Functional and Sustainable Application of Natural Antioxidant Extract Recovered from Olive Mill Wastewater on Shelf-Life Extension of “Basil Pesto”
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Production of OMWW Antioxidant Extract
2.3. Production of Basil Pesto Sauce
2.4. Physicochemical and Microbiological Analysis
2.5. Determination of Pigment Components
2.6. Antioxidant Capacity Determination
2.7. UPLC Determination of Individual Phenolic Compounds (IPC)
2.8. Accelerated Oxidative Stability Test
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Characterization of OMWW Phenolic Extract (PE) and Basil Leaves
3.2. Characterization of Basil Pesto Samples
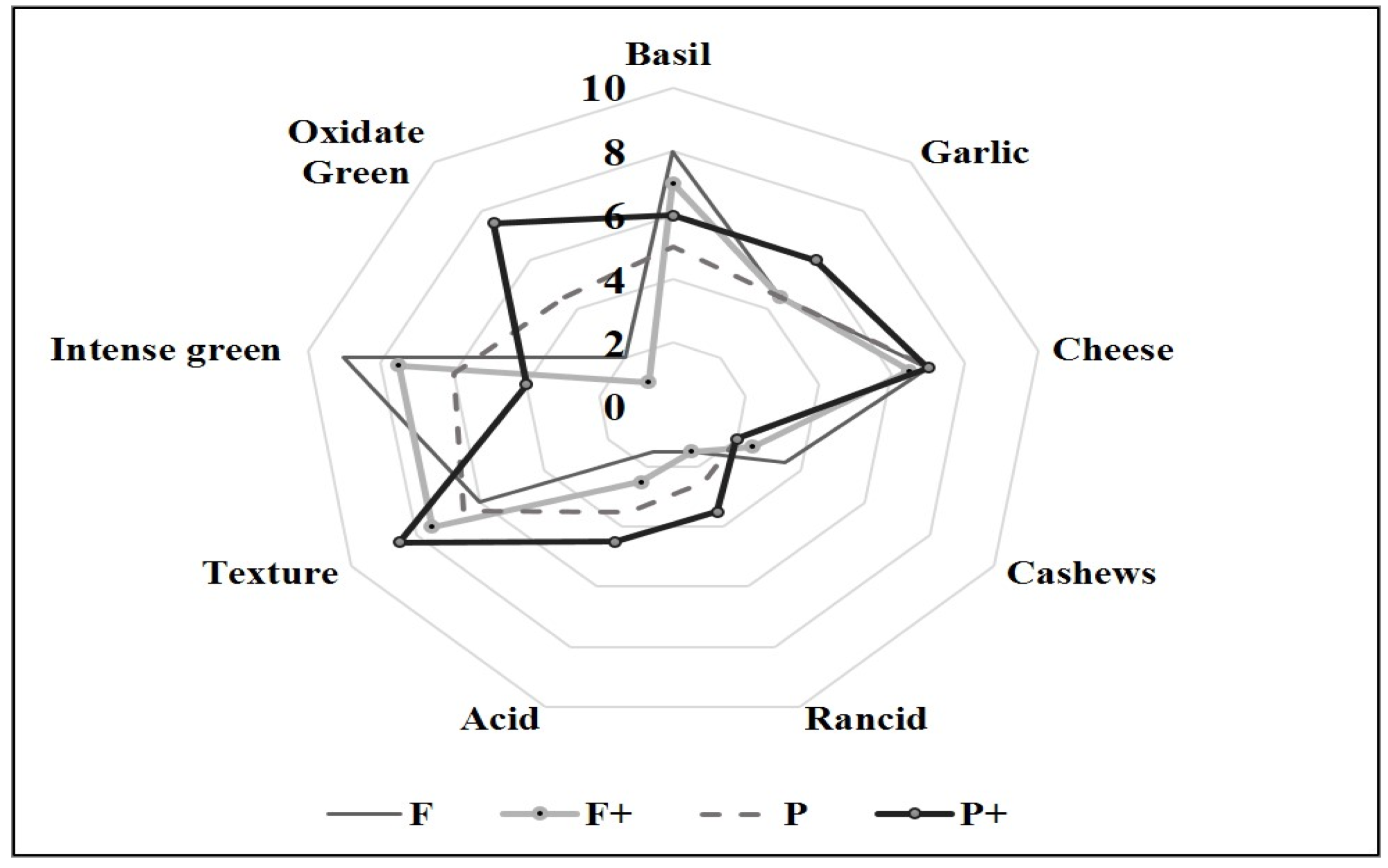
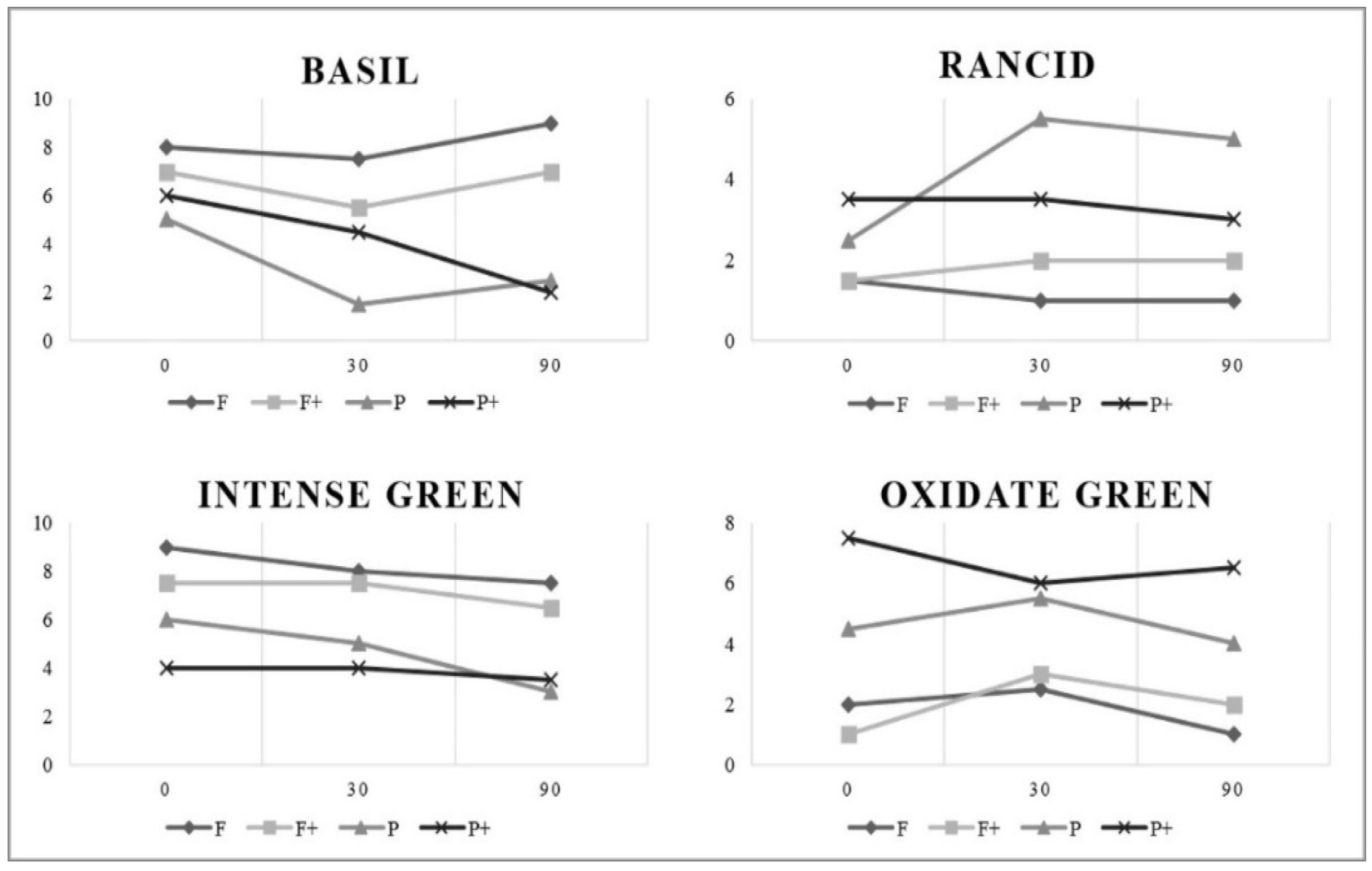
3.2.1. Physicochemical and Microbiological Aspects
3.2.2. Antioxidant Parameters
3.2.3. Oxidative Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zardetto, S.; Barbanti, D. Shelf life assessment of fresh green pesto using an accelerated test approach. Food Packag. Shelf Life 2020, 25, 100524. [Google Scholar] [CrossRef]
- Nicosia, C.; Fava, P.; Pulvirenti, A.; Antonelli, A.; Licciardello, F. Domestic Use Simulation and Secondary Shelf Life Assessment of Industrial Pesto alla genovese. Foods 2021, 10, 1948. [Google Scholar] [CrossRef] [PubMed]
- Sukardi, M.; Pulungan, H.; Purwaningsih, I.; Sita, P.F. Extraction of Phenolic Compounds from Basil (Ocimum americanum L.) Leaves with Pretreatment Using Pulsed Electric Field (PEF). In Proceedings of the International Conference on Green Agro-industry and Bioeconomy, Malang, East Java, Indonesia, 26–27 August 2019; Volume 475. [Google Scholar]
- Prinsi, B.; Morgutti, S.; Negrini, N.; Faoro, F.; Espen, L. Insight into Composition of Bioactive Phenolic Compounds in Leaves and Flowers of Green and Purple Basil. Plants 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Vicini, E.; Previdi, P. Aspetti microbiologici del pesto ligure. Ind. Conserve 1992, 67, 426–429. [Google Scholar]
- Fabiano, B.; Perego, P.; Pastorino, R.; Del Borghi, M. The extension of the shelf-life of ‘pesto’ sauce by a combination of modified atmosphere packaging and refrigeration. Int. J. Food Sci. 2020, 35, 293–303. [Google Scholar] [CrossRef]
- Franceschini, B.; Guerra, G.L.; Previdi, M.P. Influence of chemical-physical parameters on the stability of heat treated Pesto alla Genovese. Ind. Conserve 2011, 86, 143–147. [Google Scholar]
- Zunin, P.; Salvade, P.; Boggia, R.; Lanteri, S. Study of different kinds of ‘‘Pesto Genovese” by the analysis of their volatile fraction and chemometric methods. Food Chem. 2009, 114, 306–309. [Google Scholar] [CrossRef]
- Turrini, F.; Farinini, E.; Leardi, R.; Grasso, F.; Orlandi, V.; Boggia, R. A Preliminary Color Study of Different Basil-Based Semi-Finished Products during Their Storage. Molecules 2022, 27, 2059. [Google Scholar] [CrossRef]
- Galanakis, C.M. Sustainable Applications for the Valorization of Cereal Processing By-Products. Foods 2022, 11, 241. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Fedele, F.L.; Sicari, A.; Piscopo, A.; Poiana, M. Antioxidant activity shown by olive pomace extracts. J. Environ. Sci. Health B 2018, 53, 526–533. [Google Scholar] [CrossRef]
- Giuffrè, A.M.; Sicari, V.; Piscopo, A.; Louadj, L. Antioxidant activity of olive oil mill wastewater obtained from different thermal treatments. Grasas Aceites 2012, 63, 209–213. [Google Scholar] [CrossRef]
- Benamar, A.; Mahjoubi, F.Z.; Barka, N.; Kzaiber, F.; Boutoial, K.; Ali, G.A.; Oussama, A. Olive mill wastewater treatment using infiltration percolation in column followed by aerobic biological treatment. SN Appl. Sci. 2020, 2, 665. [Google Scholar] [CrossRef]
- Yangui, A.; Abderrabba, M. Towards a high yield recovery of polyphenols from olive mill wastewater on activated carbon coated with milk proteins: Experimental design and antioxidant activity. Food Chem. 2018, 262, 102–109. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive mill wastewater as renewable raw materials to generate high added-value ingredients for agro-food industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Balzan, S.; Carraro, L.; Taticchi, A.; Montemurro, F.; Novelli, E. Minimum bactericidal concentration of phenols extracted from oil vegetation water on spoilers, starters and food-borne bacteria. Ital. J. Food Saf. 2015, 4, 4519. [Google Scholar] [CrossRef][Green Version]
- Galanakis, C.M.; Tsatalas, P.; Charalambous, Z.; Galanakis, I.M. Control of microbial growth in bakery products fortified with polyphenols recovered from olive mill wastewater. Environ. Technol. Innov. 2018, 10, 1–15. [Google Scholar] [CrossRef]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Evaluation of enrichment with antioxidants from olive oil mill wastes in hydrophilic model system. J. Food Process. Preserv. 2019, 43, e14211. [Google Scholar] [CrossRef]
- Romeo, R.; De Bruno, A.; Imeneo, V.; Piscopo, A.; Poiana, M. Impact of Stability of Enriched Oil with Phenolic Extract from Olive Mill Wastewaters. Foods 2020, 9, 856. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Gattuso, A.; Piscopo, A.; Poiana, M. Functionalization of a Vegan Mayonnaise with High Value Ingredient Derived from the Agro-Industrial Sector. Foods 2021, 10, 2684. [Google Scholar] [CrossRef]
- Mitić-Ćulafić, D.S.; Pavlović, M.; Ostojić, S.; Knezević-Vukčević, J. Antimicrobial effect of natural food preservatives in fresh basil-based pesto spreads. J. Food Process. Preserv. 2014, 38, 1298–1306. [Google Scholar] [CrossRef]
- Wongsen, W.; Bodhipadma, K.; Noichinda, S.; Leung, D.W.M. Relationship between leaf position and antioxidant properties in three basil species. Int. Food Res. J. 2013, 20, 1113–1117. [Google Scholar]
- Dewanto, V.; Wu, X.Z.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- D’Antuono, I.; Kontogianni, V.G.; Kotsiou, K.; Linsalata, V.; Logrieco, A.F.; Tasioula-Margari, M.; Cardinali, A. Polyphenolic characterization of olive mill wastewaters, coming from Italian and Greek olive cultivars, after membrane technology. Food Res. Int. 2014, 65, 301–310. [Google Scholar] [CrossRef]
- Obied, H.K.; Karuso, P.; Prenzler, P.D.; Robards, K. Novel secoiridoids with antioxidant activity from Australian olive mill waste. J. Agric. Food Chem. 2007, 55, 2848–2853. [Google Scholar] [CrossRef]
- Conte, P.; Pulina, S.; Del Caro, A.; Fadda, C.; Urgeghe, P.P.; De Bruno, A.; Difonzo, G.; Caponio, F.; Romeo, R.; Piga, A. Gluten-Free Breadsticks Fortified with Phenolic-Rich Extracts from Olive Leaves and Olive Mill Wastewater. Foods 2021, 10, 923. [Google Scholar] [CrossRef]
- Centrone, M.; D’Agostino, M.; Difonzo, G.; De Bruno, A.; Di Mise, A.; Ranieri, M.; Montemurro, C.; Valenti, G.; Poiana, M.; Caponio, F.; et al. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods 2021, 10, 11. [Google Scholar] [CrossRef]
- Aggoun, M.; Arhab, R.; Cornu, A.; Portelli, J.; Barkat, M.; Graulet, B. Olive mill wastewater microconstituents composition according to olive variety and extraction process. Food Chem. 2016, 209, 72–80. [Google Scholar] [CrossRef]
- Do, T.H.; Truong, H.B.; Nguyen, H.C. Optimization of Extraction of Phenolic Compounds from Ocimum Basilicum Leaves and Evaluation of Their Antioxidant Activity. Pharm. Chem. J. 2020, 54, 162–169. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; E-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and Successive Harvests Interaction Affects Phenolic Acids and Aroma Profile of Genovese Basil for Pesto Sauce Production. Foods 2021, 10, 278. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Sestili, P.; Ismail, T.; Neugart, S.; Qamar, M.; Esatbeyoglu, T. Toxicity, Antioxidant Activity, and Phytochemicals of Basil (Ocimum basilicum L.) Leaves Cultivated in Southern Punjab, Pakistan. Foods 2022, 11, 1239. [Google Scholar] [CrossRef]
- Masino, F.; Ulrici, A.; Antonelli, A. Extraction and quantification of main pigments in pesto sauces. Eur. Food Res. Technol. 2008, 226, 569–575. [Google Scholar] [CrossRef]
- Yakhlef, W.; Arhab, R.; Romero, C.; Brenes, M.; de Castro, A.; Medina, E. Phenolic composition and antimicrobial activity of Algerian olive products and by-products. LWT Food Sci. Technol. 2018, 93, 323–328. [Google Scholar] [CrossRef]
- Maoloni, A.; Cardinali, F.; Milanović, V.; Garofalo, C.; Osimani, A.; Mozzon, M.; Aquilanti, L. Microbiological safety and stability of novel green sauces made with sea fennel (Chritmum maritimum L.). Food Res. Int. 2022, 157, 111463. [Google Scholar] [CrossRef]
- Mikdame, H.; Kharmach, E.; Mtarfi, N.E.; Alaoui, K.; Abbou, M.B.; Rokni, Y.; Majbar, Z.; Taleb, M.; Rais, Z. By-Products of Olive Oil in the Service of the Deficiency of Food Antioxidants: The Case of Butter. J. Food Qual. 2020, 2020, 6382942. [Google Scholar] [CrossRef]
- Klug, T.V.; Collado, E.; Martínez-Sánchez, A.; Gomez, P.A.; Aguayo, E.; Otón, M.; Artés, F.; Artés-Hernandez, F. Innovative Quality Improvement by Continuous Microwave Processing of a Faba Beans Pesto Sauce. Food Bioproc. Tech. 2018, 11, 561–571. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, K.B.; Choi, S.K. Quality characteristics of basil pesto added with various nuts during storage. Culin. Sci. Hosp. Res. 2018, 22, 29–43. [Google Scholar]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef]
- Friedman, M.; Jürgens, H.S. Effect of pH on the stability of plant phenolic compounds. J. Agric. Food Chem. 2020, 48, 2101–2110. [Google Scholar] [CrossRef]
- Branco, I.G.; Moraes, I.C.F.; Argandoña, E.J.S.; Madrona, G.S.; dos Santos, C.; Ruiz, A.L.T.G.; de Carvalho, J.E.; Haminiuk, C.W.I. Influence of pasteurization on antioxidant and in vitro anti-proliferative effects of jambolan (Syzygium cumini (L.) Skeels) fruit pulp. Ind. Crops Prod. 2016, 89, 225–230. [Google Scholar] [CrossRef]
- Kwee, E.M.; Niemeyer, E.D. Variations in phenolic composition and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Martillanes, S.; Rocha-Pimienta, J.; Gil, M.V.; Ayuso-Yuste, M.C.; Delgado-Adámez, J. Antioxidant and antimicrobial evaluation of rice bran (Oryza sativa L.) extracts in a mayonnaise-type emulsion. Food Chem. 2020, 308, 125633. [Google Scholar] [CrossRef] [PubMed]
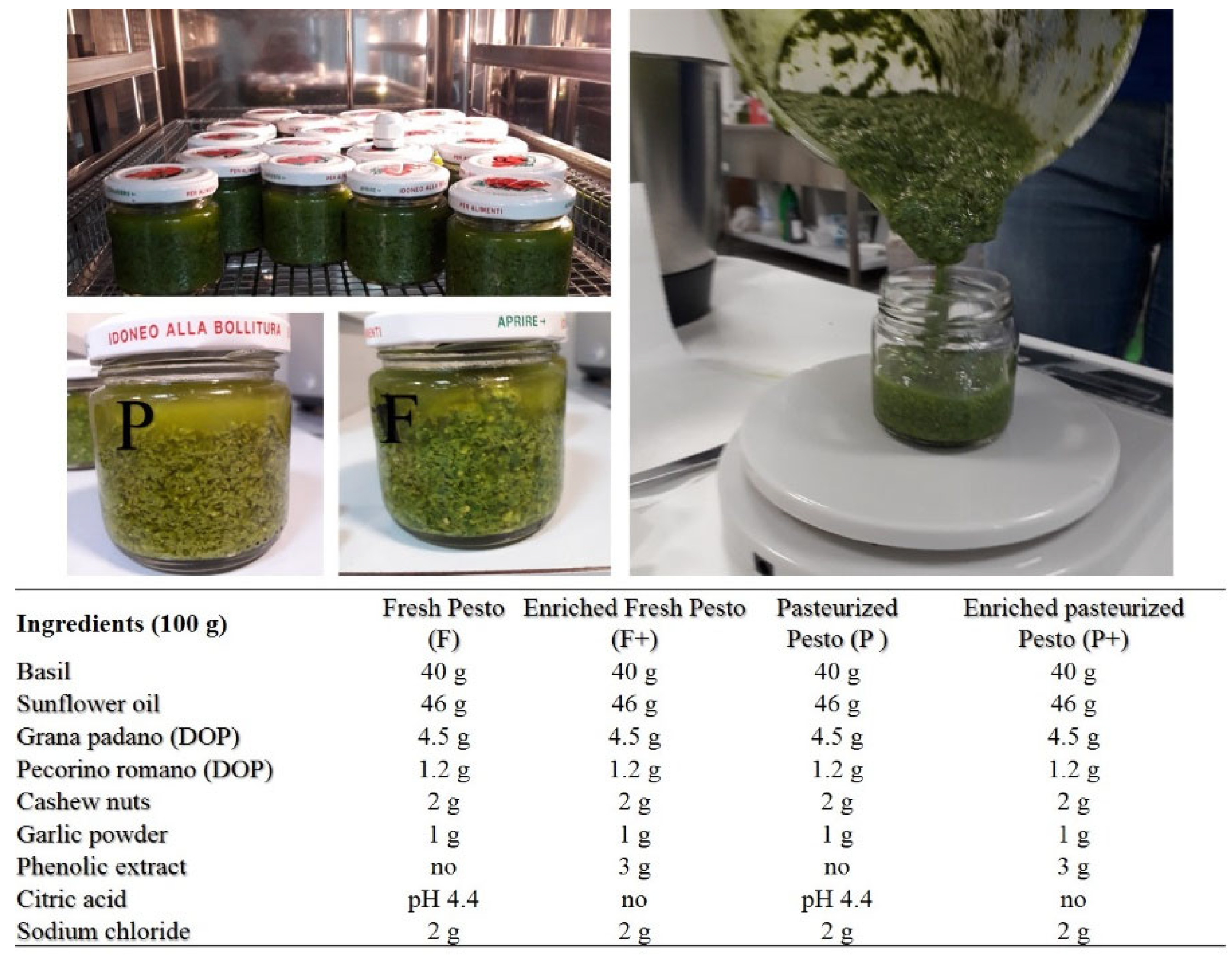
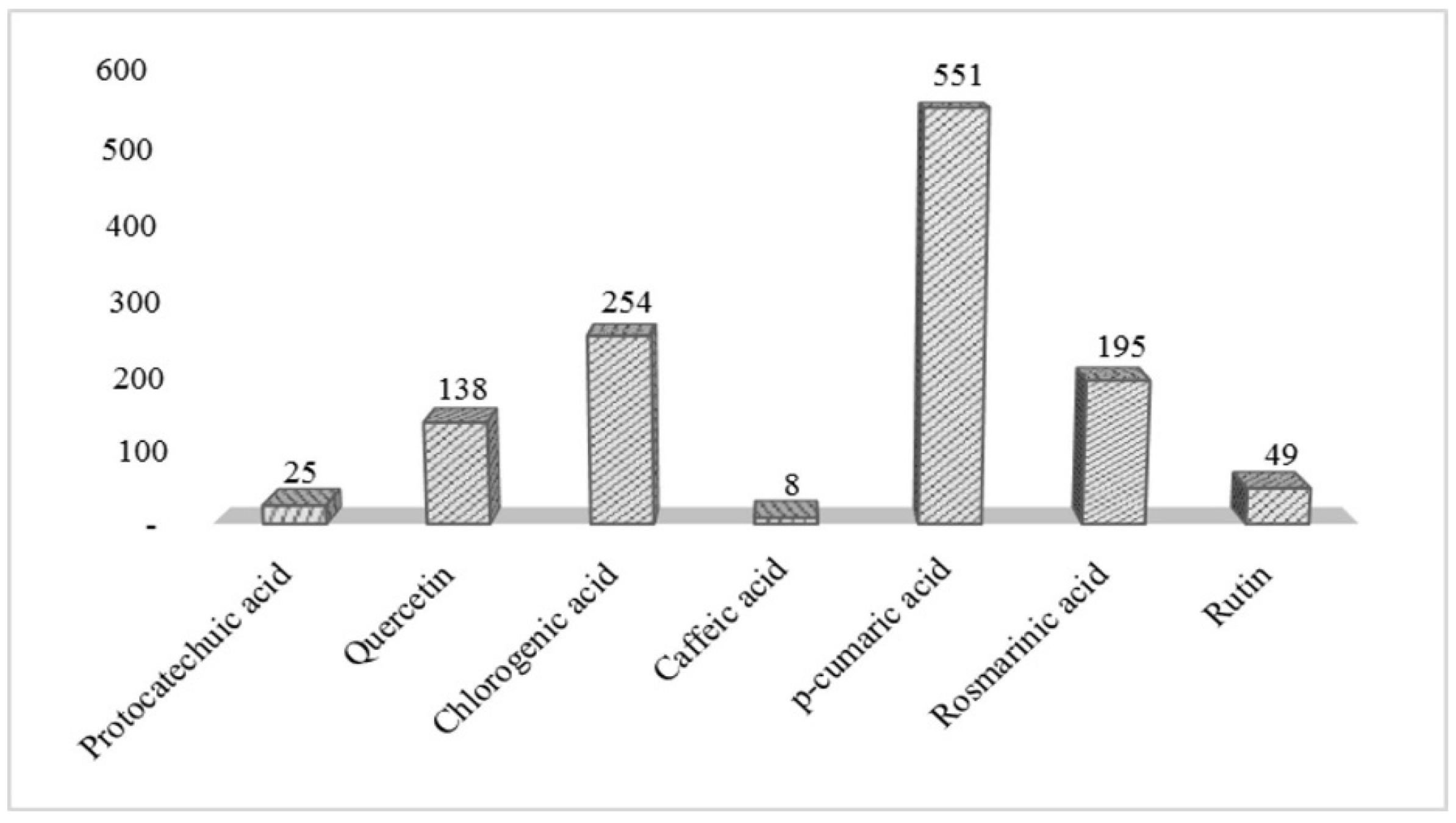
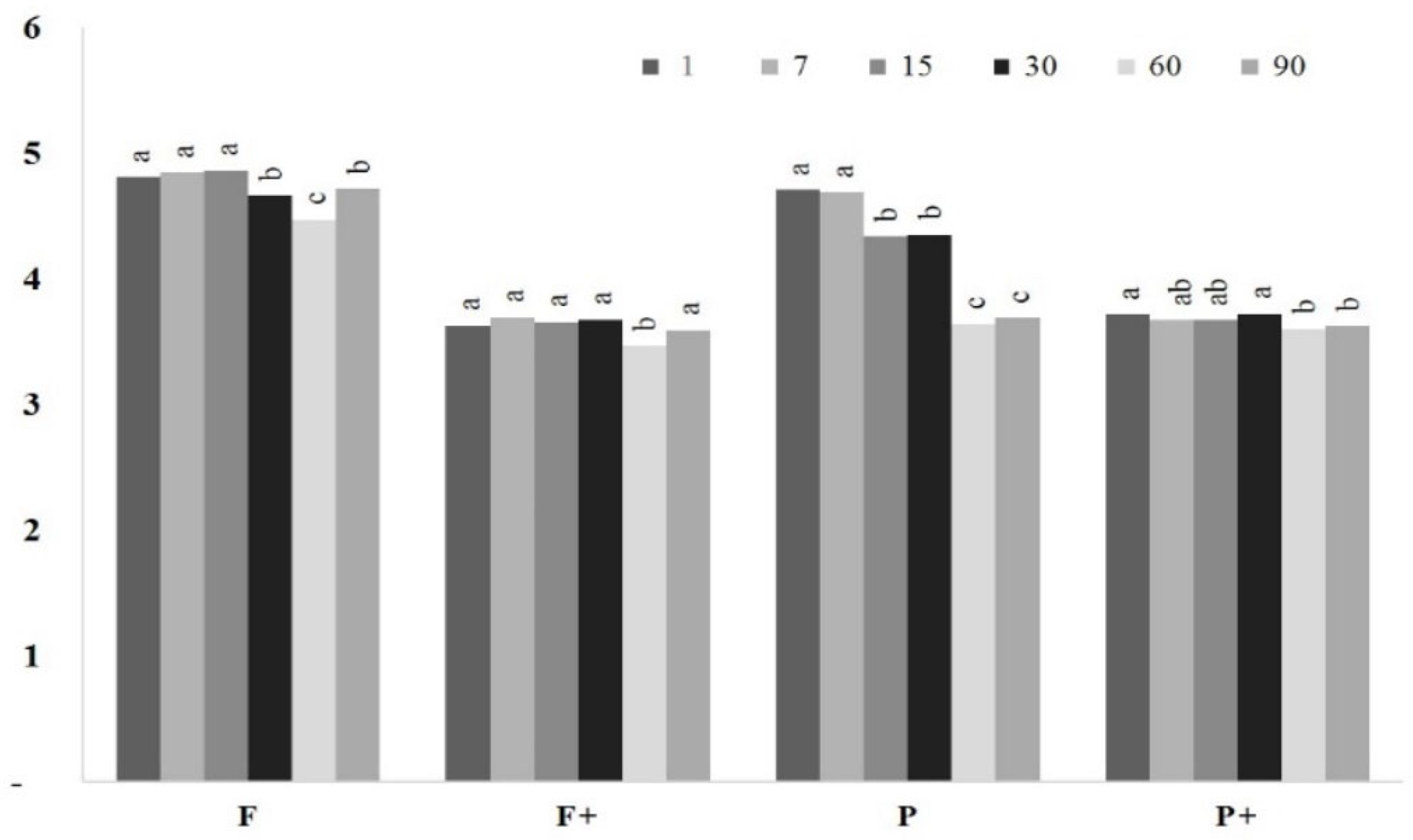
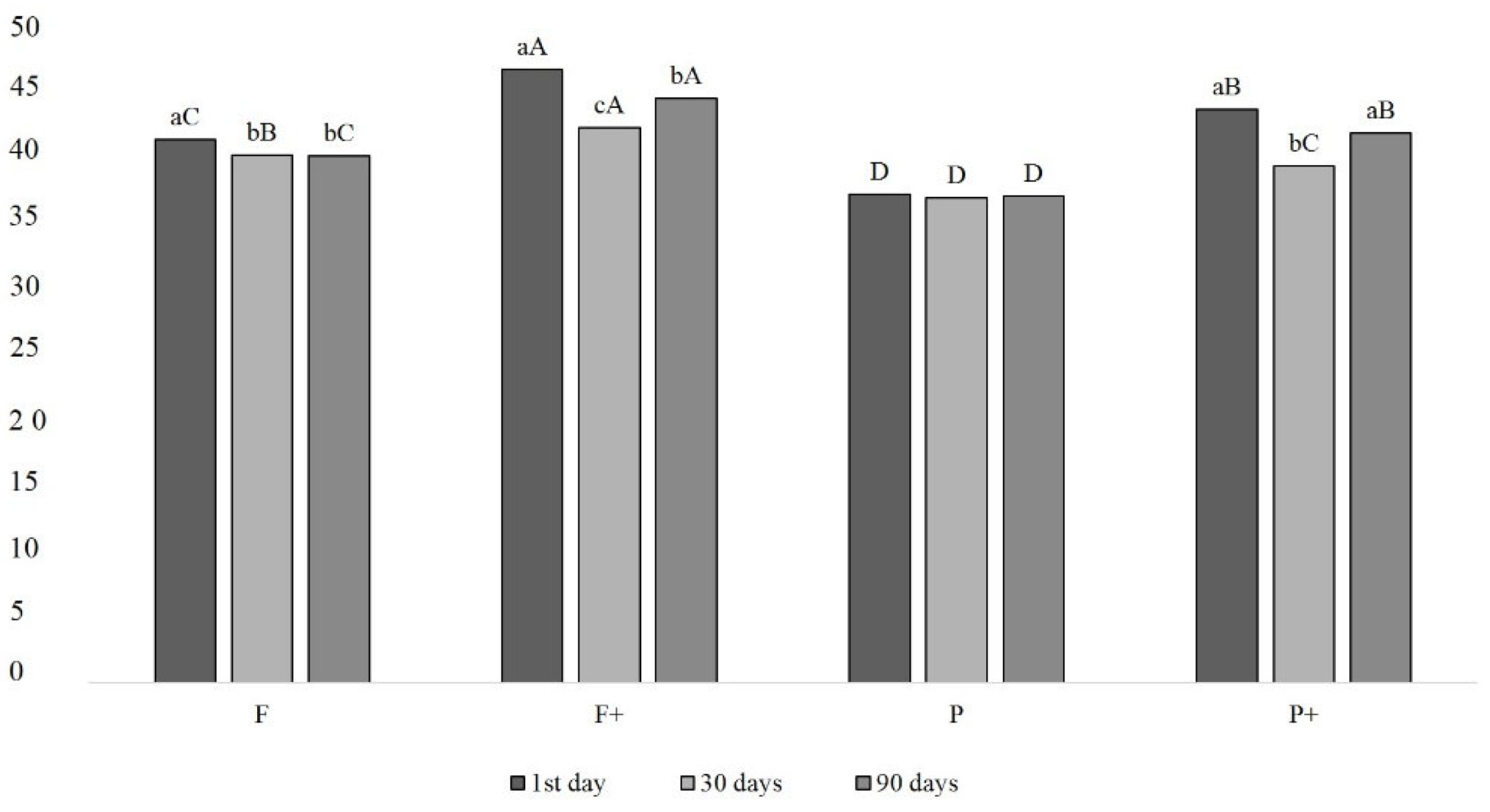
| TPC (mg g−1) | 60.22 ± 17.47 |
| Hydroxytyrosol (mg g−1) | 1.03 ± 0.09 |
| Tyrosol (mg g−1) | 0.57 ± 0.04 |
| DPPH (µmol TE g−1) | 154 ± 21 |
| ABTS (µmol TE g−1) | 3768 ± 66 |
| L* | 48.81 ± 4.40 |
| A* | −9.17 ± 1.12 |
| B* | 23.54 ± 3.44 |
| aw | 0.983 ± 0.004 |
| Moisture (%) | 90.64 ± 0.02 |
| CHLA (mg 100 g−1) | 2.68 ± 0.01 |
| CHLB (mg 100 g−1) | 4.67 ± 0.01 |
| TPC (mg GAE g−1) | 3.64 ± 0.02 |
| DPPH (µmol TE g−1) | 5.71 ± 0.17 |
| ABTS (µmol TE g−1) | 79.08 ± 5.61 |
| Days | F | F+ | P | P+ | Sign. | |
|---|---|---|---|---|---|---|
| L* | 1 | 42.09 ± 1.09 ABa | 43.33 ± 1.52 Aab | 41.42 ± 1.03 B | 42.74 ± 0.88 AB | ** |
| 7 | 39.50 ± 1.38 Cb | 42.47 ± 0.90 Bab | 40.33 ± 1.06 C | 44.33 ± 1.25 A | ** | |
| 15 | 39.04 ± 1.46 Cb | 41.89 ± 1.02 Bb | 40.14 ± 0.98 C | 44.03 ± 1.78 A | ** | |
| 30 | 40.12 ± 1.17 Bb | 42.53 ± 0.75 Aab | 40.52 ± 1.42 B | 42.78 ± 1.28 A | ** | |
| 60 | 38.99 ± 0.90 Cb | 42.85 ± 1.09 ABab | 41.83 ± 1.18 B | 43.09 ± 0.86 A | ** | |
| 90 | 39.34 ± 1.58 Bb | 43.28 ± 0.53 Aa | 41.72 ± 2.14 A | 43.27 ± 0.96 A | ** | |
| Sign. | ** | * | n.s. | n.s. | ||
| a* | 1 | −5.45 ± 0.32 Dc | −3.96 ± 0.31 Ce | −1.65 ± 0.60 Be | 1.56 ± 0.26 Aa | ** |
| 7 | −3.87 ± 0.71 Db | −2.33 ± 0.25 Cd | 0.38 ± 0.32 Bd | 2.12 ± 0.26 Ab | ** | |
| 15 | −4.09 ± 0.46 Db | −2.02 ± 0.16 Ccd | 0.77 ± 0.33 Bcd | 2.21 ± 0.28 Ab | ** | |
| 21 | −3.65 ± 0.58 Dab | −1.81 ± 0.23 Cc | 1.30 ± 0.28 Bc | 2.33 ± 0.28 Ab | ** | |
| 60 | −3.06 ± 0.48 Da | −1.40 ± 0.24 Cb | 1.90 ± 0.27 Bb | 2.33 ± 0.24 Ab | ** | |
| 90 | −3.08 ± 0.51 Ca | −0.99 ± 0.24 Ba | 2.13 ± 0.31 Aa | 2.28 ± 0.17 Ab | ** | |
| Sign. | ** | ** | ** | ** | ||
| b* | 1 | 14.98 ± 1.28 Ba | 18.13 ± 1.21 Aa | 14.97 ± 1.42 B | 17.43 ± 1.45 Abc | ** |
| 7 | 12.02 ± 1.46 Cb | 15.91 ± 2.05 Bb | 13.21 ± 1.16 C | 19.02 ± 1.10 Aab | ** | |
| 15 | 12.09 ± 2.02 Cb | 15.42 ± 1.07 Bb | 13.94 ± 1.29 BC | 19.66 ± 1.66 Aa | ** | |
| 21 | 12.72 ± 1.57 Db | 16.26 ± 0.94 Bab | 14.35 ± 0.85 C | 18.87 ± 1.21 Aab | ** | |
| 60 | 11.00 ± 1.11 Cb | 16.28 ± 2.14 Bab | 15.10 ± 1.48 B | 19.21 ± 1.96 Aab | ** | |
| 90 | 11.49 ± 1.64 Bb | 16.82 ± 0.45 Aab | 15.24 ± 2.52 A | 16.85 ± 1.08 Ac | ** | |
| Sign. | ** | ** | n.s. | ** | ||
| ΔE | 5.63 ± 2.47 A | 3.85 ± 0.95 AB | 4.56 ± 1.20 A | 2.54 ± 1.39 B | ** | |
| Storage Time (Days) | CHLA (mg 100 g−1) | CHLB (mg 100 g−1) | β-CAR (mg 100 g−1) | TPC (mg GAE g−1) | DPPH (µmol TE g−1) | ABTS (µmol TE g−1) | |
|---|---|---|---|---|---|---|---|
| F | 1st | 1.74 ± 0.02 | 0.78 ± 0.03 | 0.21 ± 0.01 | 1.00 ± 0.00 | 2.33 ± 0.55 | 52.26 ± 2.75 |
| 90th | 1.64 ± 0.01 | 0.71 ± 0.01 | 0.40 ± 0.01 | 1.64 ± 0.00 | 3.99 ± 0.07 | 66.05 ± 5.92 | |
| Sign. | * | n.s. | ** | ** | n.s. | n.s. | |
| F+ | 1st | 1.53 ± 0.01 | 0.76 ± 0.02 | 0.11 ± 0.02 | 1.66 ± 0.00 | 3.23 ± 0.38 | 69.10 ± 1.42 |
| 90th | 1.56 ± 0.00 | 0.66 ± 0.01 | 0.49 ± 0.00 | 1.88 ± 0.00 | 3.27 ± 0.28 | 65.70 ± 2.53 | |
| Sign. | n.s. | * | ** | ** | n.s. | n.s. | |
| P | 1st | 1.21 ± 0.01 | 0.57 ± 0.01 | 0.49 ± 0.02 | 1.39 ± 0.01 | 3.88 ± 0.03 | 62.58 ± 0.09 |
| 90th | 1.33 ± 0.00 | 0.28 ± 0.00 | 0.37 ± 0.00 | 1.87 ± 0.00 | 4.33 ± 0.76 | 82.72 ± 1.49 | |
| Sign. | ** | ** | ** | ** | n.s. | ** | |
| P+ | 1st | 1.30 ± 0.00 | 0.35 ± 0.01 | 0.56 ± 0.02 | 1.79 ± 0.02 | 3.70 ± 0.32 | 66.85 ± 3.27 |
| 90th | 1.46 ± 0.00 | 0.20 ± 0.00 | 0.50 ± 0.01 | 2.06 ± 0.00 | 4.10 ± 0.00 | 75.05 ± 4.03 | |
| Sign. | ** | ** | * | ** | n.s. | n.s. |
| Compounds | Regression Equation | R2 | LOD µg g−1 | LOQ µg g−1 |
|---|---|---|---|---|
| Hydroxytyrosol | y = 25.77x − 24.41 | 0.9999 | 0.0888 | 0.05 |
| Protocatechuic acid | y = 119.35x + 645.19 | 0.9998 | 0.0656 | 0.04 |
| Tyrosol | y = 41.25x − 49.09 | 0.9998 | 0.0456 | 0.02 |
| Chlorogenic acid | y = 52.15x − 100.73 | 0.9999 | 0.0532 | 0.03 |
| Caffeic acid | y = 112.33x − 110.33 | 0.9997 | 0.0782 | 0.05 |
| p-coumaric acid | y = 113.96x + 50.26 | 0.9999 | 0.0988 | 0.20 |
| Quercetin | y = 59.097x + 119.01 | 0.9999 | 0.0456 | 0.02 |
| Oleuropein | y = 46.63x + 51.21 | 0.9997 | 0.0123 | 0.04 |
| Luteolin | y = 74.58x − 136.08 | 0.9996 | 0.0234 | 0.05 |
| Apigenin | y = 100.91x − 296.57 | 0.9989 | 0.0345 | 0.02 |
| Rutin | y = 46.07x − 14.44 | 0.9999 | 0.04536 | 0.04 |
| Rosmarinic acid | y = 228.72x − 194.39 | 0.9997 | 0.1123 | 0.45 |
| Compounds | Days | F | F+ | P | P+ | FC | PC | Sign. |
|---|---|---|---|---|---|---|---|---|
| Hydroxytyrosol | 1st | nd | 10.55 | nd | 10.40 | nd | nd | n.s. |
| 90th | nd | 8.61 | nd | 8.38 | - | - | n.s. | |
| Sign. | * | * | ||||||
| Protocatechuic acid | 1st | 15.52 d | 21.60 b | 19.20 c | 18.64 c | 15.47 d | 23.96 a | ** |
| 90th | 13.40 d | 20.72 b | 36.28 a | 16.98 c | nd | nd | ** | |
| Sign. | ** | n.s. | ** | * | ||||
| Tyrosol | 1st | nd | 8.87 b | nd | 11.02 a | nd | nd | ** |
| 90th | nd | 11.37 a | nd | 8.70 b | nd | nd | ** | |
| Sign. | ** | ** | ||||||
| Quercetin | 1st | 4.15 d | 12.18 a | 7.52 c | 10.16 b | 2.14 e | 2.31 e | ** |
| 90th | 2.80 b | 7.66 a | 7.96 a | 2.29 b | nd | nd | ** | |
| Sign. | ** | ** | n.s. | ** | ||||
| Oleuropein | 1st | nd | 2.32 b | nd | 4.12 a | nd | nd | ** |
| 90th | nd | 5.10 a | nd | 1.41 b | nd | nd | ** | |
| Sign. | ** | ** | ||||||
| Luteolin | 1st | nd | 19.75 a | 17.55 b | 19.55 a | 16.87 b | nd | ** |
| 90th | nd | 21.26 a | 17.12 c | 19.80 b | - | - | ** | |
| Sign. | ** | n.s. | n.s. | |||||
| Apigenin | 1st | nd | 13.00 b | 10.42 d | 11.81 c | 7.26 e | 16.19 a | ** |
| 90th | nd | 14.72 a | 12.47 c | 12.75 b | nd | nd | ** | |
| Sign. | ** | ** | * | |||||
| Chlorogenic acid | 1st | 15.64 a | 6.05 b | 4.91 c | nd | nd | nd | ** |
| 90th | 13.81 a | 6.69 b | nd | nd | nd | nd | ** | |
| Sign. | * | * | ** | |||||
| Caffeic acid | 1st | 18.06 d | 35.47 b | 31.08 c | 31.50 c | nd | 69.33 a | ** |
| 90th | 48.61 a | 39.21 c | 35.45 d | 42.90 b | - | - | ** | |
| Sign. | ** | ** | ** | ** | ||||
| p-coumaric | 1st | 31.73 f | 49.89 c | 43.81 e | 46.18 d | 165.65 a | 54.71 b | ** |
| 90th | 27.25 d | 53.16 c | 59.22 b | 65.20 a | - | - | ** | |
| Sign. | ** | ** | ** | ** | ||||
| Rosmarinic acid | 1st | 9.94 f | 27.18 e | 36.30 d | 37.82 c | 45.41 b | 99.73 a | ** |
| 90th | 7.72 d | 21.96 c | 32.40 b | 39.40 a | - | - | ** | |
| Sign. | ** | ** | ** | ** | ||||
| Rutin | 1st | 10.30 c | 11.58 b | 9.50 c | 11.45 b | 8.30 d | 23.67 a | ** |
| 90th | 8.92 c | 11.44 a | 9.10 c | 10.67 b | - | - | ** | |
| Sign. | ** | n.s. | n.s. | n.s |
| Samples | Time | Weight | Reactor | Pressure | Temperature | Calculation | aIP |
|---|---|---|---|---|---|---|---|
| (g) | (bar) | (°C) | IP | (h:mm) | |||
| F | 1st | 30.00 | B | 6.00 | 90.0 | LSM | 12.03 |
| 90th | 30.00 | B | 6.00 | 90.0 | LSM | 8.15 | |
| P | 1st | 30.00 | B | 6.00 | 90.0 | LSM | 10.41 |
| 90th | 30.00 | B | 6.00 | 90.0 | LSM | 10.04 | |
| F+ | 1st | 30.00 | B | 6.00 | 90.0 | LSM | 12.3 |
| 90th | 30.00 | B | 6.00 | 90.0 | LSM | 11.42 | |
| P+ | 1st | 30.00 | B | 6.00 | 90.0 | LSM | 11.85 |
| 90th | 30.00 | B | 6.00 | 90.0 | LSM | 12.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bruno, A.; Gattuso, A.; Romeo, R.; Santacaterina, S.; Piscopo, A. Functional and Sustainable Application of Natural Antioxidant Extract Recovered from Olive Mill Wastewater on Shelf-Life Extension of “Basil Pesto”. Appl. Sci. 2022, 12, 10965. https://doi.org/10.3390/app122110965
De Bruno A, Gattuso A, Romeo R, Santacaterina S, Piscopo A. Functional and Sustainable Application of Natural Antioxidant Extract Recovered from Olive Mill Wastewater on Shelf-Life Extension of “Basil Pesto”. Applied Sciences. 2022; 12(21):10965. https://doi.org/10.3390/app122110965
Chicago/Turabian StyleDe Bruno, Alessandra, Antonio Gattuso, Rosa Romeo, Simone Santacaterina, and Amalia Piscopo. 2022. "Functional and Sustainable Application of Natural Antioxidant Extract Recovered from Olive Mill Wastewater on Shelf-Life Extension of “Basil Pesto”" Applied Sciences 12, no. 21: 10965. https://doi.org/10.3390/app122110965
APA StyleDe Bruno, A., Gattuso, A., Romeo, R., Santacaterina, S., & Piscopo, A. (2022). Functional and Sustainable Application of Natural Antioxidant Extract Recovered from Olive Mill Wastewater on Shelf-Life Extension of “Basil Pesto”. Applied Sciences, 12(21), 10965. https://doi.org/10.3390/app122110965










