Facile Synthesis of Hafnium Oxide Nanoparticle Decorated on Graphene Nanosheet and Its Photocatalytic Degradation of Organic Pollutants under UV-Light Irradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hafnium Oxide Nanoparticle
2.3. Preparation of Graphene
2.4. Synthesis of HfO2-Graphene Nanocomposite
2.5. Characterization Methods
2.6. Photocatalytic Experiments
3. Results and Discussion
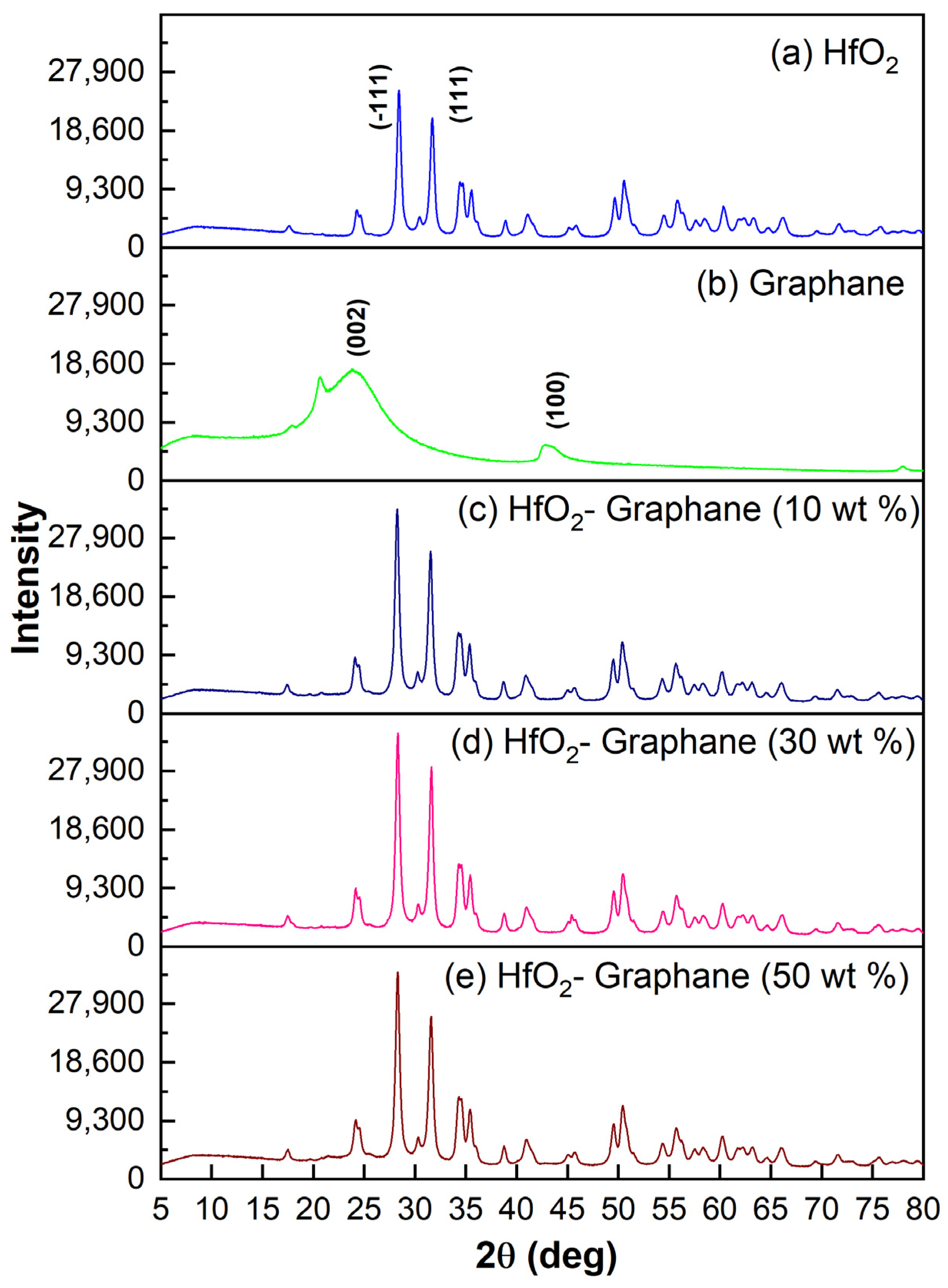
3.1. X-ray Diffraction
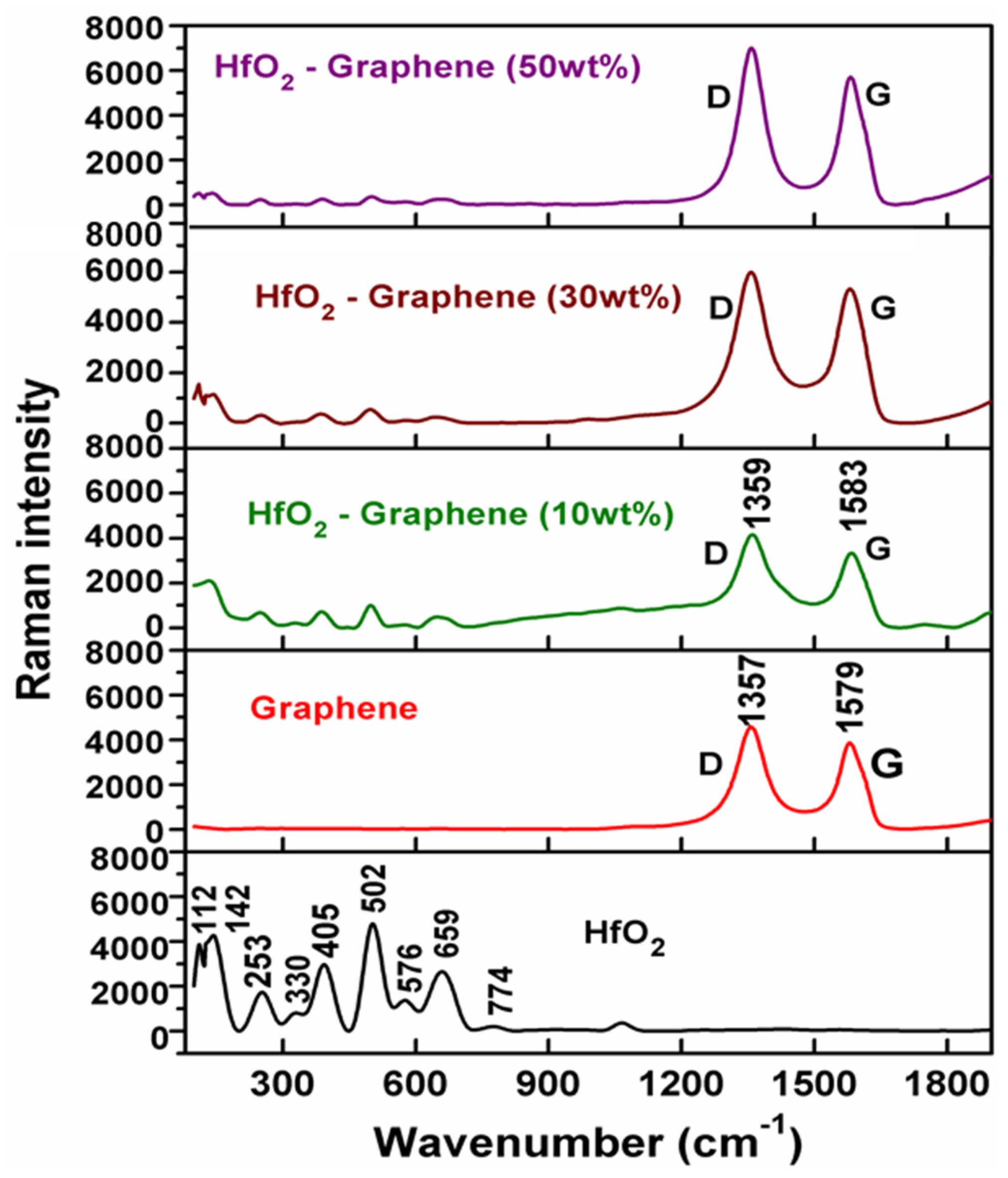
3.2. Raman Spectroscopy
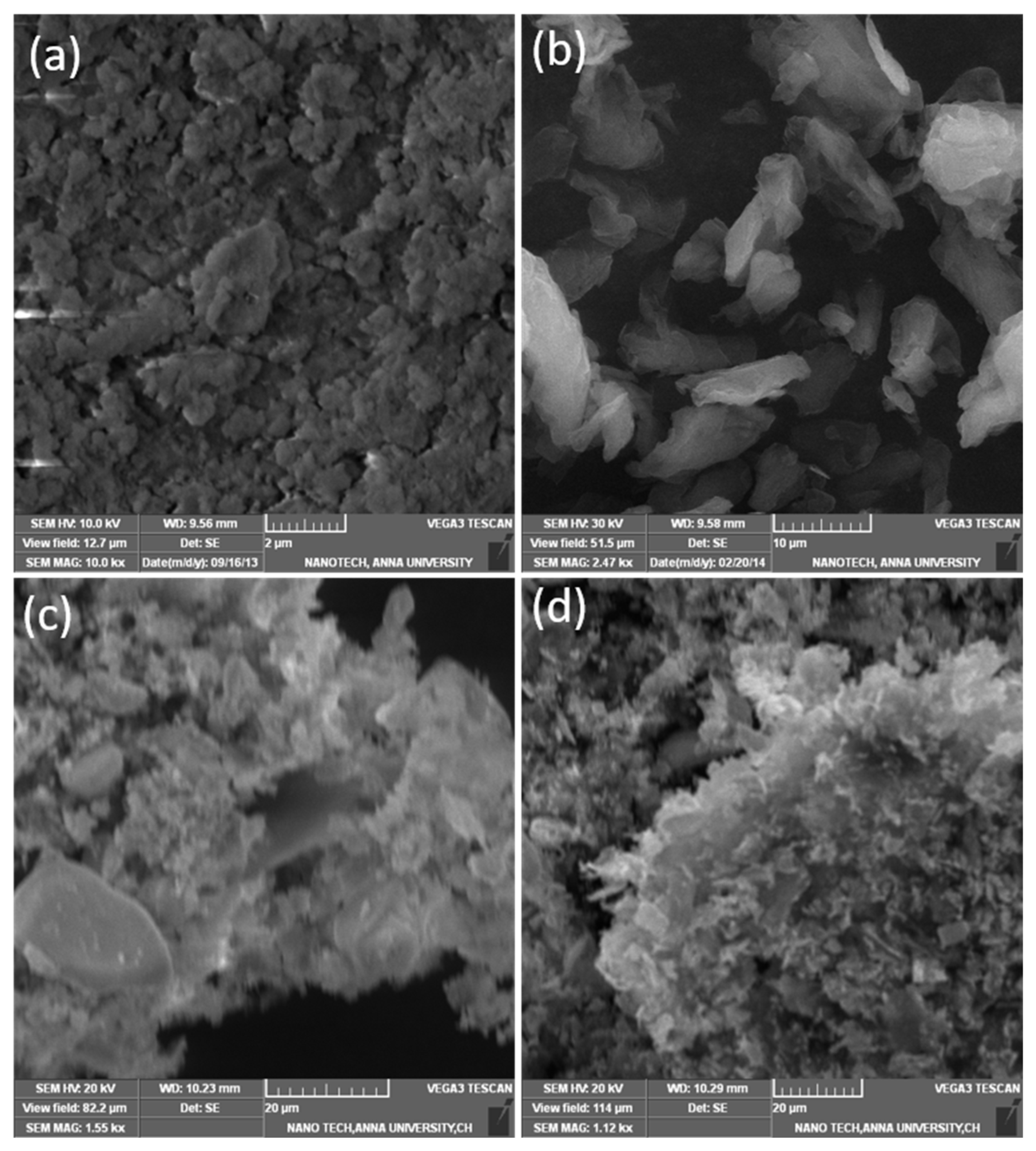
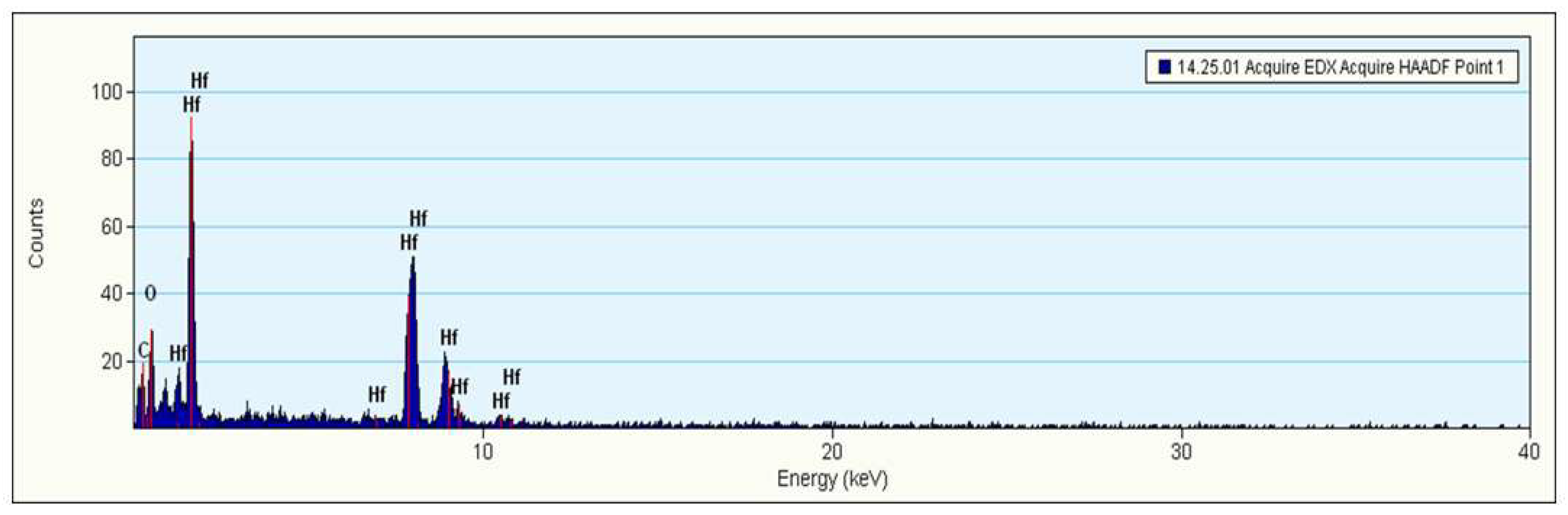
3.3. Morphology and EDX Analysis
3.4. Optical Properties
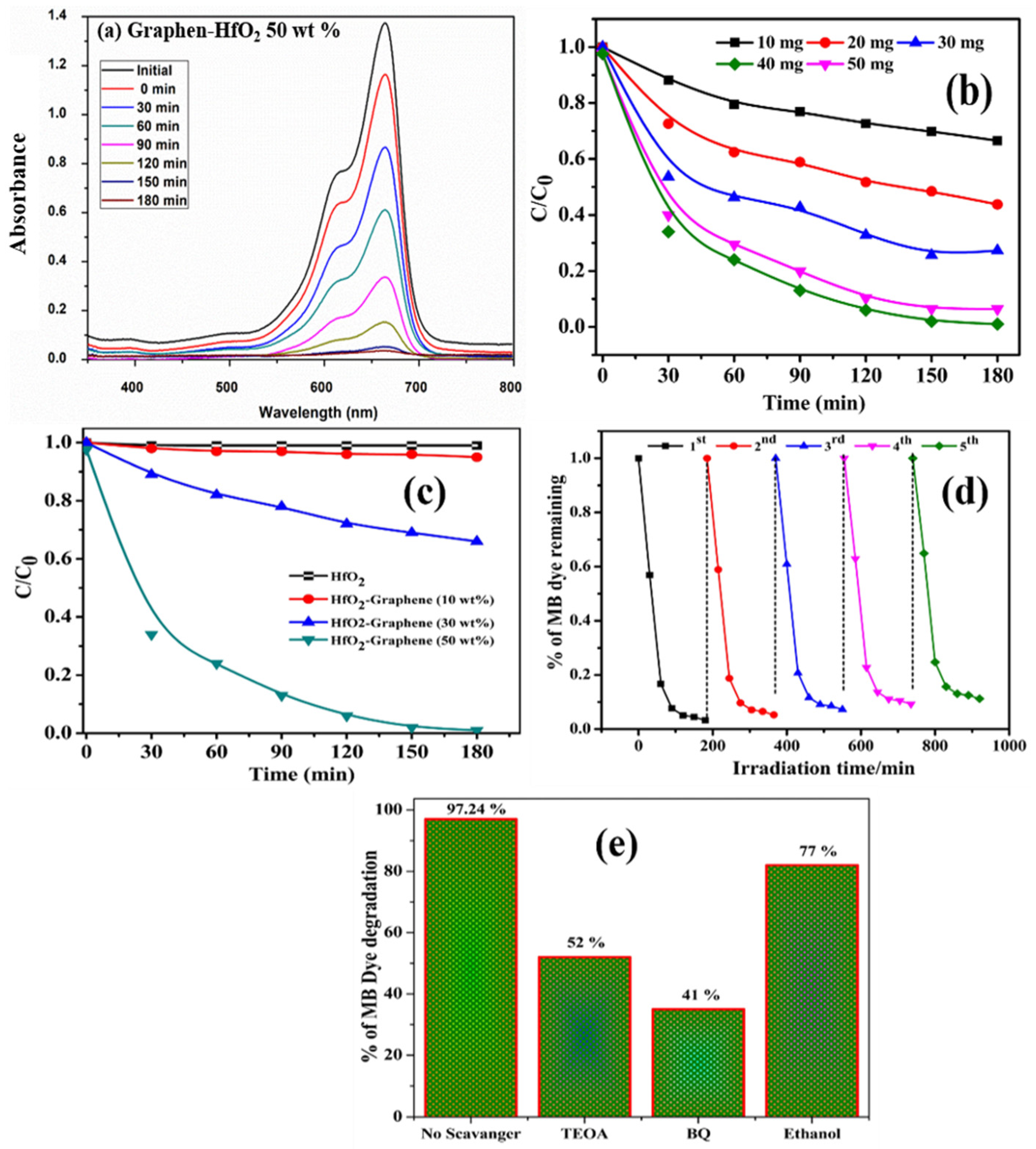
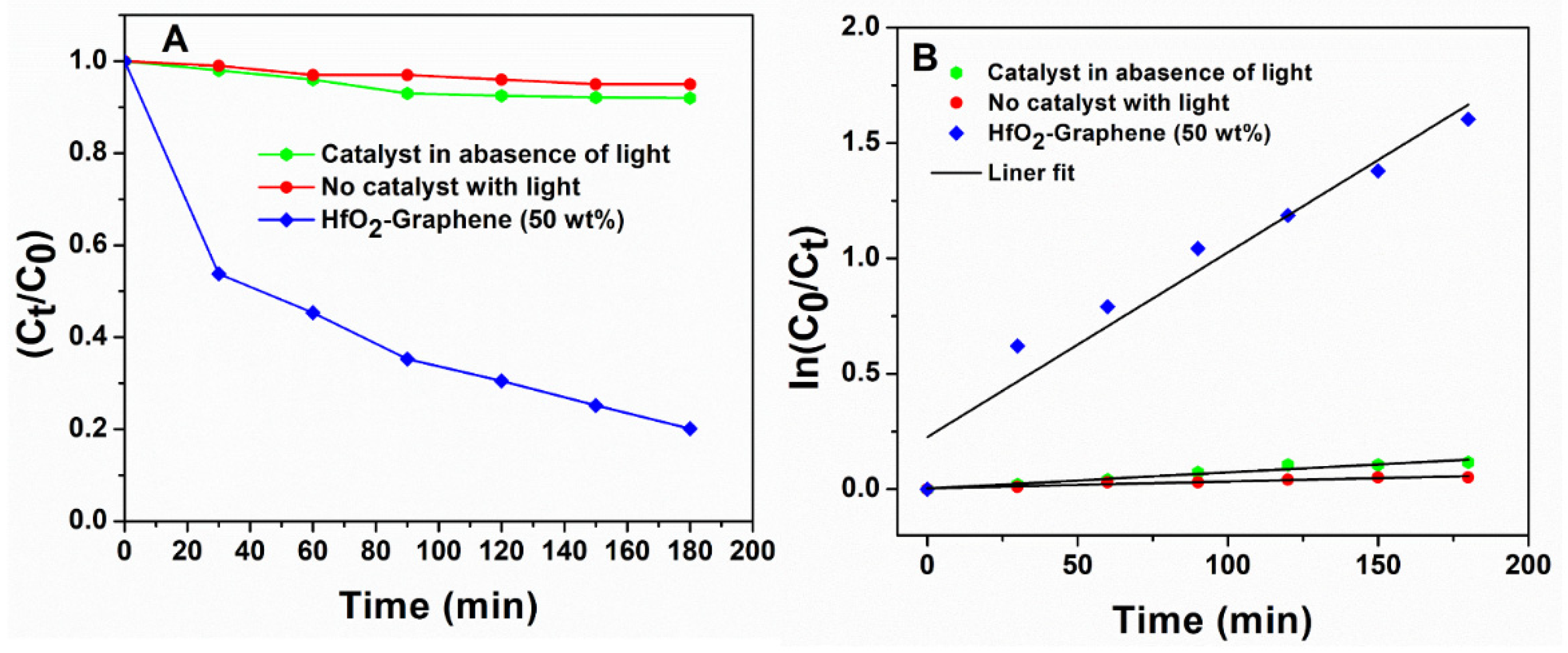
3.5. Photocatalytic Activity
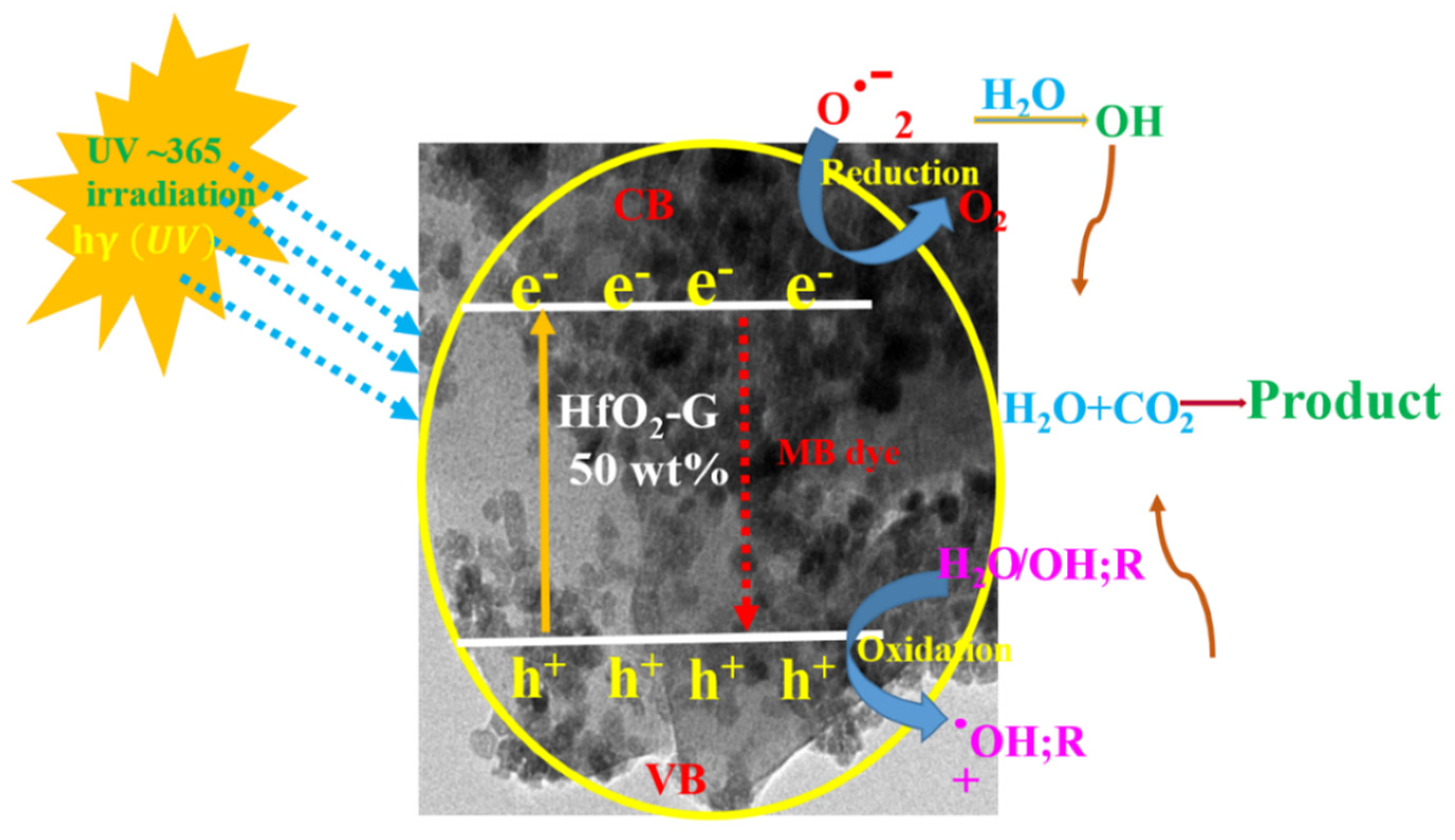
3.6. Photocatalytic Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Shahawi, M.; Hamza, A.; Bashammakh, A.S.; Al-Saggaf, W. An overview on the accumulation, distribution, transformations, toxicity and analytical methods for the monitoring of persistent organic pollutants. Talanta 2010, 80, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Osotsi, M.I.; Macharia, D.K.; Zhu, B.; Wang, Z.; Shen, X.; Liu, Z.; Zhang, L.; Chen, Z. Synthesis of ZnWO4−x nanorods with oxygen vacancy for efficient photocatalytic degradation of tetracycline. Prog. Nat. Sci. Mater. Int. 2018, 28, 408–415. [Google Scholar] [CrossRef]
- Tamminen, M.; Karkman, A.; Lohmus, A.; Muziasari, W.I.; Takasu, H.; Wada, S.; Suzuki, S.; Virta, M. Tetracycline resistance genes persist at aquaculture farms in the absence of selection pressure. Environ. Sci. Technol. 2011, 45, 386–391. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Environ. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Lü, M.; Li, J.; Yang, X.; Zhang, C.; Yang, J.; Hu, H.; Wang, X. Applications of graphene-based materials in environmental protection and detection. Chin. Sci. Bull. 2013, 58, 2698–2710. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Kim, Y.; Park, H.; Park, K. Synthesis and analysis of thermally degradable polybutadiene containing Diels–Alder adduct. Bull. Korean Chem. Soc. 2021, 42, 1602–1609. [Google Scholar] [CrossRef]
- Durai, M.; Chauhan, D.; Durai, M.; Saravanan, M.; Kumaravel, S.; Erusappan, E.; Ahn, Y.-H. Layered KTO/BiOCl nanostructures for the efficient visible light photocatalytic degradation of harmful dyes. Chemosphere 2022, 306, 135659. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, T.; Xu, P.; Zhang, L.; Liu, J.; Chen, Z. Growth of C3N4 nanosheets on carbon-fiber cloth as flexible and macroscale filter-membrane-shaped photocatalyst for degrading the flowing wastewater. Appl. Catal. B 2017, 219, 425–431. [Google Scholar] [CrossRef]
- Hiroshi, O.; Masao, K. Photochemical Reactions between Methylene Blue and Tri-, Di- and Monomethylamine. I. Bull. Chem. Soc. Jpn. 1957, 30, 136–141. [Google Scholar] [CrossRef]
- Hiroshi, O.; Kenichi, K.; Masao, K. Photochemical Reactions between Methylene Blue and Tri-, Di- and Monomethylamine. III. The Behavior of Methylene Blue in the Presence of Oxygen. Bull. Chem. Soc. Jpn. 1959, 32, 125–132. [Google Scholar] [CrossRef]
- Senthilkumaar, S.; Porkodi, K.; Gomathi, R.; Maheswari, A.G.; Manonmani, N. Sol–gel derived silver doped nanocrystalline titania catalysed photodegradation of methylene blue from aqueous solution. Dyes Pigment. 2006, 69, 22–30. [Google Scholar] [CrossRef]
- Yogi, C.; Kojima, K.; Wada, N.; Tokumoto, H.; Takai, T.; Mizoguchi, T.; Tamiaki, H. Photocatalytic degradation of methylene blue by TiO2 film and Au particles-TiO2 composite film. Thin Solid Films 2008, 516, 5881–5884. [Google Scholar] [CrossRef]
- Kiwi, J.; Pulgarin, C.; Peringer, P.; Grätzel, M. Beneficial effects of homogeneous photo-Fenton pretreatment upon the biodegradation of anthraquinone sulfonate in waste water treatment. Appl. Catal. B Environ. 1993, 3, 85–99. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Kastner, J.R.; Thompson, D.N.; Cherry, R.S. Water-soluble polymer for increasing the biodegradation of sparingly soluble vapors. Enzym. Microb. Technol. 1999, 24, 104–110. [Google Scholar] [CrossRef]
- Miller, M.J.; Allen, D.G. Modelling transport and degradation of hydrophobic pollutants in biofilter biofilms. Chem. Eng. J. 2005, 113, 197–204. [Google Scholar] [CrossRef]
- Chen, Y.; Dionysiou, D.D. A comparative study on physicochemical properties and photocatalytic behavior of macroporous TiO2-P25 composite films and macroporous TiO2 films coated on stainless steel substrate. Appl. Catal. A Gen. 2007, 317, 129–137. [Google Scholar] [CrossRef]
- Changchun, C.; Jiangfeng, L.; Ping, L.; Benhai, Y. Investigation of photocatalytic degradation of methyl orange by using nano-sized ZnO catalysts. Adv. Chem. Eng. Sci. 2011, 1, 9–14. [Google Scholar]
- Chen, Y.; Crittenden, J.C.; Hackney, S.; Sutter, L.; Hand, D.W. Preparation of a Novel TiO2-Based p−n Junction Nanotube Photocatalyst. Environ. Sci. Technol. 2005, 39, 1201–1208. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, Y.; Hou, J.; Zhao, X.; Pan, C. Preparation and Enhanced Photocatalytic Activity of TiO2 Nanocrystals with Internal Pores. ACS Appl. Mater. Interfaces 2014, 6, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- Kopidakis, N.; Schiff, E.A.; Park, N.G.; van de Lagemaat, J.; Frank, A.J. Ambipolar Diffusion of Photocarriers in Electrolyte-Filled, Nanoporous TiO2. J. Phys. Chem. B 2000, 104, 3930–3936. [Google Scholar] [CrossRef]
- Hurum, D.C.; Gray, K.A.; Rajh, T.; Thurnauer, M.C. Recombination Pathways in the Degussa P25 Formulation of TiO2: Surface versus Lattice Mechanisms. J. Phys. Chem. B 2005, 109, 977–980. [Google Scholar] [CrossRef]
- Sangpour, P.; Hashemi, F.; Moshfegh, A.Z. Photoenhanced Degradation of Methylene Blue on Cosputtered M:TiO2 (M = Au, Ag, Cu) Nanocomposite Systems: A Comparative Study. J. Phys. Chem. C 2010, 114, 13955–13961. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology; World Scientific: Singapore, 2010; pp. 11–19. [Google Scholar]
- Ye, Y.; Dai, Y.; Dai, L.; Shi, Z.; Liu, N.; Wang, F.; Fu, L.; Peng, R.; Wen, X.; Chen, Z.; et al. High-Performance Single CdS Nanowire (Nanobelt) Schottky Junction Solar Cells with Au/Graphene Schottky Electrodes. ACS Appl. Mater. Interfaces 2010, 2, 3406–3410. [Google Scholar] [CrossRef]
- Berger, C.; Song, Z.; Li, X.; Wu, X.; Brown, N.; Naud, C.; Mayou, D.; Li, T.; Hass, J.; Marchenkov, A.N. Electronic confinement and coherence in patterned epitaxial graphene. Science 2006, 312, 1191–1196. [Google Scholar] [CrossRef] [Green Version]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, L.; Cheng, H.; Zhu, Y. Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl. Catal. B Environ. 2011, 101, 382–387. [Google Scholar] [CrossRef]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H. Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef] [Green Version]
- Si, Y.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Yoo, E.; Okata, T.; Akita, T.; Kohyama, M.; Nakamura, J.; Honma, I. Enhanced Electrocatalytic Activity of Pt Subnanoclusters on Graphene Nanosheet Surface. Nano Lett. 2009, 9, 2255–2259. [Google Scholar] [CrossRef] [PubMed]
- Murugan, A.V.; Muraliganth, T.; Manthiram, A. Rapid, facile microwave-solvothermal synthesis of graphene nanosheets and their polyaniline nanocomposites for energy strorage. Chem. Mater. 2009, 21, 5004–5006. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Casalongue, H.; Chen, Z.; Dai, H. TiO2 Nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 2010, 3, 701–705. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lv, X.; Li, Y.; Wang, Y.; Li, J. P25-graphene composite as a high performance photocatalyst. ACS Nano 2010, 4, 380–386. [Google Scholar] [CrossRef]
- Seema, H.; Kemp, K.C.; Chandra, V.; Kim, K.S. Graphene–SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology 2012, 23, 355705. [Google Scholar] [CrossRef]
- Yang, Y.; Ren, L.; Zhang, C.; Huang, S.; Liu, T. Facile fabrication of functionalized graphene sheets (FGS)/ZnO nanocomposites with photocatalytic property. ACS Appl. Mater. Interfaces 2011, 3, 2779–2785. [Google Scholar] [CrossRef]
- Lee, M.; Zine, N.; Baraket, A.; Zabala, M.; Campabadal, F.; Caruso, R.; Trivella, M.G.; Jaffrezic-Renault, N.; Errachid, A. A novel biosensor based on hafnium oxide: Application for early stage detection of human interleukin-10. Sens. Actuators B Chem. 2012, 175, 201–207. [Google Scholar] [CrossRef]
- Srinivasan, V.S.; Pandya, A. Dosimetry aspects of hafnium oxide metal-oxide-semiconductor (MOS) capacitor. Thin Solid Films 2011, 520, 574–577. [Google Scholar] [CrossRef]
- Kwatra, D.; Venugopal, A.; Anant, S. Nanoparticles in radiation therapy: A summary of various approaches to enhance radiosensitization in cancer. Transl. Cancer Res. 2013, 2, 330–342. [Google Scholar]
- Jayaraman, V.; Bhavesh, G.; Chinnathambi, S.; Ganesan, S.; Aruna, P. Synthesis and characterization of hafnium oxide nanoparticles for bio-safety. Mater. Express 2014, 4, 375–383. [Google Scholar] [CrossRef]
- Ahmad, M.; Iqbal, Z.; Hong, Z.; Yang, J.; Zhang, Y.; Khalid, N.; Ahmed, E. Enhanced sunlight photocatalytic performance of hafnium doped ZnO nanoparticles for methylene blue degradation. Integr. Ferroelectr. 2013, 145, 108–114. [Google Scholar] [CrossRef]
- Cho, B.H.; Ko, W.B. Preparation of graphene-ZrO2 nanocomposites by heat treatment and photocatalytic degradation of organic dyes. J. Nanosci. Nanotechnol. 2013, 13, 7625–7630. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.S.; Gejo, F.L.; Shluger, A.; Nieminen, R.M. Vacancy and interstitial defects in hafnia. Phys. Rev. B 2002, 65, 174117. [Google Scholar] [CrossRef] [Green Version]
- Ni, J.; Zhou, Q.; Li, Z.; Zhang, Z. Oxygen defect induced photoluminescence of hf o 2 thin films. Appl. Phys. Lett. 2008, 93, 011905. [Google Scholar] [CrossRef]
- Ramadoss, A.; Krishnamoorthy, K.; Kim, S.J. Novel synthesis of hafnium oxide nanoparticles by precipitation method and its characterization. Mater. Res. Bull. 2012, 47, 2680–2684. [Google Scholar] [CrossRef]
- Shanmugam, M.; Jayavel, R. Synthesize of graphene-tin oxide nanocomposite and its photocatalytic properties for the degradation of organic pollutants under visible light. J. Nanosci. Nanotechnol. 2015, 15, 7195–7201. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. TiO2-graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef]
- Revathi, P.; Krishnasamy, K. A facile synthesis of RGO/HfO2 nanocomposite for high-performance supercapacitor. Mater. Today Proc. 2021, 47, 1–7. [Google Scholar] [CrossRef]
- Krishnakumar, B.; Imae, T.; Miras, J.; Esquena, J. Synthesis and azo dye photodegradation activity of ZrS2–ZnO nanocomposites. Sep. Purif. Technol. 2014, 132, 281–288. [Google Scholar] [CrossRef]
- Jayaraman, A.; Wang, S.; Sharma, S.; Ming, L. Pressure-induced phase transformations in HfO2 to 50 GPa studied by Raman spectroscopy. Phys. Rev. B 1993, 48, 9205. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhu, Y.; Yang, X.; Jiang, X.; Li, C. Preparation of graphene–TiO2 composites with enhanced photocatalytic activity. New J. Chem. 2011, 35, 353–359. [Google Scholar] [CrossRef]
- Dar, R.A.; Naikoo, G.A.; Srivastava, A.K.; Hassan, I.U.; Karna, S.P.; Giri, L.; Shaikh, A.M.; Rezakazemi, M.; Ahmed, W. Performance of graphene-zinc oxide nanocomposite coated-glassy carbon electrode in the sensitive determination of para-nitrophenol. Sci. Rep. 2022, 12, 117. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Ha, D.; King, T.-J. Observation of bulk HfO2 defects by spectroscopic ellipsometry. J. Vac. Sci. Technol. A Vac. Surf. Films 2004, 22, 1337–1341. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, Z.; Yao, J.; Beitler, E.; Zhu, Y.; Tour, J.M. Growth of graphene from solid carbon sources. Nature 2010, 468, 549–552. [Google Scholar] [CrossRef]
- Pouretedal, H.R.; Norozi, A.; Keshavarz, M.H.; Semnani, A. Nanoparticles of zinc sulfide doped with manganese, nickel and copper as nanophotocatalyst in the degradation of organic dyes. J. Hazard. Mater. 2009, 162, 674–681. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhang, X.; Yang, L.; Zhang, N.; Pan, G.; Yin, S.; Chen, Y.; Wei, J. Polymer photovoltaic cells based on solution-processable graphene and P3HT. Adv. Funct. Mater. 2009, 19, 894–904. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhang, X.; Zhang, N.; Yang, L.; Yin, S.; Chen, Y. Organic photovoltaic cells based on an acceptor of soluble graphene. Appl. Phys. Lett. 2008, 92, 223303. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Ren, J.; Huo, Y.; Bian, Z.; Li, H. Nanocrystalline Fe/TiO2 visible photocatalyst with a mesoporous structure prepared via a nonhydrolytic sol-gel route. J. Phys. Chem. C 2007, 111, 18965–18969. [Google Scholar] [CrossRef]
- Gawande, S.B.; Thakare, S.R. Graphene wrapped BiVO4 photocatalyst and its enhanced performance under visible light irradiation. Int. Nano Lett. 2012, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Mahalingam, S.; Ramasamy, J.; Ahn, Y.-H. Synthesis and application of graphene-αMoO3 nanocomposite for improving visible light irradiated photocatalytic decolorization of methylene blue dye. J. Taiwan Inst. Chem. Eng. 2017, 80, 276–285. [Google Scholar] [CrossRef]
- Atchudan, R.; Edison, T.N.J.I.; Perumal, S.; Karthik, N.; Karthikeyan, D.; Shanmugam, M.; Lee, Y.R. Concurrent synthesis of nitrogen-doped carbon dots for cell imaging and ZnO@ nitrogen-doped carbon sheets for photocatalytic degradation of methylene blue. J. Photochem. Photobiol. A Chem. 2018, 350, 75–85. [Google Scholar] [CrossRef]
- Das, S.; Ahn, Y.-H. Synthesis and application of CdS nanorods for LED-based photocatalytic degradation of tetracycline antibiotic. Chemosphere 2022, 291, 132870. [Google Scholar] [CrossRef]



| Nanocomposites | Irradiation Source | Dye | Irradiation Time (min) | Degradation (%) | References |
|---|---|---|---|---|---|
| P25-graphene | UV | MB dye | 60 | 85 | [36] |
| Graphene-SnO2 | UV | MB dye | 360 | 100 | [37] |
| TiO2-graphene | sunlight | MB dye | 180 | 75 | [50] |
| graphene-BiVO4 | Visible light | MB dye | 180 | 89 | [62] |
| G- αMoO3 | UV | MB dye | 180 | 97 | [63] |
| GO-TiO2 | UV | MO dye | 240 | 75 | [64] |
| HfO2-graphene | UV | MB dye | 180 | 98.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaraman, V.; Mahalingam, S.; Chinnathambi, S.; Pandian, G.N.; Prakasarao, A.; Ganesan, S.; Ramasamy, J.; Ayyaru, S.; Ahn, Y.-H. Facile Synthesis of Hafnium Oxide Nanoparticle Decorated on Graphene Nanosheet and Its Photocatalytic Degradation of Organic Pollutants under UV-Light Irradiation. Appl. Sci. 2022, 12, 11222. https://doi.org/10.3390/app122111222
Jayaraman V, Mahalingam S, Chinnathambi S, Pandian GN, Prakasarao A, Ganesan S, Ramasamy J, Ayyaru S, Ahn Y-H. Facile Synthesis of Hafnium Oxide Nanoparticle Decorated on Graphene Nanosheet and Its Photocatalytic Degradation of Organic Pollutants under UV-Light Irradiation. Applied Sciences. 2022; 12(21):11222. https://doi.org/10.3390/app122111222
Chicago/Turabian StyleJayaraman, Venkatachalam, Shanmugam Mahalingam, Shanmugavel Chinnathambi, Ganesh N. Pandian, Aruna Prakasarao, Singaravelu Ganesan, Jayavel Ramasamy, Sivasankaran Ayyaru, and Young-Ho Ahn. 2022. "Facile Synthesis of Hafnium Oxide Nanoparticle Decorated on Graphene Nanosheet and Its Photocatalytic Degradation of Organic Pollutants under UV-Light Irradiation" Applied Sciences 12, no. 21: 11222. https://doi.org/10.3390/app122111222






