Featured Application
Dentin hypersensitivity (DH) is a very common global condition which available treatment options are multiple. No gold standard product has been identified, but n-HAP-based therapy recently showed greater DH relief.
Abstract
Dentinal hypersensitivity represents one of the most widespread dental problems and symptoms in the general population. It mainly affects the age group between 18 and 65 years old, presents an incidence of 35%, and may negatively affect the oral health-related quality of life of these patients. This longitudinal study aims to measure dentinal hypersensitivity in adult patients after the use of domiciliary desensitizing devices. In this study, 52 patients were included (32 females and 20 males, mean age: 53 ± 9.4) At T0 (baseline), patients were instructed to use a desensitizing toothpaste and, at T1, 27 patients reported using sodium monofluorophosphate (1450 ppm) and arginine 8% product (product A), while 25 patients reported using a toothpaste containing n-HAp (nano-hydroxyapatite) in effective dose 2.25% and 0.15% fluoride (1500 ppm) (product B). The study was carried out through three follow up appointments, 14 days apart from one another (T1 and T2). Results showed that the air sensitivity test did not exhibit a significant difference between the time points (p > 0.05), while a significant improvement of DH was recorded for tactile, osmotic, cold thermic, acid, and omni-comprehensive tests in both groups. Both desensitizing agents were effective in reducing DH for different stimuli.
1. Introduction
Dental hypersensitivity (DH) is a challenging condition in dental practice, representing one of the most widespread dental problems and symptoms in the world population [1]. Thanks to the increasing life expectancy and to the greater attention to oral health, with healthy functional natural dentition present even in older age, it is also reasonable to assume a further increase in DH prevalence among the population. Currently, DH presents an incidence of about 35% of the population worldwide and occurs mainly between 20 and 50 years of age. Regarding prevalence, DH seems to affect women in higher number compared to men in an age range from 30 to 40 years old [2,3]. DH is also found between 72% and 98% of patients presenting gingival recession and/or periodontal disease [4]. DH is conventionally defined as an episode of short sharp pain arising from exposed dentin after the application of thermal, tactile, chemical, osmotic, or evaporative stimuli, which cannot be caused by any other dental pathology and/or defect [5]. This condition impacts oral health-related quality of life [3], causing significant impairment on patients’ daily life such as speaking, eating, drinking, and toothbrushing [6,7,8].
In the literature, many etiopathogenetic theories to try and explain DH such as the neural theory, the odontoblastic transduction theory, and the hydrodynamic theory; the latter is considered the most accepted pain theory [9,10].
According to the hydrodynamic theory, dental hypersensitivity is caused by the fluid flow in the dentinal tubules caused by the stimulus, and the consequent activation of nociceptors in the pulp/dentine border area. Therefore, the main cause of DH is the exposure of dentinal tissue with unobstructed dentinal tubules, which allows the hydrodynamic mechanism to occur [11,12].
In the literature, two other etiological theories are currently discussed: odontoblast transducer theory and neural theory. Current evidence highlights the fundamental role in DH pain mechanism of odontoblasts, representing a mechanosensory system, working as sensor cells. According to the neural theory, dental primary afferent neurons were found to respond to heat by the expression of TRP channels (temperature-sensitive transient receptor potential channels), suggesting that they may transduce only high temperatures. Odontoblasts too were found to express the same receptors class. This evidence leading to the identification of TRP channels as potential receptors provides a key information that may justify all the molecular mechanisms on which all the three DH etiological recognized theories are based [13].
Since consistent correlation is reported between tubules density and pain responses, dentinal tubules’ patency seems to be a needed condition in sensitive dentine. [11,12,13].
Several other dental defects and/or pathologies can cause similar DH pain symptoms [13,14], therefore a differential diagnosis other similar conditions is key. Some of these conditions are: chipped teeth, fractures in restorations and pulpal sensitivity response to cavities or restorative therapies [15].
An exposed dentine surface may be caused by: gingival recession, anomalies in the cementoenamel junction (CEJ) leaving uncovered areas of dentine surface, erosive phenomena, and removal or loss of tooth enamel [13,16].
Before discussing and applying any of the available DH treatment options, it is necessary to eradicate the eventual causative etiological factors that may be present in the patient, to prevent recurrence of the condition [13,14,15,16].
Treatment options can be classified according to administration modality (home treatment or professional treatment) and/or mechanism of action (modification/blocking of the pulpal nerve response or fluid flow alteration in the dentinal tubules). From the application of the latter, the effect on the clinical symptoms of DH is perceived only after a couple of weeks. A more direct approach to DH-associated pain is given by dentinal tubule orifice occlusion. Tubular occluding agents are effective if their persistence on the tooth surface is good enough to assure the occlusion of dentinal tubules, also resisting in an environment oscillating between an acid and neutral state such as the oral cavity.
In the literature, controversies are reported in desensitizing agents comparation, and no gold-standard treatment is addressed. Study results are often contradictory and clinical indexes and/or protocols for stimuli response evaluation are multiple and different; standardization in procedures and evaluation of sensitivity would be desirable for future studies [13,14,15,16,17]. The presence of a wide array of desensitizing devices and treatment modalities for DH management is strong evidence of the fact that despite being an intensely studied phenomenon in literature, no common consensus has been reached yet regarding the gold standard treatment of DH [17,18].
Recently, new technologies together with advances in nanomaterials studies allowed to introduce a new therapeutic alternative in DH treatment, represented by nano-hydroxyapatite (n-HAp) in different formulations.
Recent evidence shows that nano-sized particles of Hydroxyapatite present similar structure, morphology and crystallinity compared to natural dental hydroxyapatite. Nano-hydroxyapatite (n-HAp) is thought to be a highly bioactive and biocompatible material, widely applied in dentistry for tooth remineralization and in medicine in general as a bone substitute. N-HAp was reported to have a good remineralization potential when applied on enamel caries, but very few and contradictory information is available regarding DH treatment results.
Several studies report the application of n-HAp as biomimetic material for enamel reconstruction after tooth mineral component loss, thanks to its aforementioned unique remineralization potential [19,20,21,22,23,24,25,26]. N-HAp toothpaste showed promising results in DH management and post-bleaching-related sensitivity [27,28,29].
Despite the available literature on n-Hap, there is not yet a consensus in considering n-HAp treatment as the gold standard for DH, and no evidence is present regarding n-HAp and which is its minimum effective dose.
The objective of the present study was to evaluate the effects of desensitizing agents on DH using different tests: air, tactile, cold thermical, osmotic, and acid.
2. Materials and Methods
2.1. Patients Screening
A prospective longitudinal monocentric observational study was designed and performed within the Oral Hygiene Unit, AOUP Pisa, Italy. All patients aged between 20 and 70 years old, who underwent a first visit at Oral Hygiene Unit, AOUP Pisa, Italy, were considered eligible for the study. The single-center observational study lasted approximately 7 months. The study has been conducted according to the Helsinki Declaration (1975), as revised in 2000. The study purpose was explained to the patients, who provided their written consent and willingness to participate.
The inclusion criteria were:
- -
- Patients undergoing a first visit at the Oral Hygiene Unit of the University Hospital;
- -
- Aged between 20 and 70 years, both sexes;
- -
- Patients with molars suffering from dentinal hypersensitivity (according to internationally recognized criteria listed below);
- -
- Presence of a full permanent dentition.
The exclusion criteria were:
- -
- Patients with periodontal diseases and patients with bruxism;
- -
- Patients presenting dental caries, teeth with cracked enamel, dental erosion;
- -
- Patients who underwent a vital bleaching session within 2 months;
- -
- Patients with total or partial removable prostheses or with extensive fixed prostheses (more than 1/3 of the teeth) and patients with missing teeth and without rehabilitation;
- -
- Patients treated with anti-inflammatory drugs or daily analgesics;
- -
- Patients not resident in the territory as this factor may affect their compliance of attending regular visits at the follow-up because of the long distances;
- -
- Use of desensitizing products in progress or in the last 6 months prior to the first visit.
2.2. Study Design
Of the 1120 patients which were visited in the Oral Hygiene Unit, only 52 (32 females and 20 males, mean age: 53 ± 9.4) met the inclusion criteria and were recruited for the study (Table 1). At T0 (baseline), patients were instructed to use a desensitizing toothpaste, and at T1, 27 patients reported using sodium monofluorophosphate (1450 ppm) and arginine 8% product (product A), while 25 patients reported using a toothpaste containing n-HAp (nano-hydroxyapatite) in effective dose 2.25% and 0.15% fluoride (1500 ppm) (product B). These agents should determine a remineralization of tooth enamel and an occlusion of dentinal tubules, leading to a reduction of dental hypersensitivity.

Table 1.
Study population descriptive data.
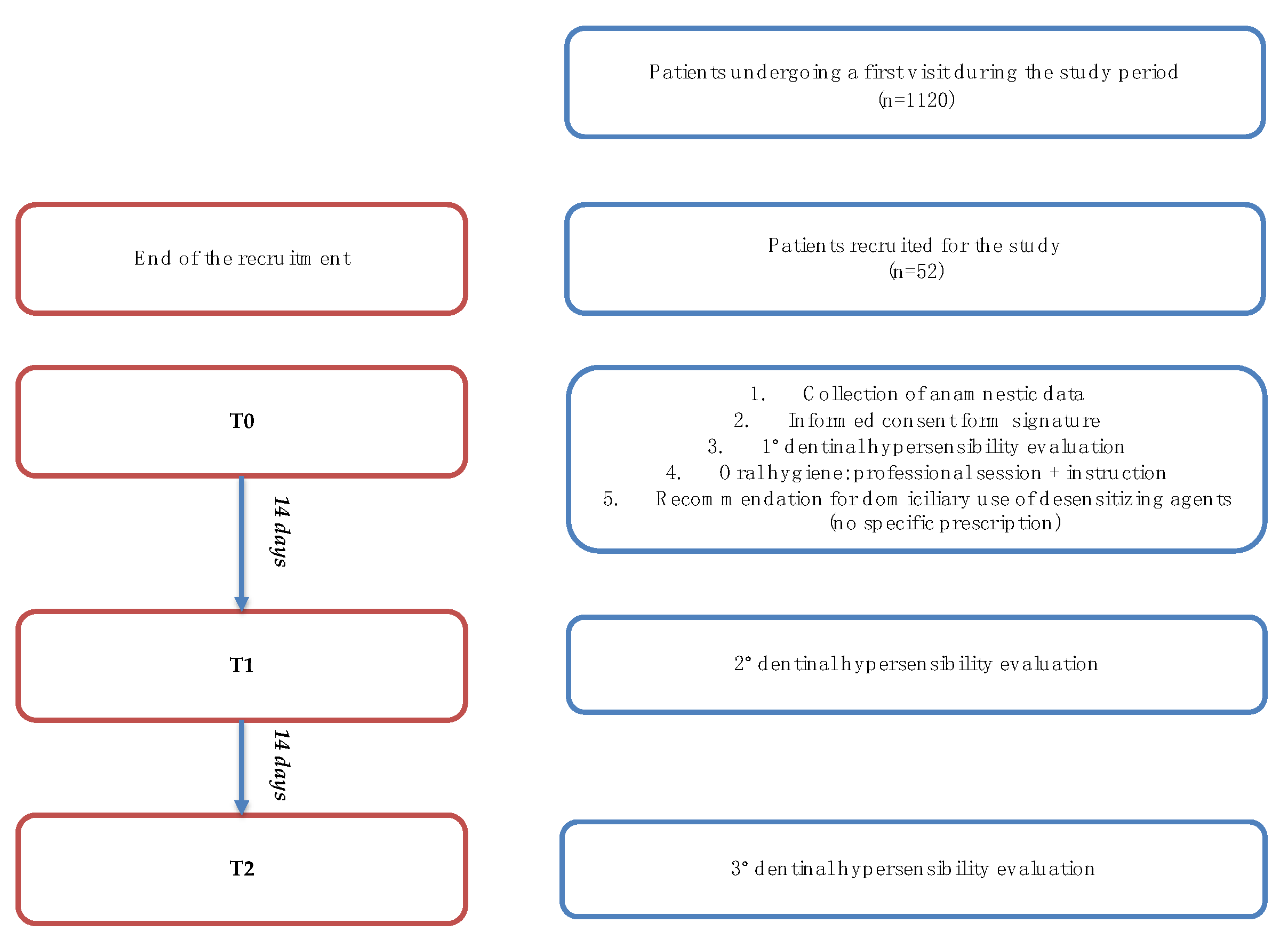
The study was carried out through three follow-up appointments, 14 days apart from one another, labelled T0 (baseline), T1, and T2 (see Figure 1) [30]. The clinical record used for each patient follow-up is reported below (Figure 2). All patients were evaluated by a single dental hygienist healthcare practitioner who had received extensive training on clinical procedures before the beginning of the study.
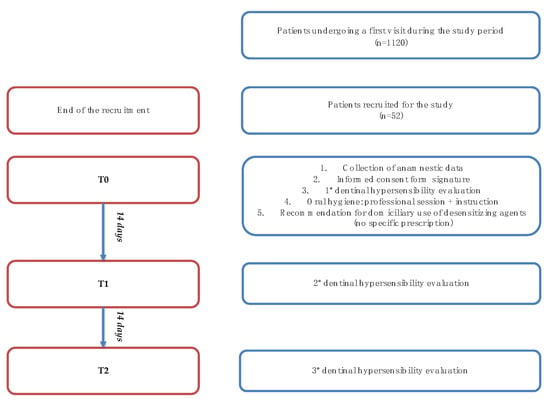
Figure 1.
Study flowchart.


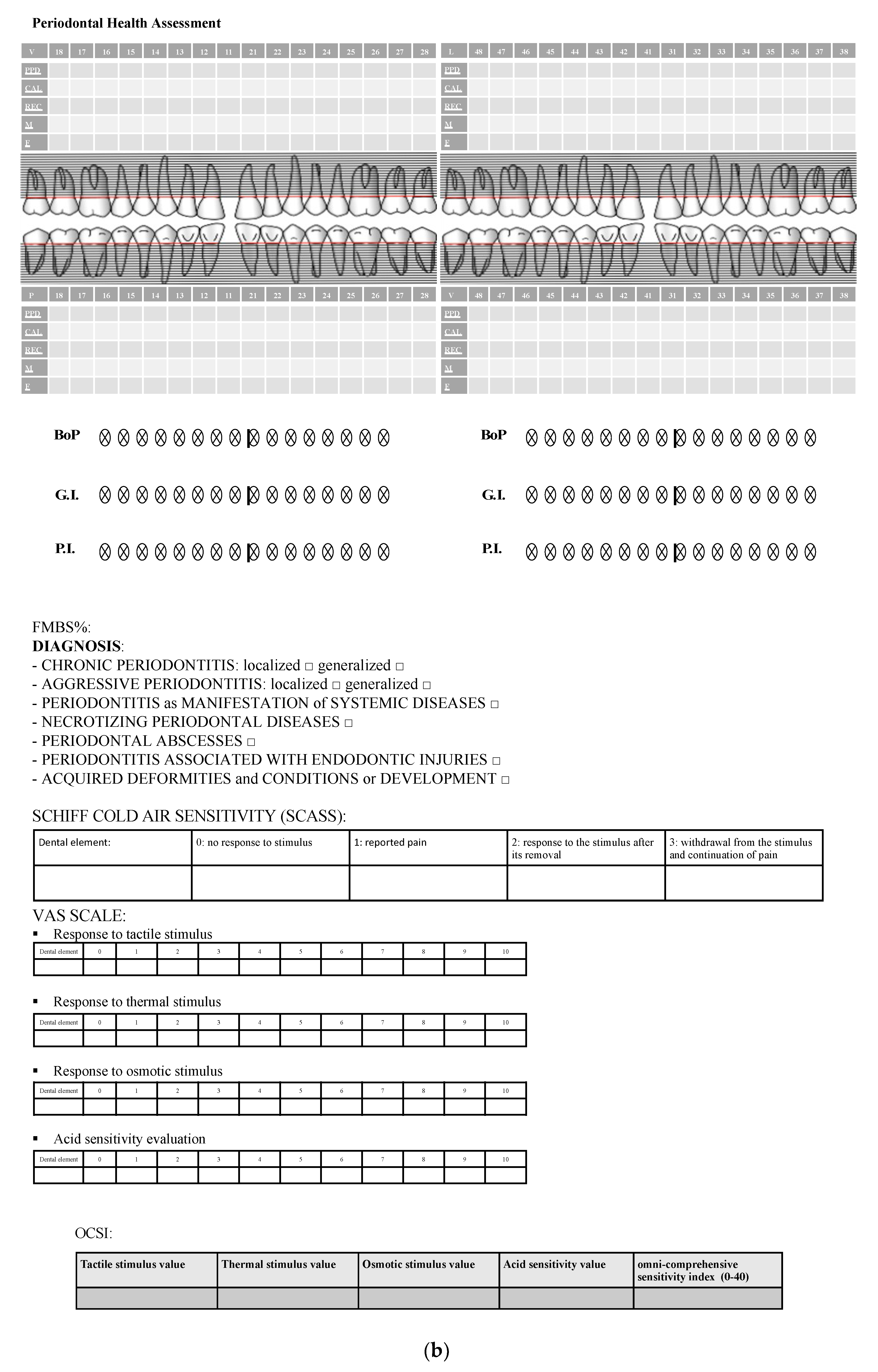
Figure 2.
(a) Medical records used for each follow-up. (b) Periodontal health records and sensitivity test form used for each follow-up.
2.3. Anamnestic and Periodontal Parameters
At T0, medical records and anamnestic data were collected, and informed consent was signed for each patient.
Prior to hypersensitivity analysis of stimuli evaluation, in order to assess the periodontal health, periodontal probing was performed with specific indices: dental mobility (according to Miller classification) [31], presence of furcations, gingival recession, plaque index and gingival index (according to Loe and Silness) [32,33,34], and bleeding index (the dichotomous one was applied for the study).
2.4. DH Assessment
DH assessment was performed through analysis of the response to different stimuli: evaporative (air) stimulus, tactile stimulus, thermic (cold) stimulus, osmotic stimulus, and acid stimulus.
Sensitivity stimuli were applied on all molar teeth for each patient, and the overall score assigned to each patient corresponded to the dental elements with greater DH. A “resting time” of five minutes was introduced between stimuli in order to avoid any interference. The evaporative stimulus was evaluated through the Air Blast Test, consisting in the application of an air jet perpendicularly to the main axis of the tooth for 1 s, at a 10 mm distance from the cementoenamel junction on the vestibular region using the air/water syringe of the dental unit (at a pressure of 3–4 atm), while the adjacent teeth were isolated using the operator’s fingers [35]. The same level of atmospheric pressure, calibrated at 4 bar, was used for all patients. The evaporative stimulus response was evaluated using the SCASS scale (Schiff Cold Air Sensitivity Scale) [36] and a 4-value index:
- -
- 0: the patient shows no response to stimulus.
- -
- 1: the patient responds to the stimulus but does not require the stimulus interruption.
- -
- 2: the patient responds to the stimulus and require the stimulus interruption or moves away from it.
- -
- 3: the patient responds to the stimulus, reports pain, require the stimulus interruption or moves away from it and still reports the continuation of pain even after the stimulus was interrupted.
Regarding tactile, thermal, osmotic, and acid stimulus response evaluation, the VAS scale was used, ranging from 0 (no pain) to 10 (maximum intensity) according to patient reported pain quantification [37,38]. The response to tactile stimulus was assessed using a blunt tip probe scratching a light pressure (10 g) along the cementoenamel junction for three seconds [39], while thermal, osmotic, and acid sensitivity evaluations were performed through a needle-free syringe with cold water, saturated saline (390 g dissolved in 1 L of water, 28%), and citric acid (pH value: 3), respectively.
Finally, an omni-comprehensive sensitivity score ranging from 0 to 40 was calculated using the sum of each different DH stimuli response values (tactile, thermic, osmotic, and acid) obtained with the VAS scale for each stimulus applied.
Additionally, at the baseline appointment (T0) after DH evaluation tests, a professional oral hygiene session was performed, followed by appropriate home oral hygiene instructions. The patients were also advised to use a toothbrush with soft bristles following the roller brushing technique, considered the most suitable since it is atraumatic and easily reproducible. At the end of T0, all patients were recommended to start desensitizing-devices application for home use at least twice a day during brushing and to apply the desensitizing products on sensitive molar teeth for at least two minutes. It was specified to apply wet desensitizer toothpaste on each tooth. In this observational study the desensitizing product choice between product A and B was made independently by each patient to help increasing patients’ compliance. A second evaluation of DH by stimuli analysis was carried out at T1 (after 14 days), with the same modalities. The last evaluation (T2) was carried out two weeks after T1. Molar teeth that showed dental hypersensitivity at T0 were taken into account for comparison in the second and third evaluations. Periodontal analysis and sensitivity tests were performed by the same operator in each evaluation phase to minimize inter-operator errors.
Furthermore, patients were instructed to perform the same oral hygiene habits which included: the use of dental floss 1 time/day, tooth brushing 3 times/day after the meals: in the morning, afternoon, and evening. Finally, a similar type of diet (3 meals/day with a small consumption of acidic foods 1 time/day) was suggested to the patients.
Daily application of toothpaste for 2 min was reported in a daily diary by each patient in order to check if the instructions were correctly followed at home.
Moreover, the patients recorded their oral and dietary habits on a daily diary, and the operator checked it at every time point.
2.5. Statistical Analysis
Regarding the statistical methodology, T test and chi-square tests were used through SPSS 22.0 software. All statistical tests were used with two-tailed hypotheses and a significance level of α = 0.05; statistically significant differences were set for p < 0.05. Moreover, all measurements were performed twice, by a second operator, in a sample of 10 patients, in order to calculate inter-operator reliability, and Cohen’s Kappa coefficient was used to calculate method error.
3. Results
3.1. Air Test
Regarding inter-operator reliability, it was found that Cohen’s Kappa coefficient was between 0.93 and 0.98. The present study provided, for each patient follow-up, DH tests assessment to evaporative, thermal, osmotic, acid, and tactile stimuli.
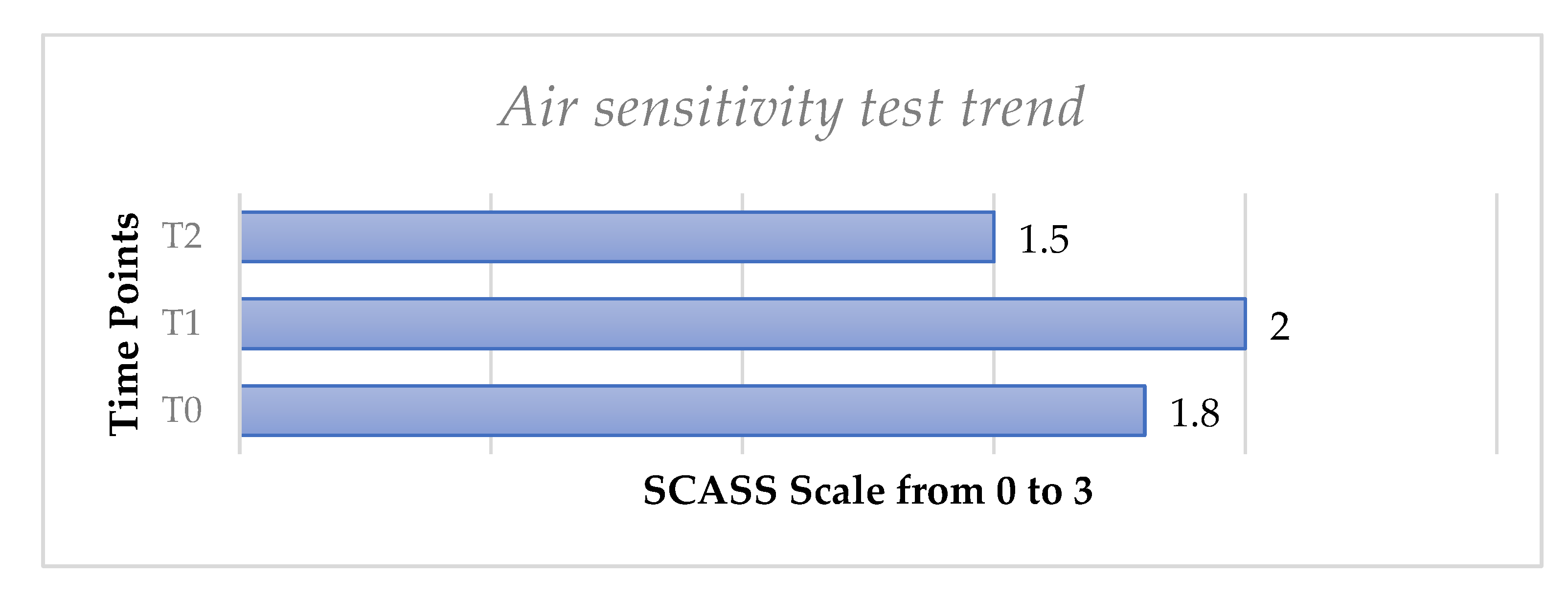
The evaporative air sensitivity test trend, performed using the SCASS scale in the whole sample of 52 patients, showed a slight increase from T0 to T1 with no statistically significant difference (p > 0.05) and then a slight decrease from T0 to T, with no significant difference (p > 0.05), (Figure 3).
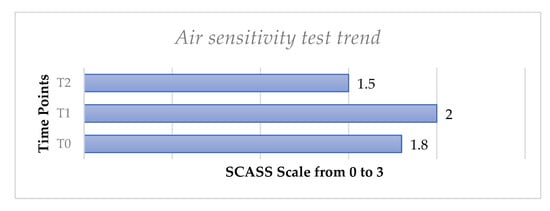
Figure 3.
Mean values of air sensitivity test from T0 to T2 for all molar teeth with hypersensitivity considered for each patient.
3.2. Tactile, Cold, Osmotic, Acid, and Omni-Comprehensive Test Performed in the Whole Sample
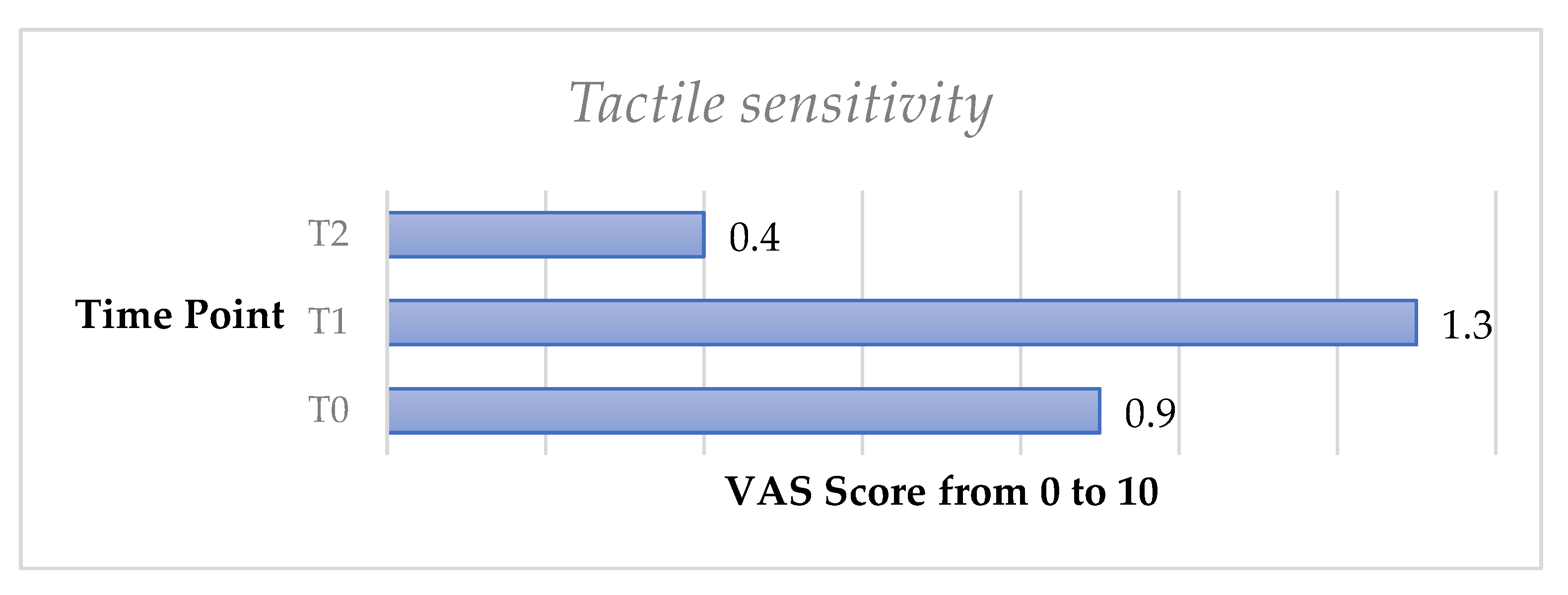
The tactile sensitivity test showed a slight increase of DH from T0 to T1; however, no significant difference was recorded (p > 0.05). DH significantly decreased from T0 to T2 (p < 0.05).
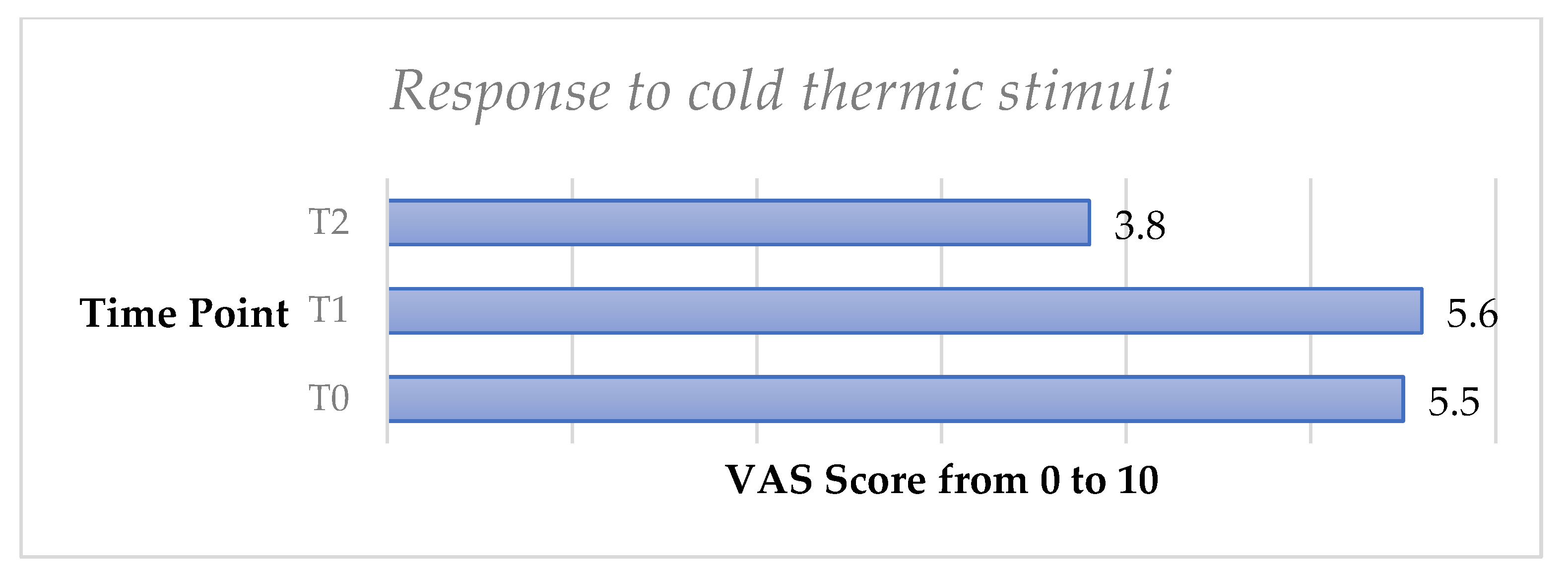
The cold thermic test showed a very slight increase of DH from T0 to T1 with no significant difference (p > 0.05). DH significantly decreased from T0 to T2, and a significant difference (p < 0.05) was observed.
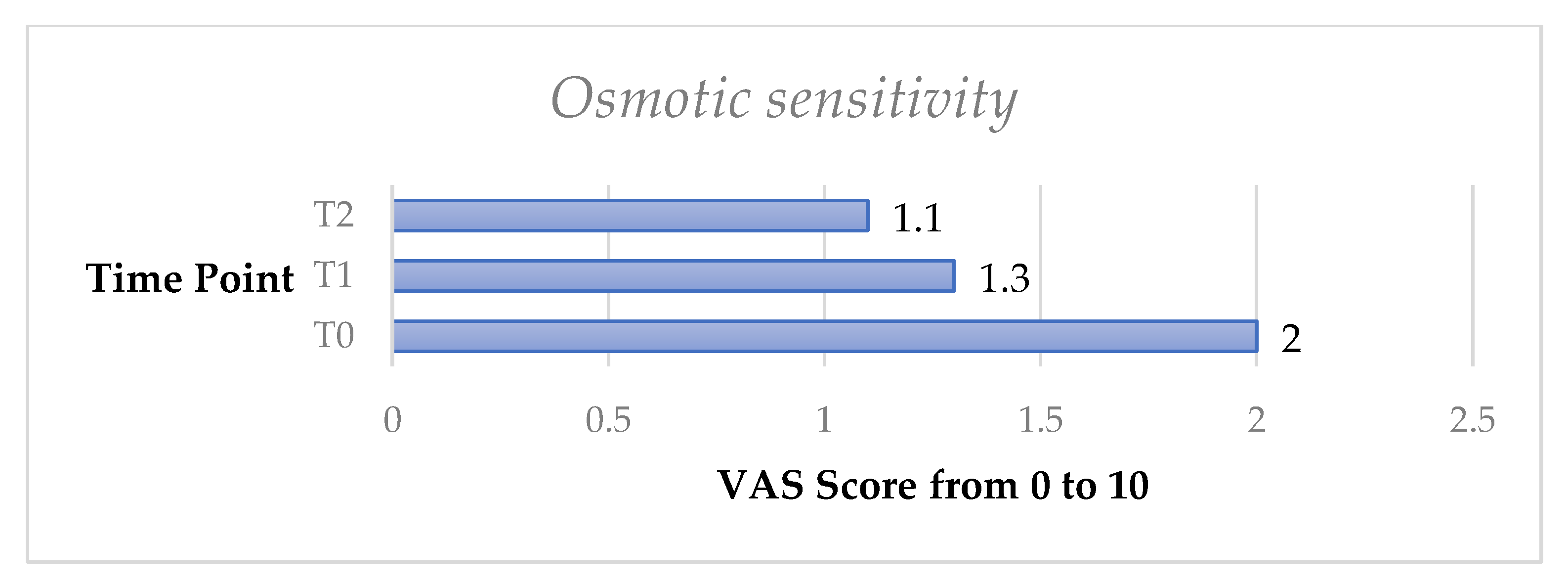
The osmotic test exhibited a significant decrease of DH both from T0 to T1 (p < 0.05) and from T0 to T2 (p < 0.05).
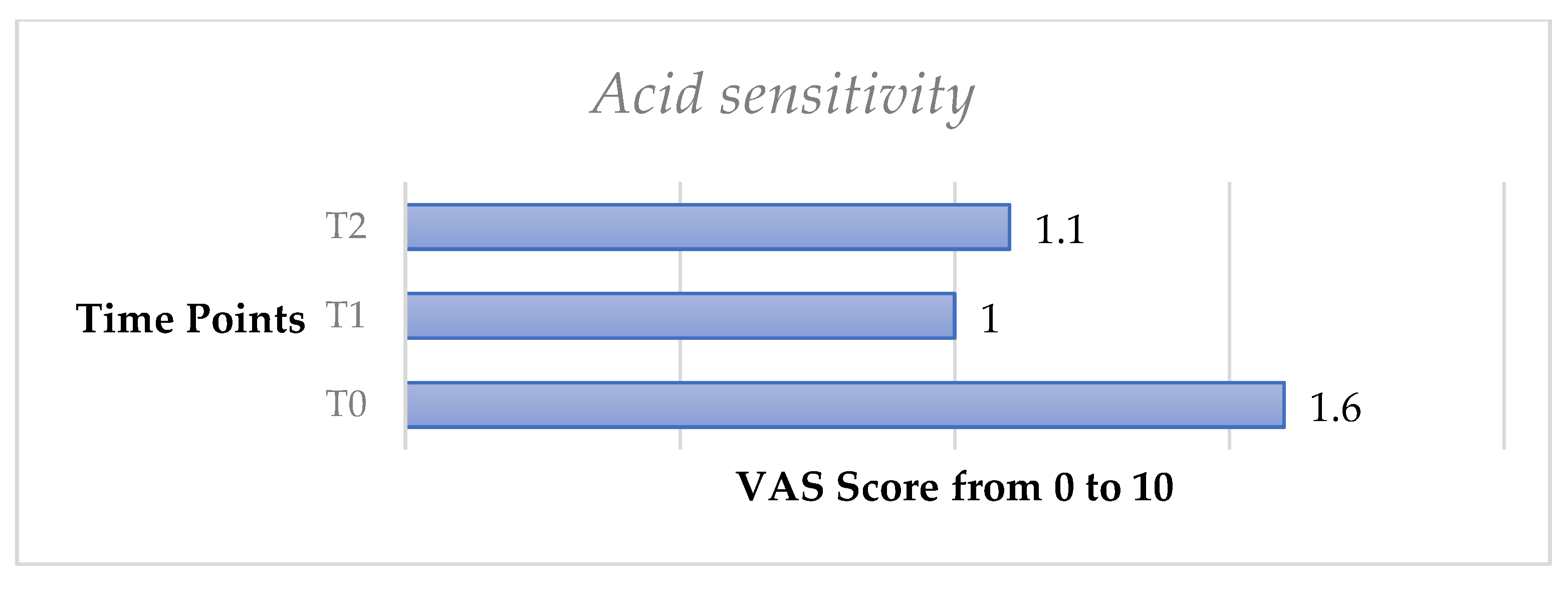
Similarly, the acid test exhibited a significant decrease of DH from T0 to T1 (p < 0.05) and from T0 to T2 (p < 0.05).
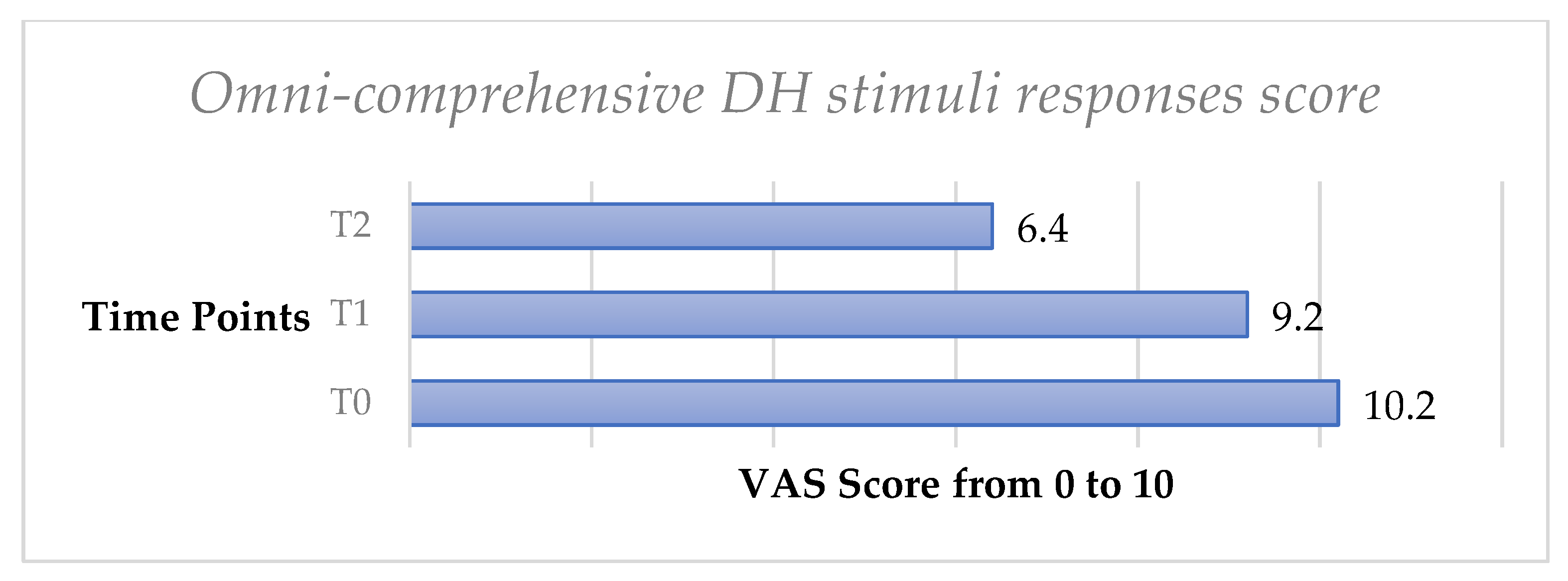
Finally, the omni-comprehensive test, calculated through the sum of the four VAS scale values obtained by the DH stimuli response clinical evaluation, showed a significant decrease of DH both from T0 to T1 (p < 0.05) and from T0 to T2 (p < 0.05) (Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8).
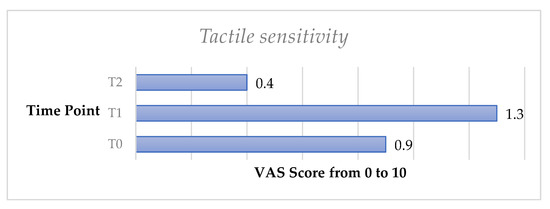
Figure 4.
Mean values of tactile sensitivity trend from T0 to T2 in the whole sample.
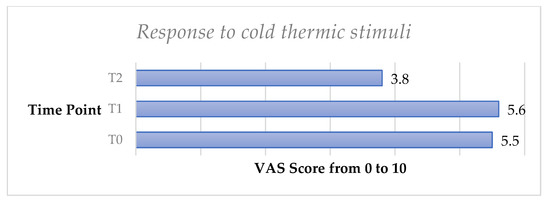
Figure 5.
Mean values of thermic sensitivity test trend from T0 to T2 in the whole sample.
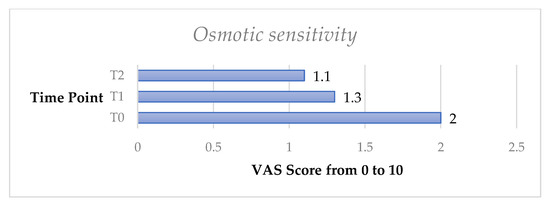
Figure 6.
Mean osmotic sensitivity test from T0 to T2 in the whole sample.
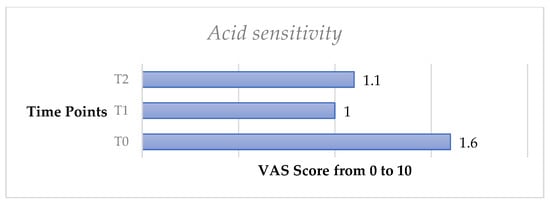
Figure 7.
Mean values of acid sensitivity test trend from T0 to T2 in the whole sample.
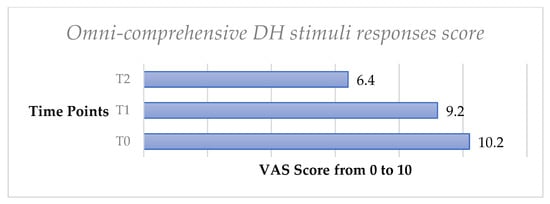
Figure 8.
Mean values of omni-comprehensive DH stimuli responses score trend from T0 to T2 in the whole sample.
Comparison between Group A and B
Starting from T1, patients reported the correct daily use of desensitizing toothpastes, at least twice a day during brushing and applied for at least 2 min on sensitive areas. A comparison was made between the two groups, differentiated by the use of desensitizing products A and B. Both products were effective in reducing DH to all stimuli from T0 to T2.
No significant differences were recorded between the two groups from T0 to T1 regarding the air test (p: 0.9; p > 0.05). Moreover, we did not record any significant difference between the two groups, from T0 to T1, with the tactile test (p: 0.84; p > 0.05), cold thermic test (p: 0.81; p > 0.05), osmotic test (p: 0.89; p > 0.05), acid test (p: 0.92; p > 0.05) and omni-comprehensive test (p: 0.89; p > 0.05). Therefore, a similar DH trend was observed in both groups treated with two different desensitizing agents after the first follow-up.
No significant differences were recorded between the two groups from T0 regarding the air test (p: 0.87; p > 0.05). Moreover, we did not record any significant difference between the two groups, from T0 to T2, with the tactile test (p: 0.88; p > 0.05), cold thermic test (p: 0.89; p > 0.05), osmotic test (p: 0.91; p > 0.05), acid test (p: 0.95; p > 0.05), and omni-comprehensive test (p: 0.9; p > 0.05). Therefore, a similar DH trend was observed in both groups treated with two different desensitizing agents at the final follow-up.
4. Discussion
In the present study, it was observed that desensitizing agents are able to significantly decrease DH in adults after a short follow-up of 30 days. Moreover, it has been reported that these agents were effective in reducing DH against different stimuli including tactile, cold thermical, osmotic, and acid agents.
In previous studies, it was reported that patients tend to overestimate DH self-perception, and it may be possible that routinary clinical tests limited to tactile or air test did not completely evaluate the full aspects of DH due to the influence psychological status and self-perception seem to have on this pathology [9,12]. For this reason, we have introduced an omni-comprehensive index that was able to evaluate all aspects linked to DH.
As previously discussed, there are several theories on the etiology of dentin hypersensitivity, such as hydrodynamic theory, modulation theory, transducer theory, and gate control theory; but none of these, however, have been fully proved. Therefore, we can conclude that all etiological factors and trigger agents linked to DH are not fully understood, and a full analysis of different agents may be helpful in the evaluation of this condition.
We have observed that the air test did not show significant differences after the follow-up. This could have been caused by the methodology applied in the present study, since for this index a scale from 0 to 3 was adopted while a VAS scale with a higher range from 0 to 10 was used for other tests. Furthermore, the subjective aspect of the tests that rely on patients’ perception should be considered.
In the present study, a slight increase at T1 was recorded for most of the tests, which can be justified by a temporary hypersensitivity following the professional oral hygiene session; moreover, a likely greater awareness of DH by the patient, following the clinical instruction of the dental professional, may have increased DH self-perception.
This slight increase of DH, however, has been reported as only temporary since DH decreased significantly at T2, seemingly because of the use of desensitizing devices.
Therefore, we may assume that these desensitizing agents need at least 30 days in order to achieve a significant DH decrease.
Regarding the differences between the two toothpastes, we can state that both were effective in reducing DH against all stimuli from T0 to T2; a slight difference in favor of product B (a toothpaste containing n-HAp in effective dose 2.25% and 0.15% fluoride, 1500 ppm) was observed, but no statistical significance was observed for this comparison.
The patients’ age seemed to affect both the SCASS scale and the final omni-comprehensive score, since significantly lower scores have been registered in older patients, with an inversely proportional ratio. This could be caused by the natural accumulation of secondary and tertiary dentin with a partial obliteration of dentinal tubules during aging. Furthermore, diet plays an important role on the onset and development of dentinal hypersensitivity because of the acidic properties of foods; younger people consume high quantities of soft and sugary drinks, which are one of the main causes of hypersensitivity along with nocturnal or diurnal bruxism, wrong brushing techniques, use of toothbrushes with hard bristles, and presence of periodontal diseases with gingival recessions [3]. At the time of onset, since this condition can occur very early in young adults, it is necessary to stop any bad habits and use specific agents that favor remineralization such as: toothpastes, mouthwashes, tooth mousse, and topical application of varnishes.
The Dental School of the University of Turin, in 2016, published a study titled “Comparative clinical evaluation of the efficacy of two professional products for the management of dentinal hypersensitivity: a split-mouth study” [40]. The authors conducted a split-mouth randomized controlled trial (RCT), including 10 patients with at least two dental elements per dental hemiarch, affected by DH. For each patient, DH degree was assessed using the Schiff Air Index and the VAS pain scale, while in the present study an omni-comprehensive score was calculated, to be more objective and provide a global evaluation of sensitivity responses to different stimuli. Like the present study, a professional oral hygiene session and subsequently an evaluation of the sensitivity tests after 4 weeks from the desensitizing treatment were performed. The analyzed dental elements were randomly divided into two groups: group A was treated with toothpaste containing arginine calcium carbonate, and group B with a suspension of nano-hydroxyapatite. Their results confirm both products’ effectiveness in reducing DH, with no statistically significant differences between the two groups, in agreement with our data. A systematic review and meta-analysis published in 2019 [27] highlighted that n-HAp-containing products showed greater performance in DH improvement, compared to other available treatments. A three-month follow-up study reported the same results regarding n-HAp treatment effectiveness: no significant difference was recorded with other desensitizing products over three months treatment, proving all to be equally effective [41]. Moreover, an RCT conducted by Anand at al. confirmed our results, showing better clinical performance of n-HAp-containing device compared to arginine-based toothpastes in reducing DH, with a non-statistically significant difference between groups [42].
5. Conclusions
The present study results highlighted that both desensitizing agents (arginine-based and n-HAp toothpastes) showed similar effects and were effective against DH, diminishing the symptoms.
The effect of desensitizing agents was significant after only 30 days, but it has been observed that a paradoxical increase of DH symptoms may be present 15 days after professional dental hygiene treatment.
Therefore, both devices can be considered valid when used to treat the typical algogenic symptomatology of DH, since a gold-standard desensitizing agent for the treatment of DH has not been identified yet. Future studies should involve a randomized controlled trial with longer follow-up periods, which would allow the study of the long-term effects of the various treatments and any eventual differences in the efficacy of the products when used for long periods of time.
Furthermore, an omni-comprehensive index may be useful for dentist and dental hygienists to evaluate all possible trigger agents on dentine; since patients describe DH symptoms to dental operators with a subjective evaluation, it is necessary to standardize DH investigations including all possible agents that may affect the patients’ quality of life.
Author Contributions
Conceptualization, M.M. and M.P.; methodology, M.P. and E.F.; software, M.P.; validation, M.R.G. and E.F.; formal analysis, M.R.G. and M.M.; investigation, M.P.; resources, M.M. and E.F.; data curation, E.F.; writing—original draft preparation, E.F.; writing—review and editing, M.P. and M.R.G.; visualization, M.M.; supervision, M.R.G. and M.M.; project administration, M.R.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of University Hospital of Santa Chiara of Pisa, Italy (protocol code 15078).
Informed Consent Statement
Informed consent was obtained from all patients involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- West, N.X. Dentine hypersensitivity: Preventive and therapeutic approaches to treatment. Periodontol. 2000 2008, 48, 31–41. [Google Scholar] [CrossRef]
- Addy, M. Dentine hypersensitivity: Definition, prevalence, distribution and etiology. In Tooth Wear and Sensitivity: Clinical Advances in Restorative Dentistry; Addy, M., Embery, G., Edgar, W.M., Orchardson, R., Eds.; Martin Dunitz: London, UK, 2000; pp. 239–248. [Google Scholar]
- Favaro Zeola, L.; Soares, P.V.; Cunha-Cruz, J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J. Dent. 2019, 81, 1–6. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Lussi, A. Age-related morphological, histological and functional changes in teeth. J. Oral Rehabil. 2017, 44, 291–298. [Google Scholar] [CrossRef]
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J. Can. Dent. Assoc. 2003, 69, 221–226. [Google Scholar]
- Gillam, D.G.; Seo, H.S.; Bulman, J.S.; Newman, H.N. Perceptions of dentine hypersensitivity in a general practice population. J. Oral Rehabil. 1999, 26, 710–714. [Google Scholar] [CrossRef]
- Douglas-de-Oliveira, D.W.; Vitor, G.P.; Silveira, J.O.; Martins, C.C.; Costa, F.O.; Cota, L.O.M. Effect of dentin hypersensitivity treatment on oral health related quality of life–a systematic review and meta-analysis. J. Dent. 2018, 71, 1–8. [Google Scholar] [CrossRef]
- Boiko, O.V.; Baker, S.R.; Gibson, B.J.; Locker, D.; Sufi, F.; Barlow, A.P.; Robinson, P.G. Construction and validation of the quality of life measure for dentine hypersensitivity (DHEQ). J. Clin. Periodontol. 2010, 37, 973–980. [Google Scholar] [CrossRef]
- Gysi, A. An attempt to explain the sensitiveness of dentin. Br. J. Dent. Sci. 1900, 43, 865–868. [Google Scholar]
- Brännström, M. A hydrodynamic mechanism in the transmission of pain producing stimuli through the dentine. In Sensory Mechanisms in Dentine; Anderson, D.J., Ed.; Pergamon Press: Oxford, UK, 1963; pp. 73–79. [Google Scholar]
- Brännström, M. The sensitivity of dentine. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 517–526. [Google Scholar] [CrossRef]
- Brännström, M.; Astrom, A. The hydrodynamics of dentin and its possible relationship to dentinal pain. Dent. J. 1972, 22, 219–227. [Google Scholar]
- West, N.; Seong, J.; Davies, M. Dentine hypersensitivity. Monogr. Oral Sci. 2014, 25, 108–122. [Google Scholar]
- Vlasova, N.; Samusenkov, V.; Novikova, I.; Nikolenko, D.; Nikolashvili, N.; Gor, I.; Danilina, A. Clinical efficacy of hydroxyapatite toothpaste containing Polyol Germanium Complex (PGC) with threonine in the treatment of dentine hypersensitivity. Saudi Dent. J. 2022, 34, 310–314. [Google Scholar] [CrossRef]
- Addy, M. Dentine hypersensitivity: New perspectives on an old problem. Int. Dent. J. 2002, 52, 367–375. [Google Scholar] [CrossRef]
- Dowell, P.; Addy, M. Dentine hypersensitivity—A review, aetiology, symptoms and theories of pain production. J. Clin. Periodontol. 1983, 10, 341–350. [Google Scholar] [CrossRef]
- Trushkowsky, R.D.; Oquendo, A. Treatment of dentin hypersensitivity. Dent. Clin. North Am. 2011, 55, 599–608. [Google Scholar] [CrossRef]
- Parolia, A.; Mohan, M. Management of dentinal hypersensitivity: A review. CDA J. 2011, 39, 167–179. [Google Scholar]
- Najibfard, K.; Ramalingam, K.; Chedjieu, I.; Amaechi, B.T. Remineralization of early caries by a nano-hydroxyapatite dentifrice. J. Clin. Dent. 2011, 22, 139–143. [Google Scholar]
- Tolentino, A.B.; Zeola, L.F.; Fernandes, M.R.U.; Pannuti, C.M.; Soares, P.V.; Aranha, A.C.C. Photobiomodulation therapy and 3% potassium nitrate gel as treatment of cervical dentin hypersensitivity: A randomized clinical trial. Clin. Oral. Investig. 2022, 25, 1–9. [Google Scholar] [CrossRef]
- Huang, S.B.; Gao, S.; Cheng, L.; Yu, H. Combined effects of nano-hydroxyapatite and Galla chinensis on remineralisation of initial enamel lesion in vitro. J. Dent. 2010, 38, 811–819. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, H.; Kim, B. Effect of dentinal tubule occlusion by dentifrice containing nano-carbonate apatite. J Oral Rehabil. 2008, 35, 847–853. [Google Scholar] [CrossRef]
- Jiang, R.; Xu, Y.; Wang, F.; Lin, H. Effectiveness and cytotoxicity of two desensitizing agents: A dentin permeability measurement and dentin barrier testing in vitro study. BMC Oral Health 2022, 22, 391. [Google Scholar]
- Roveri, N.; Battistella, E.; Foltran, I.; Foresti, E.; Iafasco, M.; Lelli, M.; Palazzo, B.; Rimondini, L. Synthetic biomimetic carbonatehydroxyapatite nanocrystals for enamel remineralization. Adv. Mater. Res. 2008, 47, 821–824. [Google Scholar] [CrossRef]
- Huang, S.; Gao, S.; Yu, H. Remineralization potential of nanohydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef]
- Roveri, N.; Battistella, E.; Bianchi, C.L.; Foltran, I.; Foresti, E.; Iafasco, M.; Lelli, M.; Naldoni, A.; Palazzo, B.; Rimondini, L. Surface enamel remineralization: Biomimetic apatite nanocrystals and fluoride ions different effects. J. Nanomater. 2009, 1, 1–9. [Google Scholar] [CrossRef]
- de Melo Alencar, C.; de Paula, B.L.F.; Guanipa Ortiz, M.I.; Barauna Magno, M.; Martins Silva, C.; Cople Maia, L. Clinical efficacy of nano-hydroxyapatite in dentin hypersensitivity: A systematic review and meta-analysis. J. Dent. 2019, 82, 11–21. [Google Scholar] [CrossRef]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano-hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral Investig. 2018, 22, 313–320. [Google Scholar] [CrossRef]
- Matsuura, T.; Mae, M.; Ohira, M.; Mihara, Y.; Yamashita, Y.; Sugimoto, K.; Yamada, S.; Yoshimura, A. The efficacy of a novel zinc-containing desensitizer CAREDYNE Shield for cervical dentin hypersensitivity: A pilot randomized controlled trial. BMC Oral Health 2022, 22, 294. [Google Scholar] [CrossRef]
- Holland, G.R.; Narhi, M.N.; Addy, M.; Gangarosa, L.; Orchardson, R. Guidelines for the design and conduct of clinical trials on dentine hypersensitivity. J. Clin. Periodontol. 1997, 24, 808–813. [Google Scholar] [CrossRef]
- Miller, S.C. Textbook of Periodontia, 3rd ed.; TheBlakiston Co.: Philadelphia, PA, USA, 1950. [Google Scholar]
- Loe, H. The Gingival Index, The Plaque Index and The Retention Index Systems. J Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Loe, H.; Silness, J. Periodontal Disease in Pregnancy. I. Prevalence and Severity. Acta Odontol Scand. 1963, 21, 533–551. [Google Scholar] [CrossRef]
- Silness, J.; Loe, H. Periodontal disease in pregnancy. II. Correlation Between Oral Hygiene and Periodontal Condition. Acta Odontol. Scand. 1964, 22, 121–135. [Google Scholar] [CrossRef]
- He, T.; Barker, M.L.; Biesbrock, A.R.; Miner, M.; Qaqish, J.; Sharma, N. A clinical study to assess the effect of a stabilized stannous fluoride dentifrice on hypersensitivity relative to a marketed sodium fluoride/triclosan control. J. Clin. Dent. 2014, 25, 13–18. [Google Scholar]
- Schiff, T.; Dotson, M.; Cohen, S.; De Vizio, W.; McCool, J.; Volpe, A. Efficacy of a dentifrice containing potassium nitrate, soluble pyrophosphate, PVM/MA copolymer, and sodium fluoride on dentinal hypersensitivity: A twelve-week clinical study. J. Clin. Dent. 1994, 5, 87–92. [Google Scholar]
- Jalaluddin, M.; Hashmi, A.; Devi, K.B.; Abushanan, A.; Hashem, Q.; Uthman, U.S. Assessment of the Efficacy of Different Desensitizing Agents on Dentinal Tubules Occlusion-An In vitro Study. J. Pharm. Bioallied. Sci. 2022, 14, S585–S588. [Google Scholar] [CrossRef]
- Matarazzo, L.; Nastasio, S.; Sciveres, M.; Maggiore, G. Pregnancy outcome in women with childhood onset autoimmune hepatitis and autoimmune sclerosing cholangitis on long-term immunosuppressive treatment. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 268, 7–11. [Google Scholar] [CrossRef]
- Heft, M.W.; Litaker, M.S.; Kopycka-Kedzierawski, D.T.; Meyerowitz, C.; Chonowski, S.; Yardic, R.L.; Gordan, V.V.; Mungia, R.; Gilbert, G.H.; National Dental PBRN Collaborative Group The National Dental PBRN Collaborative Group. Patient-Centered Dentinal Hypersensitivity Treatment Outcomes: Results from the National Dental PBRN. JDR Clin. Trans. Res. 2018, 3, 76–82. [Google Scholar] [CrossRef]
- Italian Dental Hygiene Magazine. Available online: https://www.rivistaitalianaigienedentale.it/valutazione-clinica-comparativa-dellefficacia-due-prodotti-professionali-la-gestione-dellipersensibilita-dentinale-uno-studio-split-mouth-2/ (accessed on 19 August 2022).
- Wang, L.; Magalhães, A.C.; Francisconi-Dos-Rios, L.F.; Calabria, M.P.; Araújo, D.F.G.; Buzalaf, M.A.R.; Lauris, J.R.P.; Pereira, J.C. Treatment of Dentin Hypersensitivity Using Nano-Hydroxyapatite Pastes: A Randomized Three-Month Clinical Trial. Oper. Dent. 2016, 41, E93–E101. [Google Scholar] [CrossRef]
- Anand, S.; Rejula, F.; Sam, J.V.G.; Christaline, R.; Nair, M.G.; Dinakaran, S. Comparative Evaluation of Effect of Nano-hydroxyapatite and 8% Arginine Containing Toothpastes in Managing Dentin Hypersensitivity: Double Blind Randomized Clinical Trial. Acta Med. 2017, 60, 114–119. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).