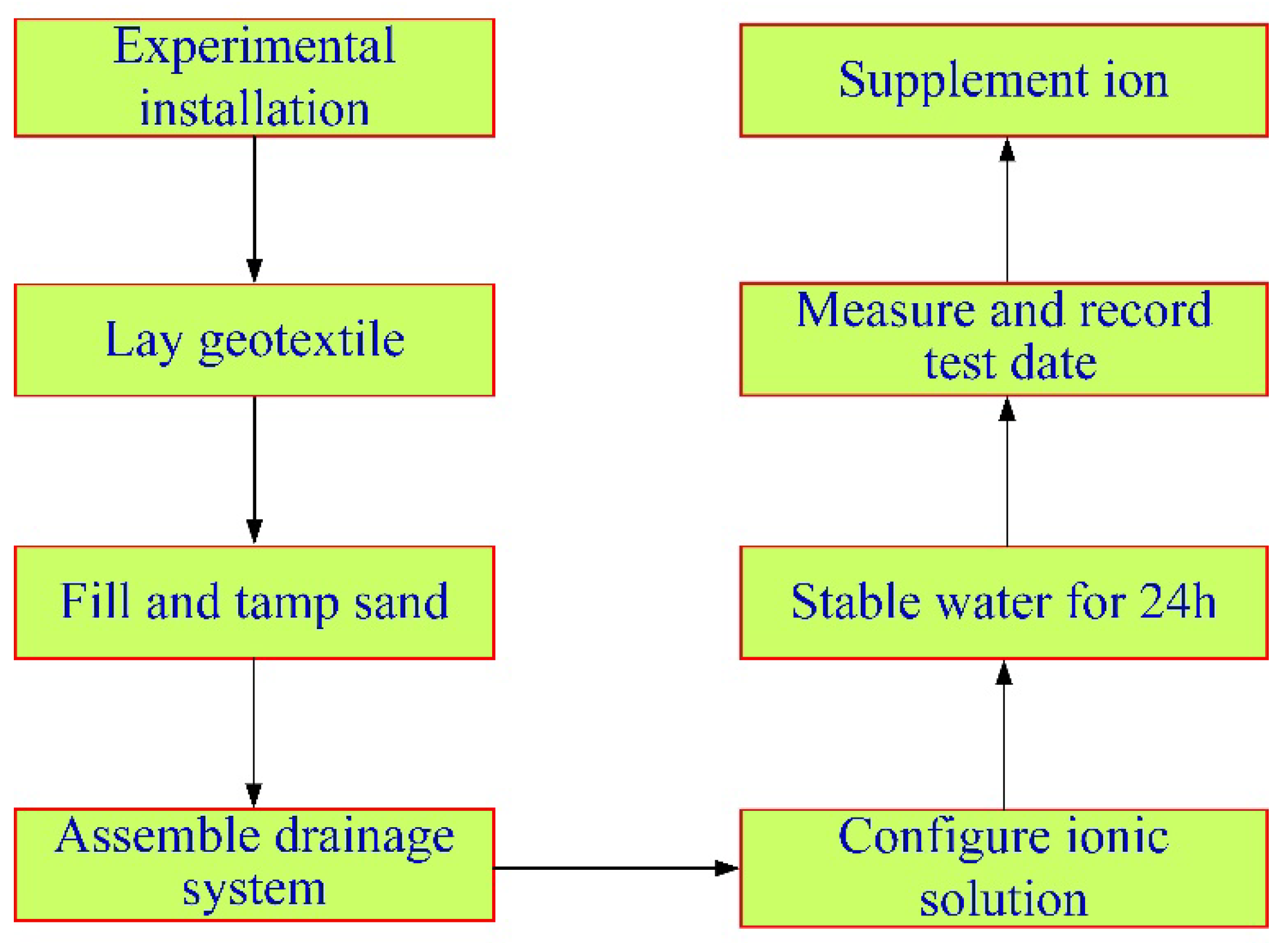
3.3. Experiment Procedure
3.4. Experimental Conditions
4. Results and Analysis
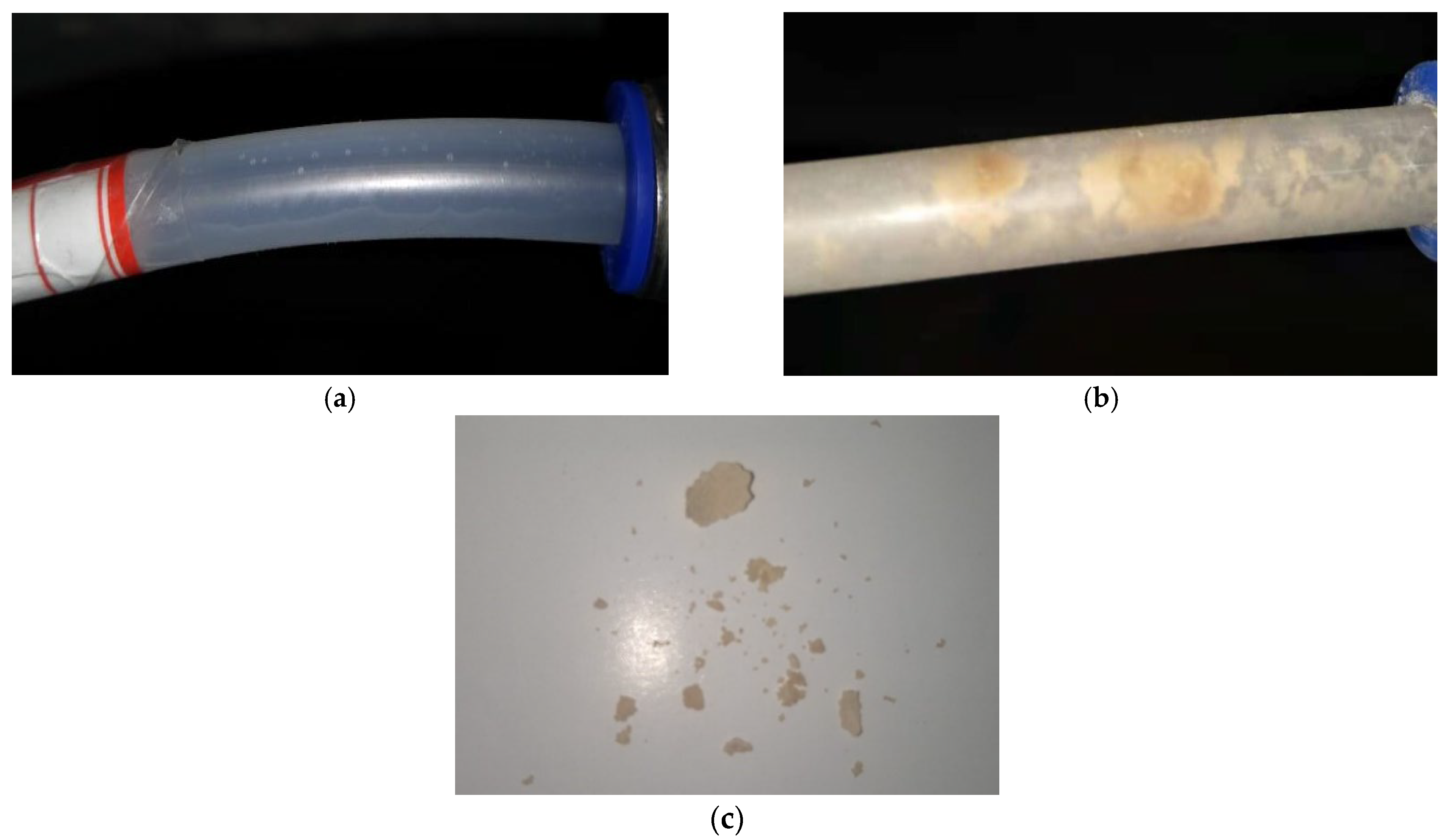
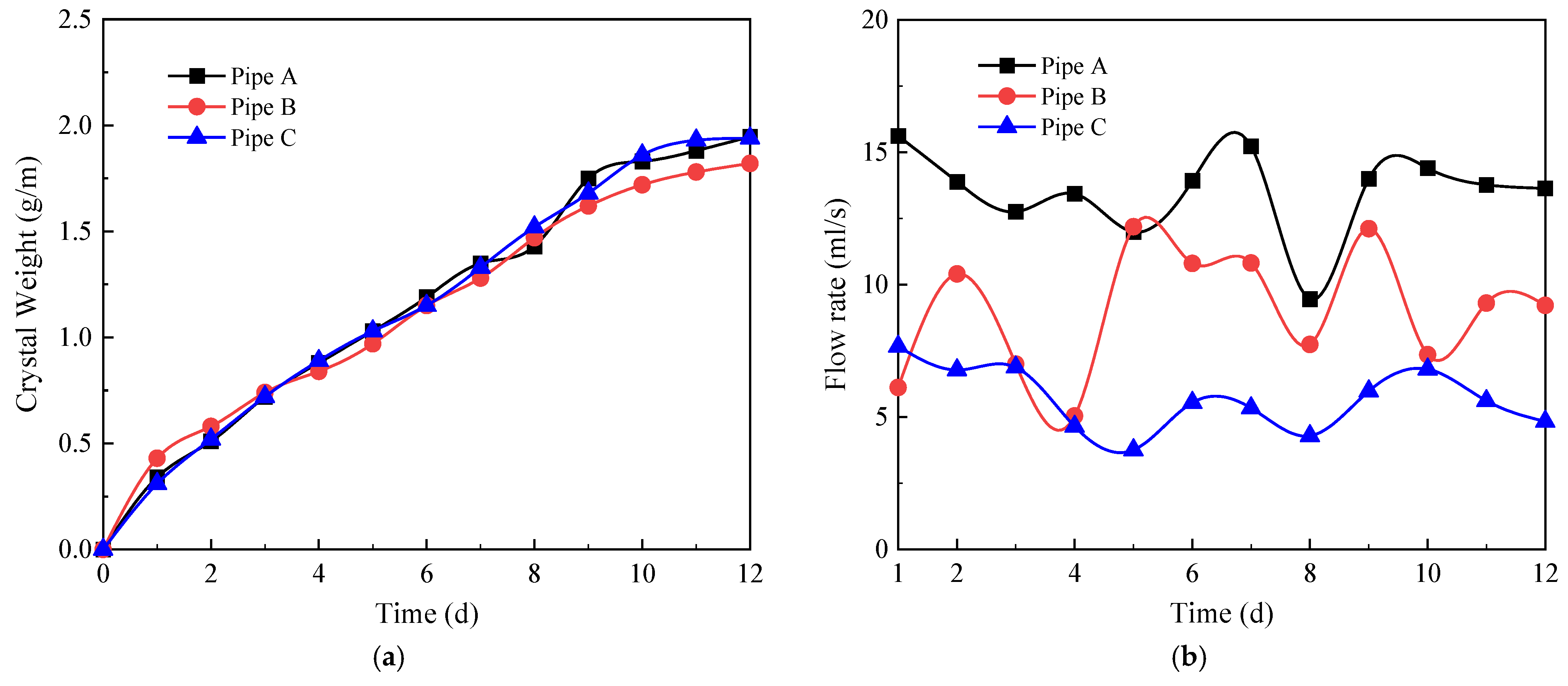
4.1. Standard Condition
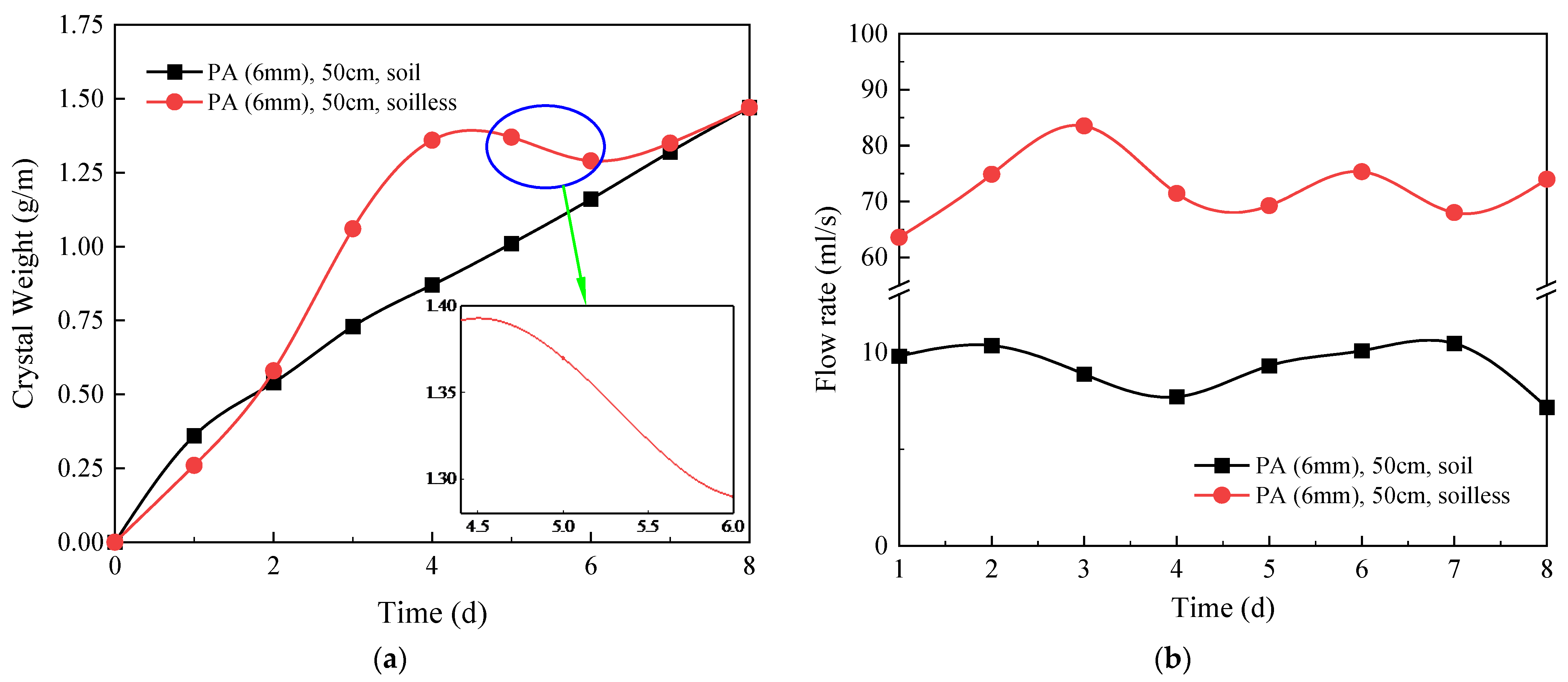
4.2. Influence of Overlaying Soil
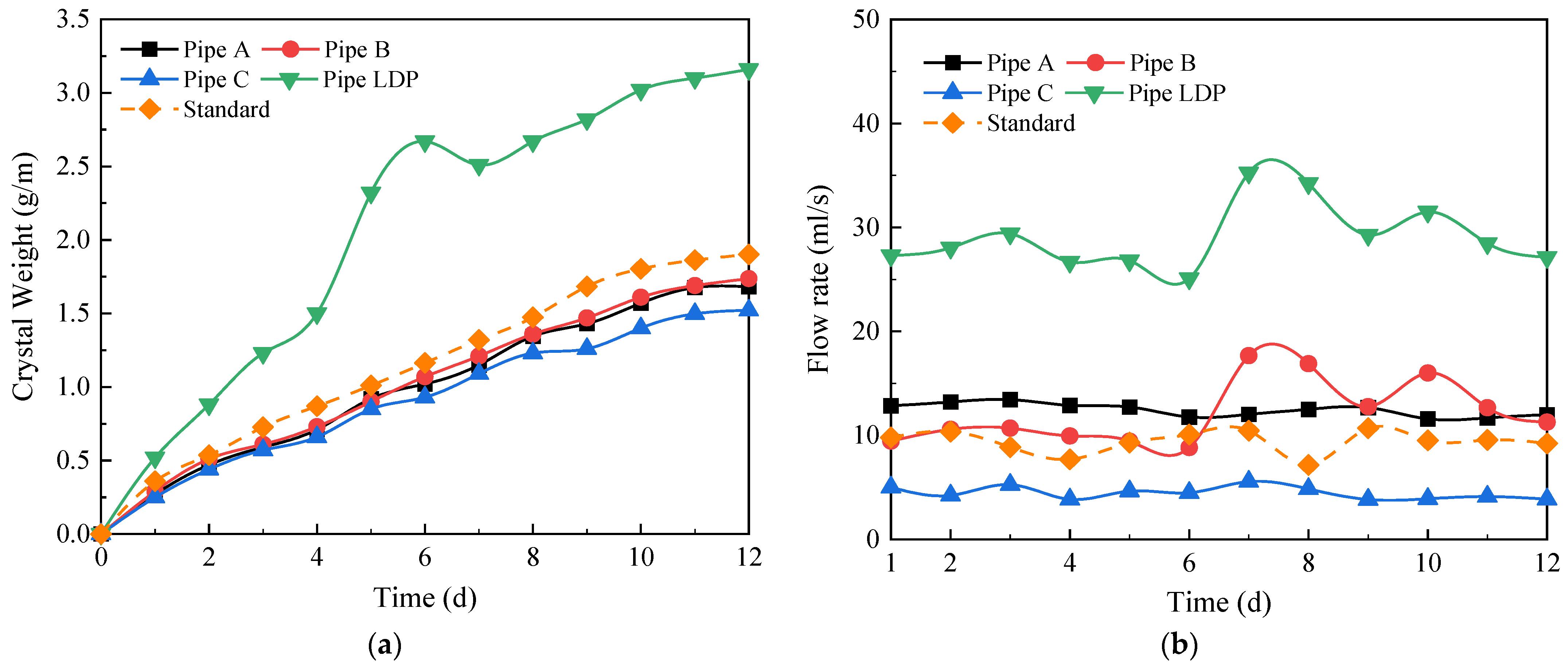
4.3. Influence of the Longitudinal Drainage Pipe
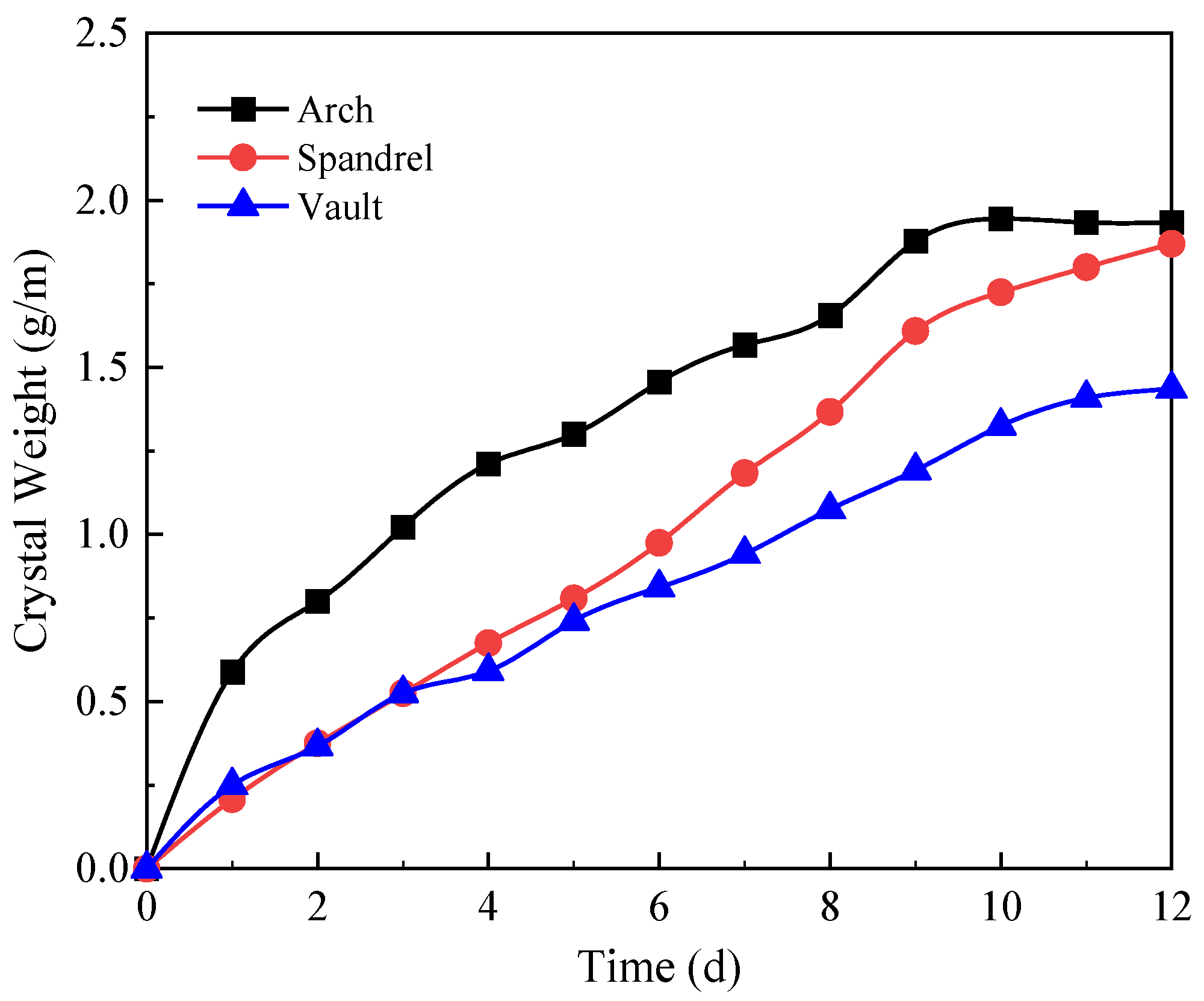
4.4. Influence of the Diameter of the Circular Drainage Pipe
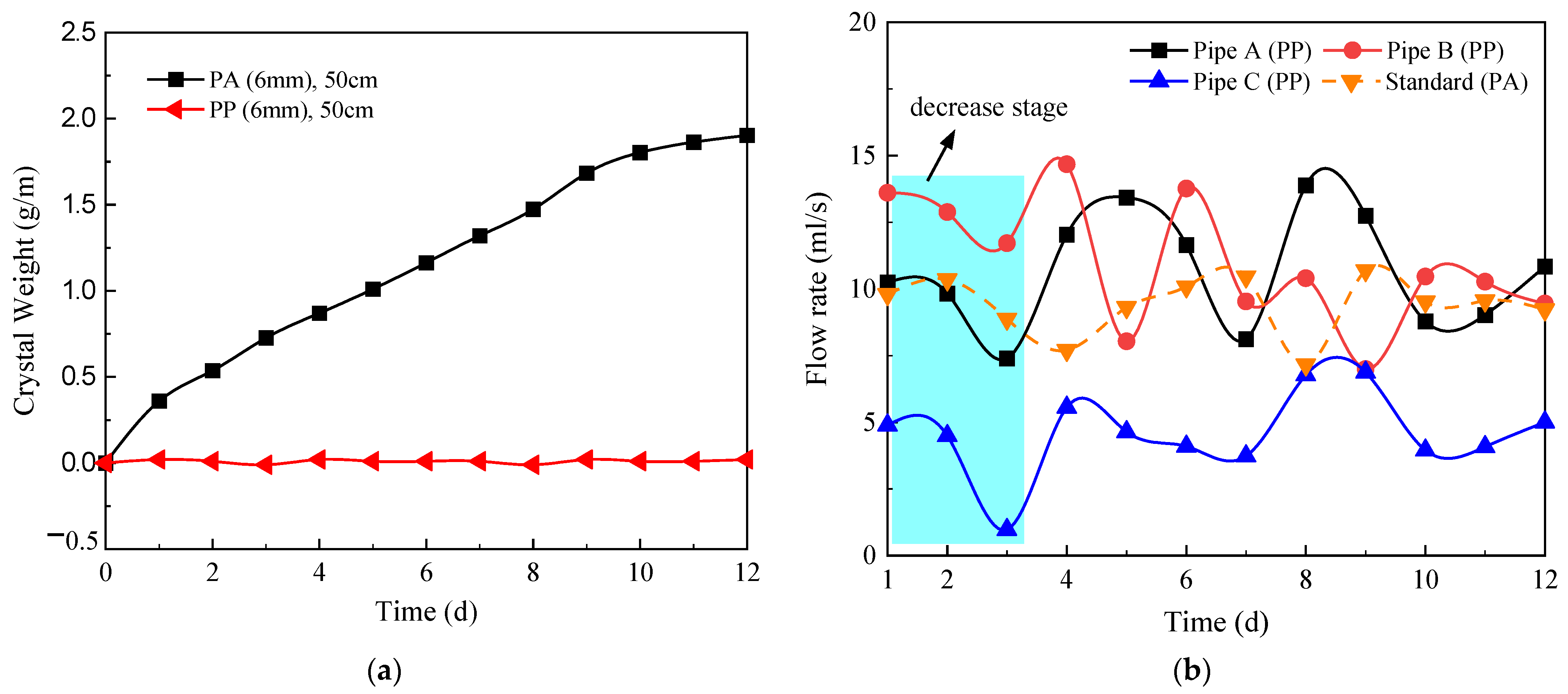
4.5. Influence of the Spacing of the Circular Drainage Pipe
4.6. Influence of the Material of the Circular Drainage Pipe
5. Conclusions
- (1)
- In the presence of the overburden layer, the development pattern of crystallization of drainage pipes can be divided into a growth stage and a stabilization stage. First, the crystallization showed a linear growth trend during the first 9 days. Subsequently, when the crystallization rate of the pipe wall is approximately equal to the scouring rate of the water flow on the inner wall of the pipe, the crystallization amount reaches a peak state, and the drainage performance of the tunnel remains stable. The flow rate of water is an important factor affecting crystallization.
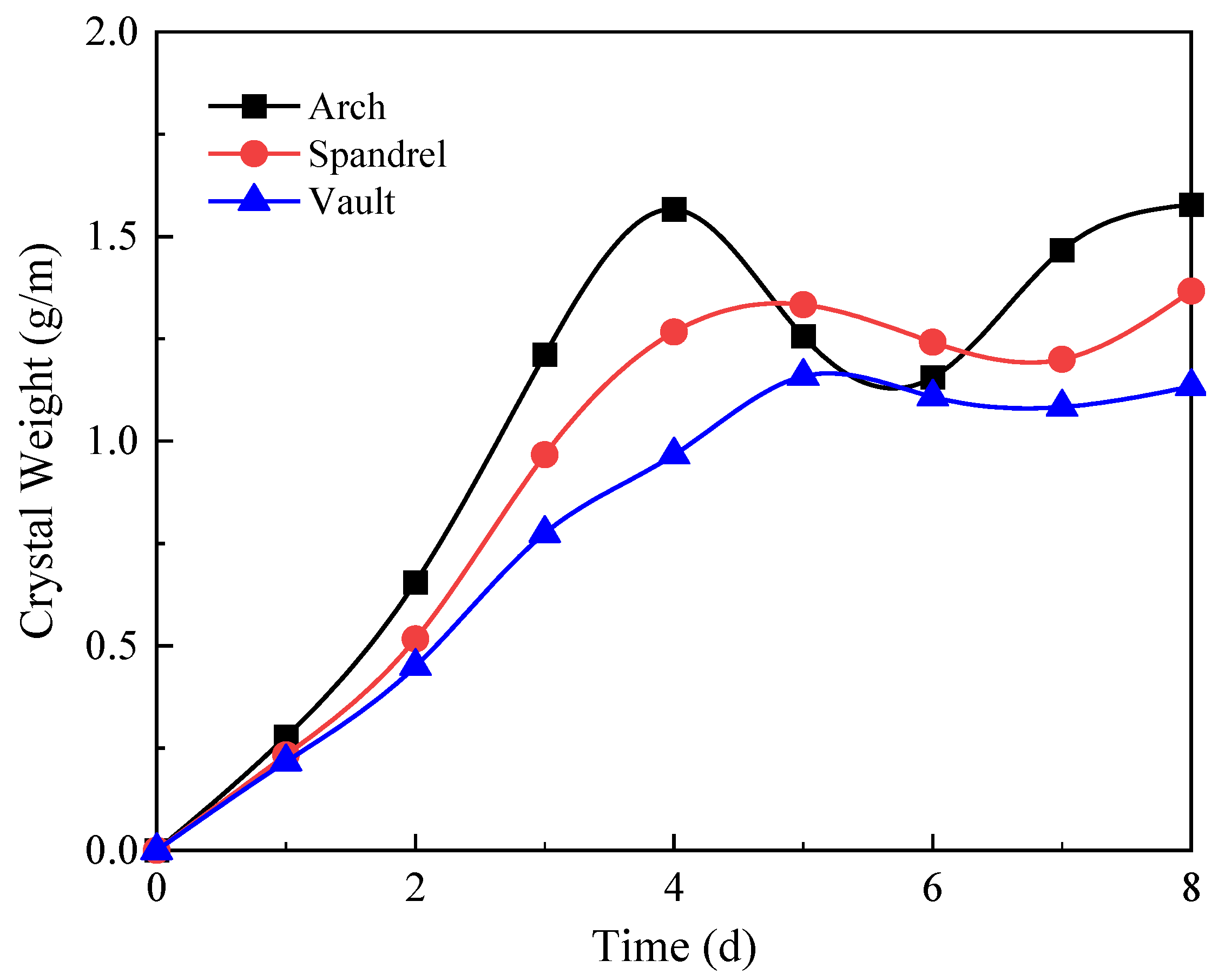
- (2)
- The sequence of the locations where crystals are generated in descending order is the arch, the shoulder, and the vault. In the design of circular drainage pipes, priority should be given to the degree of blockage in the arch. The crystallization process without filling soil can be divided into the growth stage, the decline stage, and the stable stage. The higher the groundwater flow, the higher the crystallization rate. However, under the soil-free condition, the large water flow has a strong scouring effect on the crystallization, and the crystallization amount shows a decreasing trend in the short term.
- (3)
- The presence of the longitudinal drainage pipes has no significant effect on the blocking performance of the circular drainage pipes. Longitudinal drainage pipes are more likely to form crystals and sediment than circular drains due to high water flow and low slope, causing drain clogging. It is recommended that an appropriate slope of the longitudinal drainage pipes should be selected to reduce crystallization and sediment deposition in actual projects.
- (4)
- The crystallization curve and flow curve are basically consistent with the standard operating conditions when the diameter of the circular drainage pipe is increased from 6.0 mm to 8.0 mm. This indicates that when the drainage system does not reach 100% full flow, the increase in drainage pipe diameter does not have a significant effect on the formation of crystallization and the drainage efficiency of the tunnel. Reducing the spacing between circular drainage pipes can effectively reduce the amount of crystallization of individual drainage pipes and improve the overall drainage efficiency of the tunnel.
- (5)
- The resistance of PP pipes to CaCO3 crystallization is significantly greater than that of PA pipes. In a short period of time, the diameter of the circular drainage pipe and the spacing of the circular drainage pipe have little effect on the resistance to crystallization of the drainage pipes. However, by reducing the spacing of drainage pipes, the drainage efficiency of the tunnel can be significantly improved. It is suggested that reasonable parameters of circular drainage pipes should be selected in the design of drainage systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dietzel, M.; Rinder, T.; Leis, A.; Reichl, P.; Sellner, P.; Draschitz, C.; Schöfer, H. Koralm tunnel as a case study for sinter formation in drainage systems–precipitation mechanisms and retaliatory action. Geomech. Und Tunn. Geomech. Und Tunn. 2008, 1, 271–278. [Google Scholar] [CrossRef]
- Li, P.; Liu, H.; Zhao, Y.; Li, Z. A bottom-to-up drainage and water pressure reduction system for railway tunnels. Tunn. Undergr. Space Technol. 2018, 81, 296–305. [Google Scholar] [CrossRef]
- Shin, J.H.; Addenbrooke, T.I.; Potts, D.M. A numerical study of the effect of groundwater movement on long-term tunnel behaviour. Geotechnique 2002, 52, 391–403. [Google Scholar] [CrossRef]
- Shin, J.H.; Potts, D.M.; Zdravkovic, L. The effect of pore-water pressure on NATM tunnel linings in decomposed granite soil. Can. Geotech. J. 2005, 42, 1585–1599. [Google Scholar] [CrossRef]
- Shin, J.H.; Lee, I.K.; Joo, E.J. Behavior of double lining due to long-term hydraulic deterioration of drainage system. Struct. Eng. Mech. Int. J. 2014, 52, 1257–1271. [Google Scholar] [CrossRef]
- Zhang, C.; Li, W.; Zhu, W.; Tan, Z. Face stability analysis of a shallow horseshoe-shaped shield tunnel in clay with a linearly increasing shear strength with depth. Tunn. Undergr. Space Technol. 2020, 97, 103291. [Google Scholar] [CrossRef]
- Liu, K.; Liu, X.; Zhong, Z.; Yi, L. Analysis on Stress and Stability of Lining in Partially-blocked Tunnel Drainage System. J. Eng. Sci. Technol. Rev. 2017, 10, 139–149. [Google Scholar] [CrossRef]
- Hager, B.; Foelsche, U. Stable isotope composition of precipitation in Austria. Austrian J. Earth Sci. 2015, 108, 2–13. [Google Scholar] [CrossRef]
- Li, P.; Chen, K.; Wang, F.; Li, Z. An upper-bound analytical model of blow-out for a shallow tunnel in sand considering the partial failure within the face. Tunn. Undergr. Space Technol. 2019, 91, 102989. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, N.; Liu, Y.; Lu, Y.T.; Kan, A.T.; Tomson, M.B. Application of a novel tube reactor for investigation of calcium carbonate mineral scale deposition kinetics. Chem. Eng. Res. Des. 2018, 137, 113–124. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Wei, L.; Liu, S.; Zhang, B.; Zhou, C. Experimental study on prevention of calcium carbonate crystallizing in drainage pipe of tunnel engineering. Adv. Civ. Eng. 2018, 2018, 9430517. [Google Scholar] [CrossRef]
- Chen, Y.; Cui, Y.; Barrett, A.G.; Chille, F.; Lassalle, S. Investigation of calcite precipitation in the drainage system of railway tunnels. Tunn. Undergr. Space Technol. 2019, 84, 45–55. [Google Scholar] [CrossRef]
- Yoon, S.H.; Park, E.H.; Lee, J.H.; Chun, B.S. Laboratory test of molecular vibration for preventing drainage pipe blockage in deteriorated tunnel. J. Korean Geotech. Soc. 2012, 28, 69–77. [Google Scholar] [CrossRef]
- Larsen, T.; Lioliou, M.; Ostvold, T.; Josang, L.O.; Randhol, P. Kinetics of CaCO3 scale formation during core flooding. In Proceedings of the SPE International Oilfield Scale Conference, Geilo, Norway, 9–12 March 2008. [Google Scholar]
- Yoo, C. Hydraulic deterioration of geosynthetic filter drainage system in tunnels–its impact on structural performance of tunnel linings. Geosynth. Int. 2016, 23, 463–480. [Google Scholar] [CrossRef]
- Xu, J.; Li, J. Influence of CO2 Release on Process of Precipitation of Calcium Carbonate. J. Tongji Univ. 2004, 32, 1173–1177. [Google Scholar]
- Li, P.; Feng, C.; Liu, H.; Zhao, Y.; Li, Z.; Xiong, H. Development and assessment of a water pressure reduction system for lining invert of underwater tunnels. Mar. Georesources Geotechnol. 2021, 39, 365–371. [Google Scholar] [CrossRef]
- Xing, X.; Ma, C.; Chen, Y. An experimental study of influence of pH on calcium carbonate crystallization fouling. Petro-Chem. Eq. 2004, 33, 11–15. [Google Scholar]
- Jung, H.S.; Han, Y.S.; Chung, S.R.; Chun, B.S.; Lee, Y.J. Evaluation of advanced drainage treatment for old tunnel drainage system in Korea. Tunn. Undergr. Space Technol. 2013, 38, 476–486. [Google Scholar] [CrossRef]
- Rinder, T.; Dietzel, M.; Leis, A. Calcium carbonate scaling under alkaline conditions–case studies and hydrochemical modelling. Appl. Geochem. 2013, 35, 132–141. [Google Scholar] [CrossRef]
- Boch, R.; Dietzel, M.; Reichl, P.; Leis, A.; Baldermann, A.; Mittermayr, F.; Pölt, P. Rapid ikaite (CaCO3·6H2O) crystallization in a man-made river bed: Hydrogeochemical monitoring of a rarely documented mineral formation. Appl. Geochem. 2015, 63, 366–379. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Zhang, B.; Zhou, Y.; Liu, S. Investigation and analysis on crystallization of tunnel drainage pipes in Chongqing. Adv. Mater. Sci. Eng. 2018, 2018, 7042693. [Google Scholar] [CrossRef]
- Zhai, M. Study on the Regularity of Crystallization and Blocking of Tunnel Drainage System in Limestone Area. Chongqing Jiaotong Univ. 2016, 10, 54–55. [Google Scholar]
- Li, W.; Zhang, C. Face stability analysis for a shield tunnel in anisotropic sands. Int. J. Geomech. 2020, 20, 04020043. [Google Scholar] [CrossRef]
- Zhang, C.; Han, K.; Zhang, D. Face stability analysis of shallow circular tunnels in cohesive–frictional soils. Tunn. Undergr. Space Technol. 2015, 50, 345–357. [Google Scholar] [CrossRef]
- Liu, S.; Gao, F.; Zhang, X.; Han, F.; Zhou, Y.; Xiang, K.; Xiao, D. Experimental study on anti-crystallization law of tunnel transverse flocking drainpipe at different velocities. Asia-Pac. J. Chem. Eng. 2020, 15, e2470. [Google Scholar] [CrossRef]
- Fang, Q.; Liu, X.; Zeng, K.; Zhang, X.; Zhou, M.; Du, J. Centrifuge modelling of tunnelling below existing twin tunnels with different types of support. Undergr. Space 2022, 7, 1125–1138. [Google Scholar] [CrossRef]
- Zheng, H.; Li, P.; Ma, G.; Zhang, Q. Experimental investigation of mechanical characteristics for linings of twins tunnels with asymmetric cross-section. Tunn. Undergr. Space Technol. 2022, 119, 104209. [Google Scholar] [CrossRef]




| Condition | Sand | LDP | Diameter | Spacing | Material |
|---|---|---|---|---|---|
| Standard condition | Yes | No | 6 mm | 50 cm | PA |
| No filling sand | No | No | 6 mm | 50 cm | PA |
| Install LDP | Yes | Yes | 6 mm | 50 cm | PA |
| Enlarge pipe diameter | No | No | 8 mm | 50 cm | PA |
| Narrow section space | No | No | 6 mm | 25 cm | PA |
| Change pipe material | No | No | 6 mm | 50 cm | PP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Chen, Y.; Yang, Y.; Li, P.; Wang, S.; Li, L. Experimental Investigation of Crystal Blocking in Drainage Pipes for Tunnels in the Karst Region. Appl. Sci. 2022, 12, 10928. https://doi.org/10.3390/app122110928
Xu C, Chen Y, Yang Y, Li P, Wang S, Li L. Experimental Investigation of Crystal Blocking in Drainage Pipes for Tunnels in the Karst Region. Applied Sciences. 2022; 12(21):10928. https://doi.org/10.3390/app122110928
Chicago/Turabian StyleXu, Chongbang, Yang Chen, Yunxuan Yang, Pengfei Li, Siqing Wang, and Lei Li. 2022. "Experimental Investigation of Crystal Blocking in Drainage Pipes for Tunnels in the Karst Region" Applied Sciences 12, no. 21: 10928. https://doi.org/10.3390/app122110928
APA StyleXu, C., Chen, Y., Yang, Y., Li, P., Wang, S., & Li, L. (2022). Experimental Investigation of Crystal Blocking in Drainage Pipes for Tunnels in the Karst Region. Applied Sciences, 12(21), 10928. https://doi.org/10.3390/app122110928







