Synthesis of Hydroxyapatite (HAp)-Zirconia Nanocomposite Powder and Evaluation of Its Biocompatibility: An In Vitro Study
Abstract
1. Introduction
1.1. The Significance of Biocompatible Materials in Biomedical Applications
1.2. Hydroxyapatite
1.3. Composites of Hydroxyapatite
1.4. The Hydroxyapatite-Zironia Composite
1.5. Methods of Preparation
1.6. Drawbacks of the Previous Approaches
1.7. Objectives and Advantages of the Present Work
2. Materials and Methods
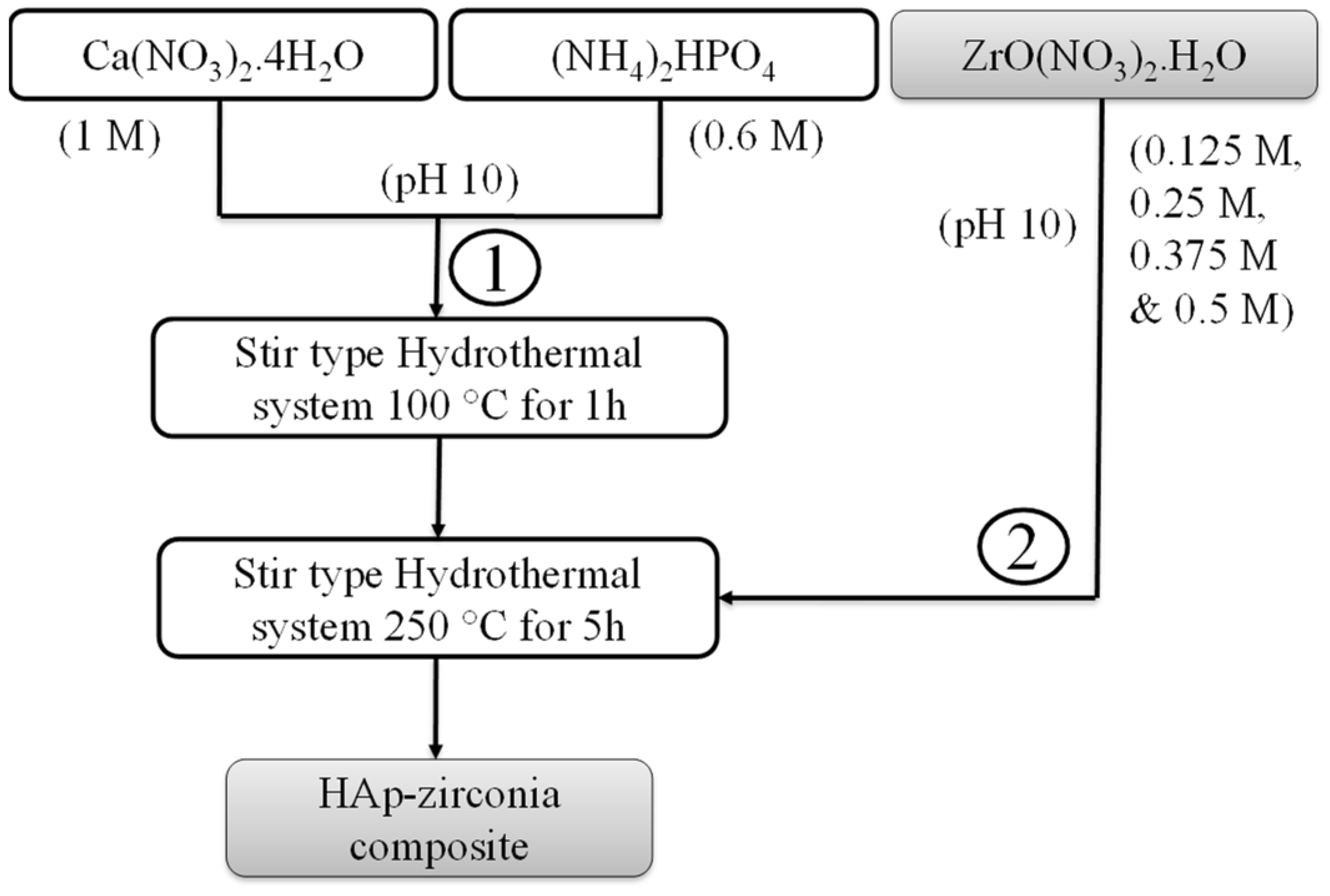
2.1. Synthesis of the HAp-Zirconia Composite
2.2. Characterization of the HAp-Zirconia Nanocomposite
3. Results
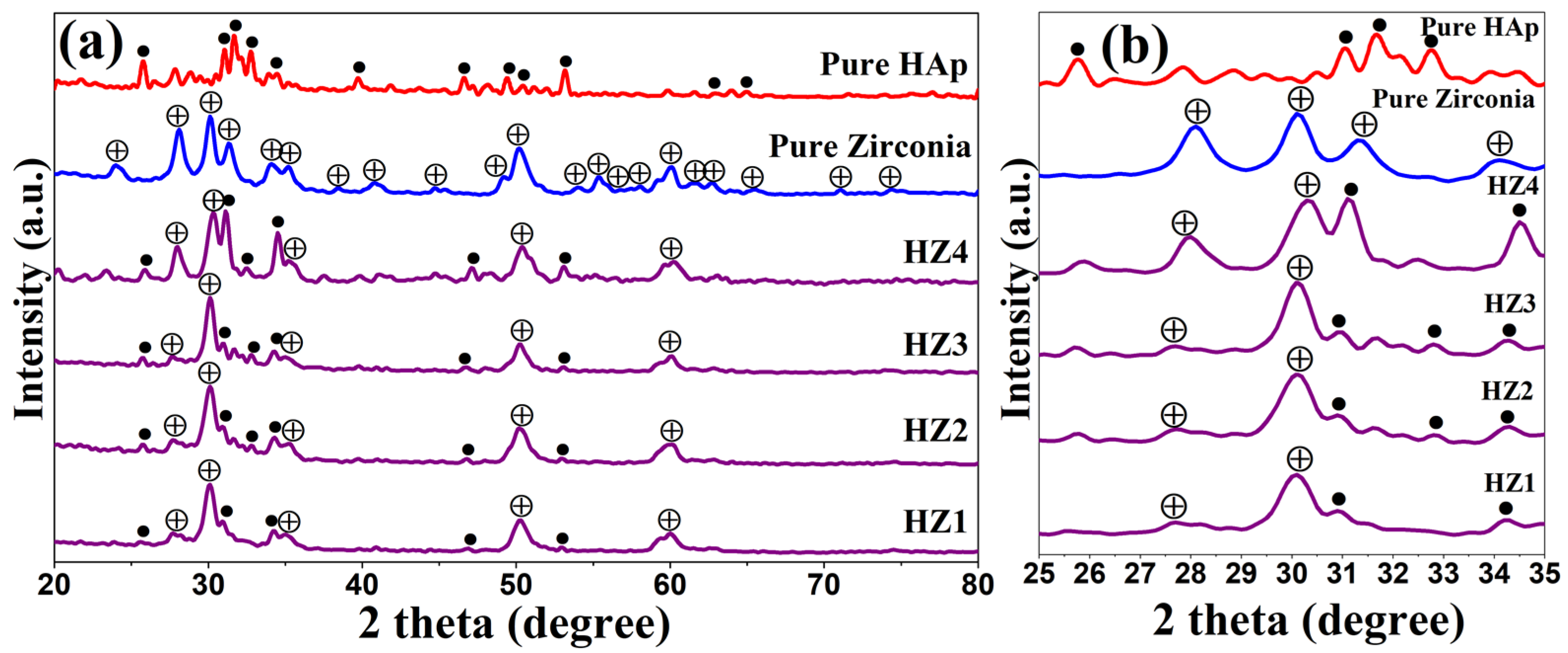
3.1. X-ray Diffraction Analysis
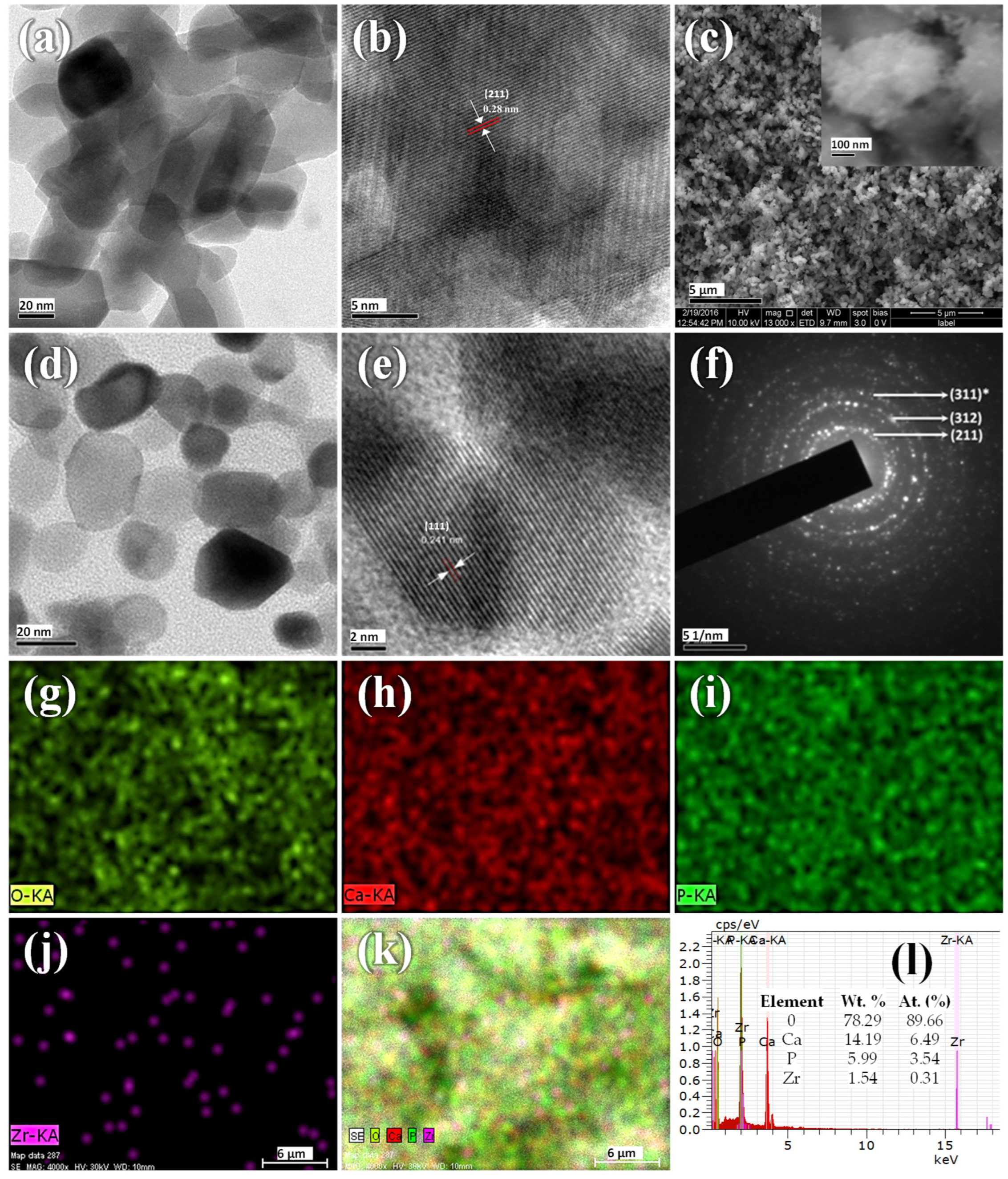
3.2. Electron Microscopy Analysis
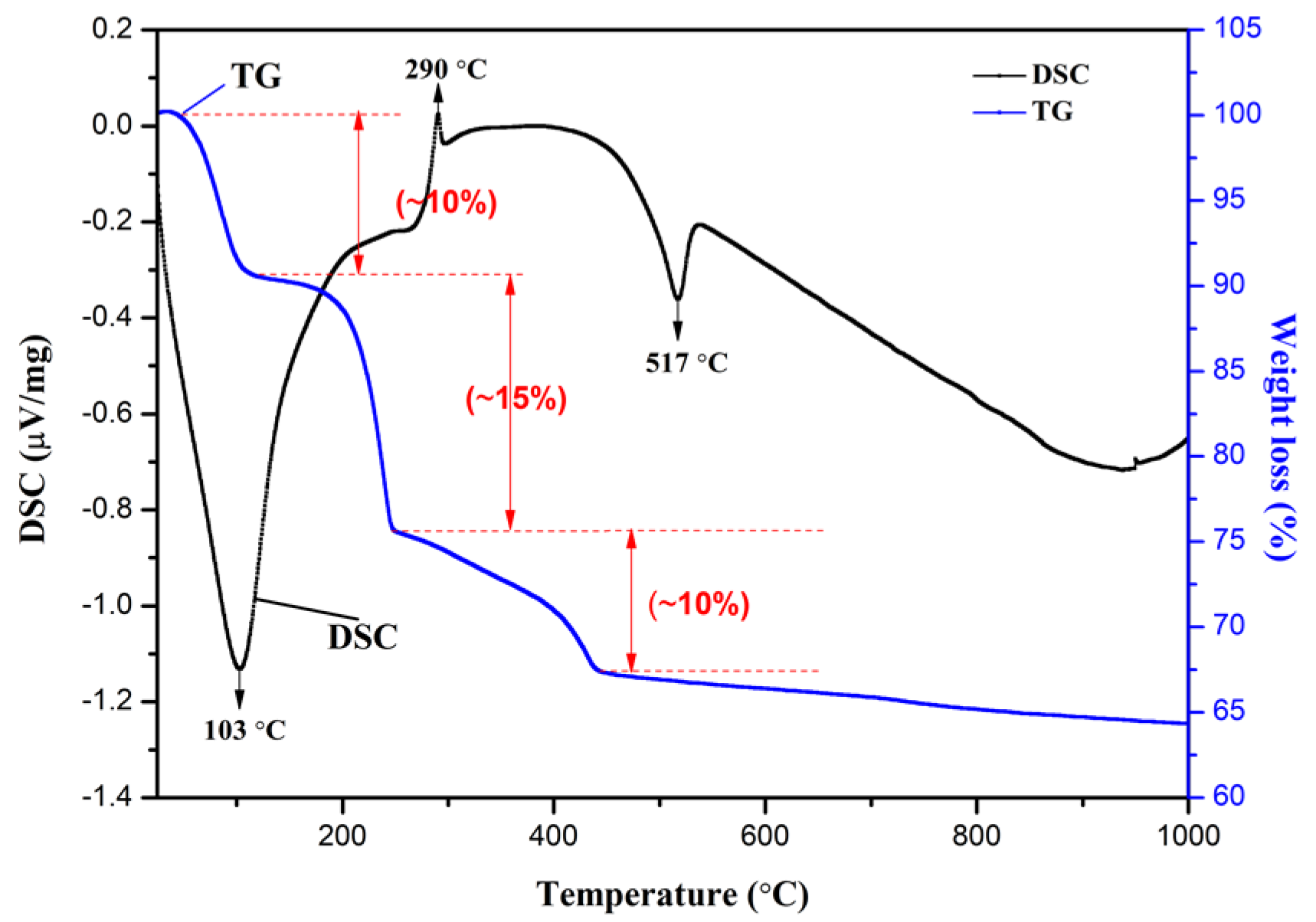
3.3. TG/DSC Analysis
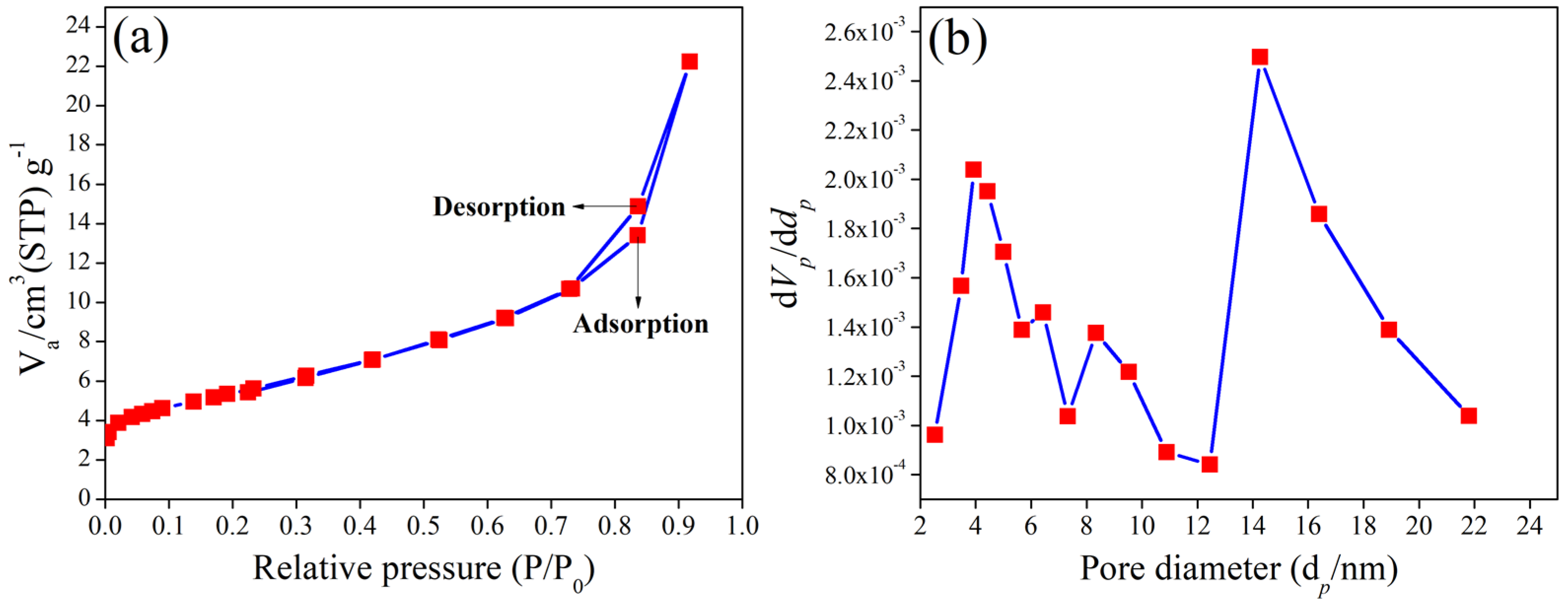
3.4. BET Analysis
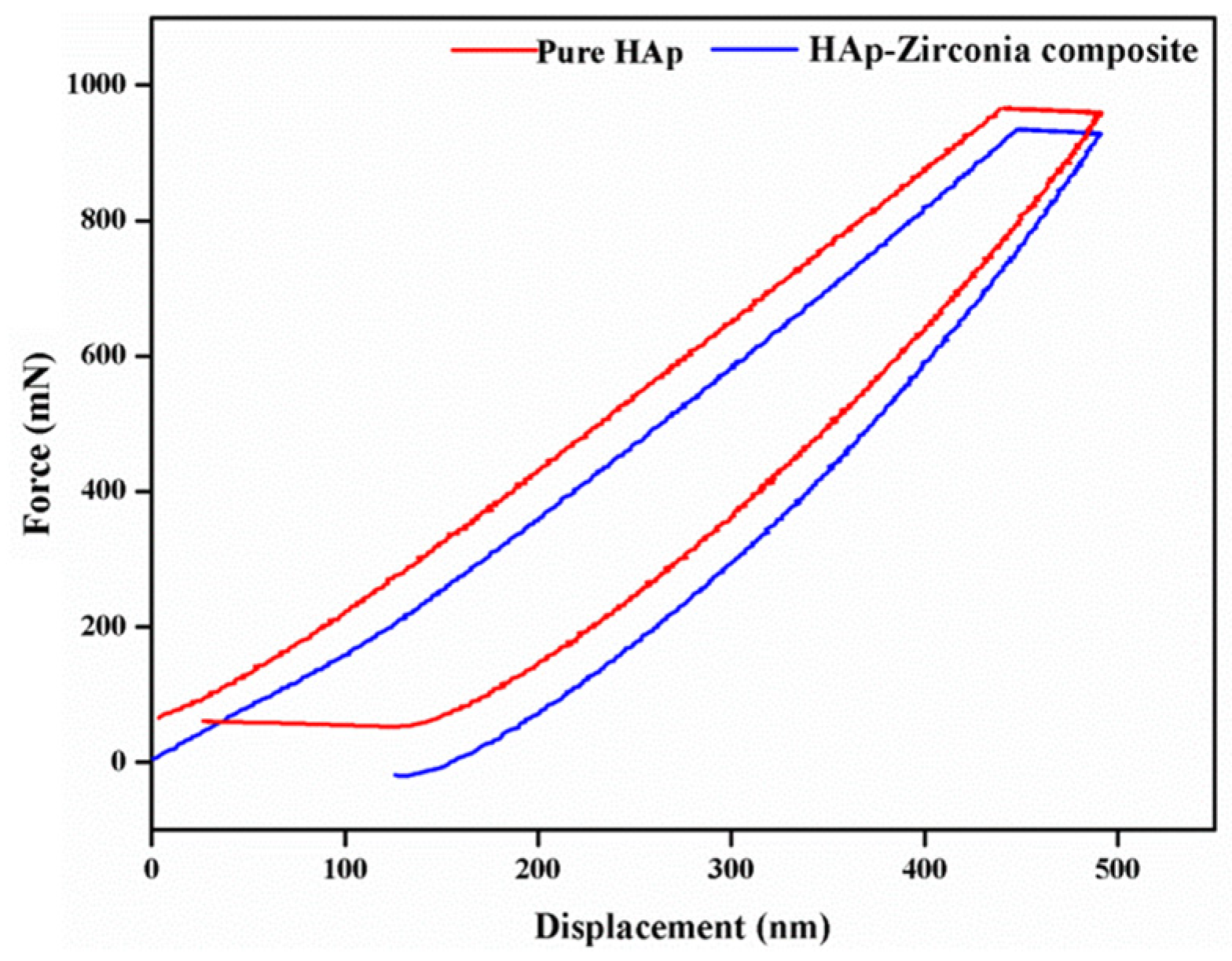
3.5. Nanoindentation Study
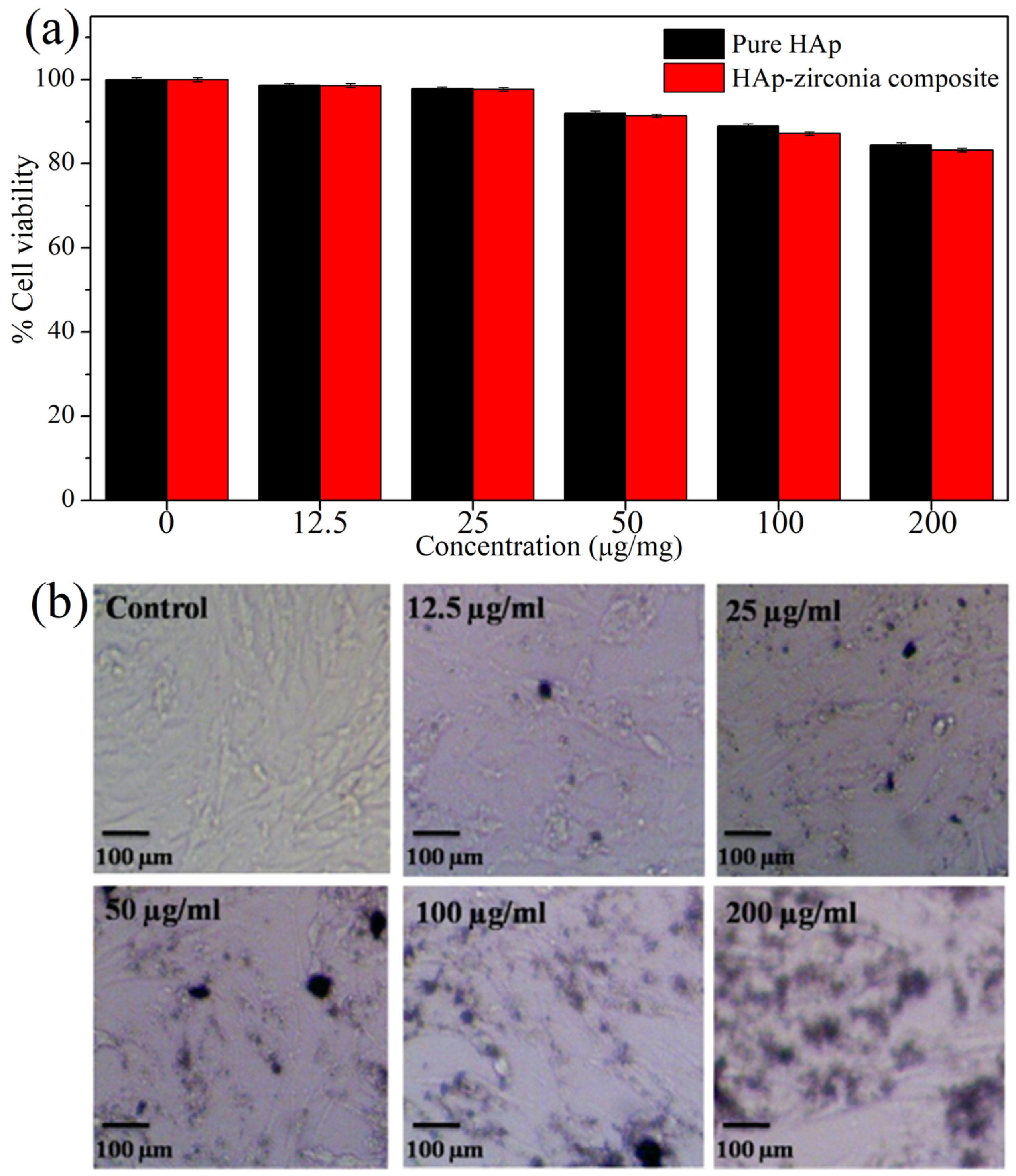
3.6. In Vitro Biocompatibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kiradzhiyska, D.; Mantcheva, R.D. Overview of biocompatible materials and their use in medicine. Folia Med. 2019, 61, 34–40. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Knabe, C.; Gildenhaar, R.; Berger, G.; Ostapowicz, W.; Fitzner, R.; Radlanski, R.; Gross, U. Morphological evaluation of osteoblasts cultured on different calcium phosphate ceramics. Biomaterials 1997, 18, 1339–1347. [Google Scholar] [CrossRef]
- Matsuoka, H.; Akiyama, H.; Okada, Y.; Ito, H.; Shigeno, C.; Konishi, J.; Kokubo, T.; Nakamura, T. In vitro analysis of the stimulation of bone formation by highly bioactive apatite-and wollastonite-containing glass-ceramic: Released calcium ions promote osteogenic differentiation in osteoblastic ROS17/2.8 cells. J. Biomed. Mater. Res. 1999, 47, 176–188. [Google Scholar] [CrossRef]
- Ylänen, H.O.; Helminen, T.; Helminen, A.; Rantakokko, J.; Karlsson, K.H.; Aro, H. Porous bioactive glass matrix in reconstruction of articular osteochondral defects. Annales Chir. Gynaecol. 1999, 88, 237–245. [Google Scholar]
- Ullah, I.; Zhang, W.; Yang, L.; Ullah, M.W.; Atta, O.M.; Khan, S.; Wu, B.; Wu, T.; Zhang, X. Impact of structural features of Sr/Fe co-doped HAp on the osteoblast proliferation and osteogenic differentiation for its application as a bone substitute. Mater. Sci. Eng. C 2020, 110, 110633. [Google Scholar] [CrossRef]
- Akao, M.; Aoki, H.; Kato, K. Mechanical properties of sintered hydroxyapatite for prosthetic applications. J. Mater. Sci. 1981, 16, 809–812. [Google Scholar] [CrossRef]
- Zhou, H.; Lee, J. Nanoscale hydroxyapatite particles for bone tissue engineering. Acta Biomater. 2011, 7, 2769–2781. [Google Scholar] [CrossRef]
- Hayashi, S.; Kuroda, Y.; Nakano, N.; Matsumoto, T.; Kamenaga, T.; Maeda, T.; Niikura, T.; Kuroda, R. Peri-prosthetic bone remodeling of hydroxyapatite-coated compaction short stem was not affected by stem alignment. J. Orthop. Surg. Res. 2022, 17, 1–11. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Feng, P.; Peng, S.; Pan, H. Accelerated degradation of HAP/PLLA bone scaffold by PGA blending facilitates bioactivity and osteoconductivity. Bioact. Mater. 2020, 6, 490–502. [Google Scholar] [CrossRef]
- Mucalo, M. Animal-bone derived hydroxyapatite in biomedical applications. In Hydroxyapatite (HAp) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 307–342. [Google Scholar] [CrossRef]
- Boer, F.C.D.; Wippermann, B.W.; Blokhuis, T.J.; Patka, P.; Bakker, F.C.; Haarman, H.J.T.M. Healing of segmental bone defects with granular porous hydroxyapatite augmented with recombinant human osteogenic protein-I or autologous bone marrow. J. Orthop. Res. 2003, 21, 521–528. [Google Scholar] [CrossRef]
- Rao, R.R.; Kannan, T. Synthesis and sintering of hydroxyapatite-Zirconia composites. Mater. Sci. Eng. C 2002, 20, 187–193. [Google Scholar] [CrossRef]
- Masseli, M.R.; Kuffner, B.H.B.; Vasconcelos, L.V.B.; Silva, G.; Sachs, D. Mechanical and physical characterization of hydroxyapatite/alumina biocomposites produced by the powder metallurgy route for biomedical applications. Matéria 2021, 26, 1382. [Google Scholar] [CrossRef]
- Orekhov, E.V.; Arbenin, A.Y.; Zemtsova, E.G.; Sokolova, D.N.; Ponomareva, A.N.; Shevtsov, M.A.; Yudintceva, N.M.; Smirnov, V.M. Template Electrochemical Synthesis of Hydroxyapatite on a Titania–Silver Composite Surface for Potential Use in Implantology. Coatings 2022, 12, 266. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, Y.; Lu, T.; He, F.; Zhang, L.; He, Q.; Ye, J. Enhancing the bioactivity of hydroxyapatite bioceramic via encapsulating with silica-based bioactive glass sol. J. Mech. Behav. Biomed. Mater. 2022, 128, 105104. [Google Scholar] [CrossRef]
- Almotiri, R.A.; Alkhamisi, M.M. Physico-chemical behavior and microstructural manipulation of nanocomposites containing hydroxyapatite, alumina, and graphene oxide. Appl. Phys. A 2022, 128, 1–13. [Google Scholar] [CrossRef]
- Sen, D.; Patil, V.; Smriti, K.; Varchas, P.; Ratnakar, R.; Shetty, D.K.; Naik, N.; Kumar, S. Nanotechnology and Nanomaterials in Dentistry: Present and Future Perspectives in Clinical Applications. Eng. Sci. 2022, 20, es8d703. [Google Scholar] [CrossRef]
- Sharifianjazi, F.; Pakseresht, A.H.; Asl, M.S.; Esmaeilkhanian, A.; Khoramabadi, H.N.; Jang, H.W.; Shokouhimehr, M. Hydroxyapatite consolidated by zirconia: Applications for dental implant. J. Compos. Compd. 2019, 2, 26–34. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Hsu, P.-Y.F.N.H.-C.; Tuan, W.-H. Effect of zirconia addition on the microstructural evolution of porous hydroxyapatite. Ceram. Int. 2007, 33, 715–718. [Google Scholar] [CrossRef]
- Nayak, Y.; Rana, R.; Pratihar, S.; Bhattacharyya, S. Low-Temperature Processing of Dense HydroxyapatiteZirconia Composites. Int. J. Appl. Ceram. Technol. 2008, 5, 29–36. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Effect of zirconia on the formation of calcium phosphate bioceramics under microwave irradiation. Mater. Lett. 2004, 58, 230–234. [Google Scholar] [CrossRef]
- Afzal, A. Implantable zirconia bioceramics for bone repair and replacement: A chronological review. Mater. Express 2014, 4, 1148. [Google Scholar] [CrossRef]
- Mezahi, F.Z.; Oudadesse, H.; Harabi, A.; Le Gal, Y. Effect of ZrO2, TiO2, and Al2O3 Additions on Process and Kinetics of Bonelike Apatite Formation on Sintered Natural Hydroxyapatite Surfaces. Int. J. Appl. Ceram. Technol. 2012, 9, 529–540. [Google Scholar] [CrossRef]
- Pandey, A.K.; Pati, F.; Mandal, D.; Dhara, S.; Biswas, K. In vitro evaluation of osteoconductivity and cellular response of zirconia and alumina based ceramics. Mater. Sci. Eng. C 2013, 33, 3923–3930. [Google Scholar] [CrossRef]
- Zhang, J.; Iwasa, M.; Kotobuki, N.; Tanaka, T.; Hirose, M.; Ohgushi, H.; Jiang, D. Fabrication of hydroxyapatite? Zirconia composites for orthopedic applications. J. Am. Ceram. Soc. 2006, 89, 3348–3355. [Google Scholar] [CrossRef]
- Silva, V.V.; Domingues, R.Z. Hydroxyapatite-Zirconia composites preparedby precipitation method. J. Mater. Sci. Mater. Med. 1997, 8, 907–910. [Google Scholar] [CrossRef]
- Kumar, P.N.; Kannan, S. Quantitative analysis of the structural stability and degradation ability of hydroxyapatite and zirconia composites synthesized in situ. RSC Adv. 2014, 4, 29946–29956. [Google Scholar] [CrossRef]
- Yoshimura, M.; Byrappa, K. Hydrothermal processing of materials: Past, present and future. J. Mater. Sci. 2007, 43, 2085–2103. [Google Scholar] [CrossRef]
- Sivaperumal, V.R.; Mani, R.; Nachiappan, M.S.; Arumugam, K. Direct hydrothermal synthesis of hydroxyapatite/alumina nanocomposite. Mater. Charact. 2017, 134, 416–421. [Google Scholar] [CrossRef]
- Agalya, P.; Kumar, G.S.; Prabu, K.; Cholan, S.; Karunakaran, G.; Hakami, J.; Shkir, M.; Ramalingam, S. One-pot ultrasonic-assisted synthesis of magnetic hydroxyapatite nanoparticles using mussel shell biowaste with the aid of trisodium citrate. Ceram. Int. 2022, 48, 28299–28307. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Das, R.M.; Kumar, M. Synthesis of Hydroxyapatite Nanoparticles by Modified Co-Precipitation Technique and Investigation of the Ceramic Characteristics upon Thermal Treatment for Their Potential Applications for Water Treatment; CRC Press: Boca Raton, FL, USA, 2022; pp. 151–169. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abu Ali, O.A.; Saleh, D.I.; Abu-Saied, M.A.; Ahmed, M.K.; Abdel-Fattah, E.; Mansour, S.F. Microstructure, morphology and physicochemical properties of nanocomposites containing hydroxyapatite/vivianite/graphene oxide for biomedical applications. Luminescence 2021, 37, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W. Physisorption of nitrogen by porous materials. J. Porous Mater. 1995, 2, 5–8. [Google Scholar] [CrossRef]
- Sing, K.S. The use of gas adsorption for the characterization of porous solids. Colloids Surf. 1989, 38, 113–124. [Google Scholar] [CrossRef]
- Rapacz-Kmita, A.; Ślósarczyk, A.; Paszkiewicz, Z. Mechanical properties of HAp–ZrO2 composites. J. Eur. Ceram. Soc. 2006, 26, 1481–1488. [Google Scholar] [CrossRef]
- Rodríguez-Clemente, R.; López-Macipe, A.; Gómez-Morales, J.; Torrent-Burgués, J.; Castaño, V. Hydroxyapatite precipitation: A case of nucleation-aggregation-agglomeration-growth mechanism. J. Eur. Ceram. Soc. 1998, 18, 1351–1356. [Google Scholar] [CrossRef]
- Leong, C.; Lim, K.; Muchtar, A.; Yahaya, N. Decomposition of Hydroxyapatite in Hydroxyapatite/Zirconia Composites for Dental Applications. Adv. Mater. Res. 2013, 750–752, 1664–1668. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer: Berlin, Germany, 2006; pp. 212–232. ISBN 978-1-4020-2303-3. [Google Scholar]
- Moritz, M.; Geszke-Moritz, M. Mesoporous materials as multifunctional tools in biosciences: Principles and applications. Mater. Sci. Eng. C 2015, 49, 114–151. [Google Scholar] [CrossRef]
- Garskaite, E.; Gross, K.-A.; Yang, S.-W.; Yang, T.C.-K.; Yang, J.-C.; Kareiva, A. Effect of processing conditions on the crystallinity and structure of carbonated calcium hydroxyapatite (CHAp). CrystEngComm 2014, 16, 3950–3959. [Google Scholar] [CrossRef]
- Sofronia, A.M.; Baies, R.; Anghel, E.M.; Marinescu, C.A.; Tanasescu, S. Thermal and structural characterization of synthetic and natural nanocrystalline hydroxyapatite. Mater. Sci. Eng. C 2014, 43, 153–163. [Google Scholar] [CrossRef]
- Isfahani, T.D.; Javadpour, J.; Khavandi, A.; Dinnebier, R.; Rezaie, H.R.; Goodarzi, M. Mechanochemical synthesis of zirconia nanoparticles: Formation mechanism and phase transformation. Int. J. Refract. Met. Hard Mater. 2012, 31, 21–27. [Google Scholar] [CrossRef]
- Deshmane, V.; Adewuyi, Y.G. Synthesis of thermally stable, high surface area, nanocrystalline mesoporous tetragonal zirconium dioxide (ZrO2): Effects of different process parameters. Microporous Mesoporous Mater. 2012, 148, 88–100. [Google Scholar] [CrossRef]
- Ming, J.; Zuo, B. A novel electrospun silk fibroin/hydroxyapatite hybrid nanofibers. Mater. Chem. Phys. 2012, 137, 421–427. [Google Scholar] [CrossRef]
| Sample Code | HAp (Weight %) | Zirconia (Weight %) |
|---|---|---|
| Pure HAp | 100 | 0 |
| Pure zirconia | 0 | 100 |
| HZ1 | 95.01 | 4.99 |
| HZ2 | 90.49 | 9.51 |
| HZ3 | 86.39 | 13.61 |
| HZ4 | 82.64 | 17.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivaperumal, V.R.; Mani, R.; Polisetti, V.; Aruchamy, K.; Oh, T. Synthesis of Hydroxyapatite (HAp)-Zirconia Nanocomposite Powder and Evaluation of Its Biocompatibility: An In Vitro Study. Appl. Sci. 2022, 12, 11056. https://doi.org/10.3390/app122111056
Sivaperumal VR, Mani R, Polisetti V, Aruchamy K, Oh T. Synthesis of Hydroxyapatite (HAp)-Zirconia Nanocomposite Powder and Evaluation of Its Biocompatibility: An In Vitro Study. Applied Sciences. 2022; 12(21):11056. https://doi.org/10.3390/app122111056
Chicago/Turabian StyleSivaperumal, Vignesh Raj, Rajkumar Mani, Veerababu Polisetti, Kanakaraj Aruchamy, and Taehwan Oh. 2022. "Synthesis of Hydroxyapatite (HAp)-Zirconia Nanocomposite Powder and Evaluation of Its Biocompatibility: An In Vitro Study" Applied Sciences 12, no. 21: 11056. https://doi.org/10.3390/app122111056
APA StyleSivaperumal, V. R., Mani, R., Polisetti, V., Aruchamy, K., & Oh, T. (2022). Synthesis of Hydroxyapatite (HAp)-Zirconia Nanocomposite Powder and Evaluation of Its Biocompatibility: An In Vitro Study. Applied Sciences, 12(21), 11056. https://doi.org/10.3390/app122111056





