Walking and Sitting Time after a Stroke: A Comparison of Shifts and Changes over Time within an Acute Care Setting
Abstract
:Featured Application
Abstract
1. Introduction
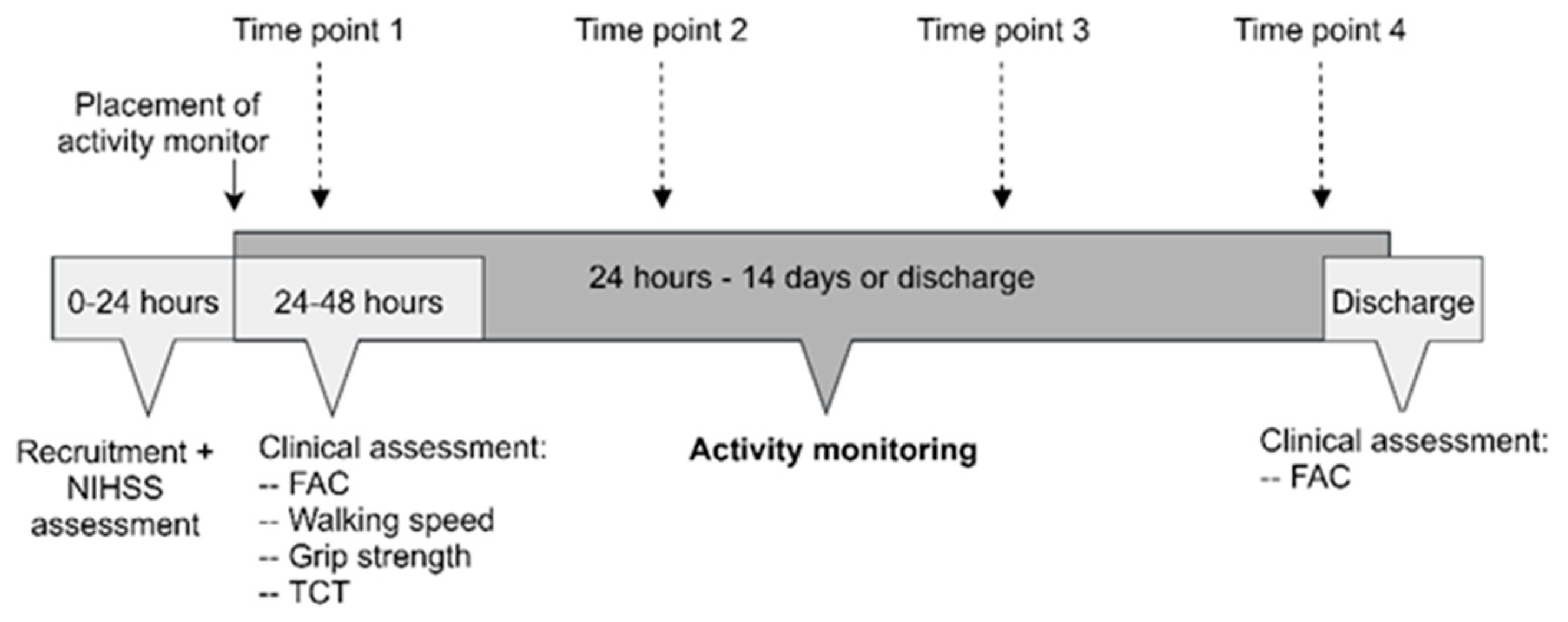
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Study Setting
2.4. Stroke Department Activity-Related Care Practices and Policies
2.5. Study Procedures
2.6. Clinical Assessment of Stroke Severity and Motor Function
2.7. Activity Monitoring
2.8. Data Management and Outcome Variables
2.9. Statistical Analyses
3. Results
3.1. Participants
3.2. Activity Monitoring
3.2.1. Sitting
3.2.2. Upright Position
3.2.3. Walking
3.2.4. Longest Sitting Period
3.2.5. Longest Walking Period
3.3. Correlations between Patients’ Activity, Stroke Severity, and Functional Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Zhang, X.; Wang, K.; Wen, J. Effects of Early Mobilization after Acute Stroke: A Meta-Analysis of Randomized Control Trials. J. Stroke Cerebrovasc. Dis. 2018, 27, 1326–1337. [Google Scholar] [CrossRef] [PubMed]
- Cumming, T.B.; Thrift, A.G.; Collier, J.M.; Churilov, L.; Dewey, H.M.; Donnan, G.A.; Bernhardt, J. Very Early Mobilization after Stroke Fast-Tracks Return to Walking. Stroke 2011, 42, 153–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Askim, T.; Bernhardt, J.; Salvesen, O.; Indredavik, B. Physical Activity Early after Stroke and Its Association to Functional Outcome 3 Months Later. J. Stroke Cerebrovasc. Dis. 2014, 23, e305–e312. [Google Scholar] [CrossRef] [PubMed]
- Cumming, T.B.; Collier, J.; Thrift, A.G.; Bernhardt, J. The Effect of Very Early Mobilization after Stroke on Psychological Well-Being. J. Rehabil. Med. 2008, 40, 609–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, A.G.; Bowen, A.; Young, G.R.; James, M.A. National Clinical Guideline for Stroke: 2016. Clin. Med. 2017, 17, 154–155. [Google Scholar] [CrossRef] [Green Version]
- Langhorne, P.; Stott, D.; Knight, A.; Bernhardt, J.; Barer, D.; Watkins, C. Very Early Rehabilitation or Intensive Telemetry after Stroke: A Pilot Randomised Trial. Cerebrovasc. Dis. 2010, 29, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Zisberg, A.; Shadmi, E.; Gur-Yaish, N.; Tonkikh, O.; Sinoff, G. Hospital-Associated Functional Decline: The Role of Hospitalization Processes Beyond Individual Risk Factors. J. Am. Geriatr. Soc. 2015, 63, 55–62. [Google Scholar] [CrossRef]
- Covinsky, K.E.; Pierluissi, E.; Johnston, C.B. Hospitalization-Associated Disability. JAMA 2011, 306, 1782–1793. [Google Scholar] [CrossRef]
- Wang, J.; Ren, D.; Liu, Y.; Wang, Y.; Zhang, B.; Xiao, Q. Effects of Early Mobilization on the Prognosis of Critically Ill Patients: A Systematic Review and Meta-Analysis. Int. J. Nurs. Stud. 2020, 110, 103708. [Google Scholar] [CrossRef]
- Kalisch, B.J.; Lee, S.; Dabney, B.W. Outcomes of Inpatient Mobilization: A Literature Review. J. Clin. Nurs. 2014, 23, 1486–1501. [Google Scholar] [CrossRef]
- Baldwin, C.; van Kessel, G.; Phillips, A.; Johnston, K. Accelerometry Shows Inpatients with Acute Medical or Surgical Conditions Spend Little Time Upright and Are Highly Sedentary: Systematic Review. Phys. Ther. 2017, 97, 1044–1065. [Google Scholar] [CrossRef] [Green Version]
- Fazio, S.; Stocking, J.; Kuhn, B.; Doroy, A.; Blackmon, E.; Young, H.M.; Adams, J.Y. How Much Do Hospitalized Adults Move? A Systematic Review and Meta-Analysis. Appl. Nurs. Res. 2020, 51, 151189. [Google Scholar] [CrossRef] [PubMed]
- Valkenet, K.; Bor, P.; van Delft, L.; Veenhof, C. Measuring Physical Activity Levels in Hospitalized Patients: A Comparison between Behavioural Mapping and Data from an Accelerometer. Clin. Rehabil. 2019, 33, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Dewey, H.; Thrift, A.; Donnan, G. Inactive and Alone: Physical Activity within the First 14 Days of Acute Stroke Unit Care. Stroke 2004, 35, 1005–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åstrand, A.; Saxin, C.; Sjöholm, A.; Skarin, M.; Linden, T.; Stoker, A.; Roshandel, S.; Dedering, Å.; Halvorsen, M.; Bernhardt, J.; et al. Poststroke Physical Activity Levels No Higher in Rehabilitation than in the Acute Hospital. J. Stroke Cerebrovasc. Dis. 2016, 25, 938–945. [Google Scholar] [CrossRef]
- Van de Port, I.G.L.; Valkenet, K.; Schuurmans, M.; Visser-Meily, J.M.A. How to Increase Activity Level in the Acute Phase after Stroke. J. Clin. Nurs. 2012, 21, 3574–3578. [Google Scholar] [CrossRef]
- Mattlage, A.E.; Redlin, S.A.; Rippee, M.A.; Abraham, M.G.; Rymer, M.M.; Billinger, S.A. Use of Accelerometers to Examine Sedentary Time on an Acute Stroke Unit. J. Neurol. Phys. Ther. 2015, 39, 166–171. [Google Scholar] [CrossRef]
- West, T.; Bernhardt, J. Physical Activity Patterns of Acute Stroke Patients Managed in a Rehabilitation Focused Stroke Unit. BioMed Res. Int. 2013, 2013, 438679. [Google Scholar] [CrossRef]
- Williams, L.S.; Yilmaz, E.Y.; Lopez-Yunez, A.M. Retrospective Assessment of Initial Stroke Severity With the {NIH} Stroke Scale. Stroke 2000, 31, 858–862. [Google Scholar] [CrossRef] [Green Version]
- National Stroke Foundation. Clinical Guidelines for Stroke Management 2010; National Stroke Foundation: Melbourne, Australia, 2010. [Google Scholar]
- Holden, M.K.; Gill, K.M.; Magliozzi, M.R.; Nathan, J.; Piehl-Baker, L. Clinical Gait Assessment in the Neurologically Impaired. Phys. Ther. 1984, 64, 35–40. [Google Scholar] [CrossRef]
- Bohannon, R.W. Comfortable and Maximum Walking Speed of Adults Aged 20–79 Years: Reference Values and Determinants. Age Ageing 1997, 26, 15–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collin, C.; Wade, D. Assessing Motor Impairment after Stroke: A Pilot Reliability Study. J. Neurol. Neurosurg. Psychiatry 1990, 53, 576–579. [Google Scholar] [CrossRef] [Green Version]
- Fokkenrood, H.J.P.; Verhofstad, N.; van den Houten, M.M.L.; Lauret, G.J.; Wittens, C.; Scheltinga, M.R.M.; Teijink, J.A.W. Physical Activity Monitoring in Patients with Peripheral Arterial Disease: Validation of an Activity Monitor. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valkenet, K.; Veenhof, C. Validity of Three Accelerometers to Investigate Lying, Sitting, Standing and Walking. PLoS ONE 2019, 14, e0217545. [Google Scholar] [CrossRef] [PubMed]
- de Groot, S.; Nieuwenhuizen, M.G. Validity and Reliability of Measuring Activities, Movement Intensity and Energy Expenditure with the DynaPort MoveMonitor. Med. Eng. Phys. 2013, 35, 1499–1505. [Google Scholar] [CrossRef]
- Storm, F.A.; Heller, B.W.; Mazzà, C. Step Detection and Activity Recognition Accuracy of Seven Physical Activity Monitors. PLoS ONE 2015, 10, e0118723. [Google Scholar] [CrossRef]
- Dijkstra, B.; Kamsma, Y.; Zijlstra, W. Detection of Gait and Postures Using a Miniaturised Triaxial Accelerometer-Based System: Accuracy in Community-Dwelling Older Adults. Age Ageing 2010, 39, 259–262. [Google Scholar] [CrossRef] [Green Version]
- van Schooten, K.S.; Pijnappels, M.; Rispens, S.M.; Elders, P.J.M.; Lips, P.; van Dieën, J.H. Ambulatory Fall-Risk Assessment: Amount and Quality of Daily-Life Gait Predict Falls in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Alt Murphy, M.; Andersson, S.; Danielsson, A.; Wipenmyr, J.; Ohlsson, F. Comparison of Accelerometer-Based Arm, Leg and Trunk Activity at Weekdays and Weekends during Subacute Inpatient Rehabilitation after Stroke. J. Rehabil. Med. 2019, 51, 426–433. [Google Scholar] [CrossRef] [Green Version]
- Norvang, O.P.; Hokstad, A.; Taraldsen, K.; Tan, X.; Lydersen, S.; Indredavik, B.; Askim, T. Time Spent Lying, Sitting, and Upright during Hospitalization after Stroke: A Prospective Observation Study. BMC Neurol. 2018, 18, 138. [Google Scholar] [CrossRef]
- Lay, S.; Bernhardt, J.; West, T.; Churilov, L.; Dart, A.; Hayes, K.; Cumming, T.B. Is Early Rehabilitation a Myth? Physical Inactivity in the First Week after Myocardial Infarction and Stroke. Disabil. Rehabil. 2015, 38, 1493–1499. [Google Scholar] [CrossRef]
- van Wijk, R.; Cumming, T.; Churilov, L.; Donnan, G.; Bernhardt, J. An Early Mobilization Protocol Successfully Delivers More and Earlier Therapy to Acute Stroke Patients: Further Results from Phase II of AVERT. Neurorehabil. Neural Repair 2012, 26, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Churilov, L.; Ellery, F.; Collier, J.; Chamberlain, J.; Langhorne, P.; Lindley, I.; Moodie, M.; Dewey, H.; Thrift, A.G. Prespecified Dose-Response Analysis for A Very Early Rehabilitation Trial (AVERT). Neurology 2016, 86, 2138–2145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, Y.; Zisberg, A.; Chayat, Y.; Gur-Yaish, N.; Gil, E.; Levin, C.; Rand, D.; Agmon, M. Walking for Better Outcomes and Recovery: The Effect of WALK-FOR in Preventing Hospital-Associated Functional Decline Among Older Adults. J. Gerontol. Ser. A 2019, 74, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Drolet, A.; DeJuilio, P.; Harkless, S.; Henricks, S.; Kamin, E.; Leddy, E.A.; Lloyd, J.M.; Waters, C.; Williams, S. Move to Improve: The Feasibility of Using an Early Mobility Protocol to Increase Ambulation in the Intensive and Intermediate Care Settings. Phys. Ther. 2013, 93, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Kirk, J.W.; Bodilsen, A.C.; Sivertsen, D.M.; Husted, R.S.; Nilsen, P.; Tjørnhøj-Thomsen, T. Disentangling the Complexity of Mobility of Older Medical Patients in Routine Practice: An Ethnographic Study in Denmark. PLoS ONE 2019, 14, e0214271. [Google Scholar] [CrossRef]
- Zisberg, A.; Agmon, M.; Gur-Yaish, N.; Rand, D.; Hayat, Y.; Gil, E.; WALK-FOR Team. No One Size Fits All—The Development of a Theory-Driven Intervention to Increase in-Hospital Mobility: The “WALK-FOR” Study. BMC Geriatr. 2018, 18, 91. [Google Scholar] [CrossRef]

| Characteristic | Mean ± SD or n (%) |
|---|---|
| Age, y | 69.4 ± 33.4 |
| Sex | |
| Males | 13 (62%) |
| Females | 8 (38%) |
| Pre-stroke mRS | |
| Independent (score 0–1) | 16 (76%) |
| Slight disability (score 2) | 1 (5%) |
| Moderate disability (score 3) | 3 (14%) |
| Moderately severe disability (score 4) | 1 (5%) |
| Side of stroke | |
| Right | 15 (71%) |
| Left | 6 (29%) |
| Stroke territory | |
| MCA | 9 (43%) |
| ACA | 5 (24%) |
| PCA | 6 (28%) |
| Subcortical | 1 (5%) |
| Acute stroke treatment | |
| tPA | 10 (48%) |
| Endovascular procedure | 4 (19%) |
| Family/caregiver support during hospitalization | |
| Yes | 18 (86%) |
| No | 3 (14%) |
| Patient | NIHSS (Score) | FAC Admission (Score) | FAC Discharge (Score) | Grip Strength (% from Unaffected Hand) | Walking Speed (m/s) | TCT (Score) |
|---|---|---|---|---|---|---|
| 1 | 6 | 5 | 5 | 28% | 0.51 | 87.5 |
| 2 | 5 | 2 | 2 | 40% | N/A | 62.5 |
| 3 | 7 | 0 | 0 | 0% | N/A | 12.5 |
| 4 | 8 | 0 | 0 | 0% | N/A | 37.5 |
| 5 | 5 | 4 | 4 | 0% | 1 | 100 |
| 6 | 10 | 1 | 5 | 8% | 0.87 | 75 |
| 7 | 10 | 2 | 2 | 33% | N/A | 75 |
| 8 | 6 | 2 | 2 | 54% | N/A | 87.5 |
| 9 | 18 | 5 | 5 | 81% | 0.87 | 100 |
| 10 | 8 | 0 | 0 | 71% | N/A | 50 |
| 11 | 6 | 4 | 5 | 71% | 0.83 | 100 |
| 12 | 10 | 0 | 0 | 50% | N/A | 37.5 |
| 13 | 6 | 4 | 4 | 107% | 1.11 | 100 |
| 14 | 5 | 2 | 2 | 80% | N/A | 87.5 |
| 15 | 17 | 0 | 0 | 0% | N/A | 37.5 |
| 16 | 5 | 3 | 4 | 90% | 0.82 | 100 |
| 17 | 6 | 3 | 4 | 89% | 0.58 | 100 |
| 18 | 5 | 3 | 5 | 67% | 0.51 | 100 |
| 19 | 18 | 2 | 3 | 80% | 0.33 | 87.5 |
| 20 | 5 | 2 | 2 | 59% | N/A | 100 |
| 21 | 6 | 2 | 2 | 122% | N/A | 87.5 |
| Summary | 6 (5–18) | 2 (0–5) | 2.5 (0–5) | 63% (0–150) | 0 (0–1.11) | 87.5 (12.5–100) |
| Shift | Time Point | Steps | Longest Sitting Time (Minutes) | Longest Walking Time (Minutes) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Average | Standard Error | Median | Minimum | Maximum | Median | Minimum | Maximum | ||
| Morning | 1 | 38 | 22.28 | 63.29 | 0.00 | 181.85 | 0.11 | 0.00 | 6.21 |
| 2 | 54.5 | 31.32 | 59.24 | 0.00 | 303.52 | 0.11 | 0.00 | 6.01 | |
| 3 | 59.2 | 35.93 | 109.00 | 0.84 | 314.55 | 0.06 | 0.00 | 1.37 | |
| 4 | 94.2 | 55.29 | 89.77 | 31.84 | 220.15 | 0.12 | 0.00 | 1.09 | |
| Evening | 1 | 20.2 | 12.16 | 8.40 | 0.00 | 255.02 | 0.05 | 0.00 | 1.15 |
| 2 | 26.3 | 15.6 | 35.26 | 0.00 | 284.25 | 0.09 | 0.00 | 1.22 | |
| 3 | 28.2 | 16.96 | 29.18 | 0.00 | 187.03 | 0.05 | 0.00 | 1.48 | |
| 4 | 57.5 | 35.61 | 44.91 | 0.00 | 84.65 | 0.00 | 0.00 | 1.69 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaron Katz, T.; Hallevi, H.; Molad, J.; Kafri, M. Walking and Sitting Time after a Stroke: A Comparison of Shifts and Changes over Time within an Acute Care Setting. Appl. Sci. 2022, 12, 10945. https://doi.org/10.3390/app122110945
Yaron Katz T, Hallevi H, Molad J, Kafri M. Walking and Sitting Time after a Stroke: A Comparison of Shifts and Changes over Time within an Acute Care Setting. Applied Sciences. 2022; 12(21):10945. https://doi.org/10.3390/app122110945
Chicago/Turabian StyleYaron Katz, Tammuz, Hen Hallevi, Jeremy Molad, and Michal Kafri. 2022. "Walking and Sitting Time after a Stroke: A Comparison of Shifts and Changes over Time within an Acute Care Setting" Applied Sciences 12, no. 21: 10945. https://doi.org/10.3390/app122110945
APA StyleYaron Katz, T., Hallevi, H., Molad, J., & Kafri, M. (2022). Walking and Sitting Time after a Stroke: A Comparison of Shifts and Changes over Time within an Acute Care Setting. Applied Sciences, 12(21), 10945. https://doi.org/10.3390/app122110945







