Prediction of Residual NPK Levels in Crop Fruits by Electronic-Nose VOC Analysis following Application of Multiple Fertilizer Rates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Plant Growth Medium, Experimental Design, and Fruit Samples
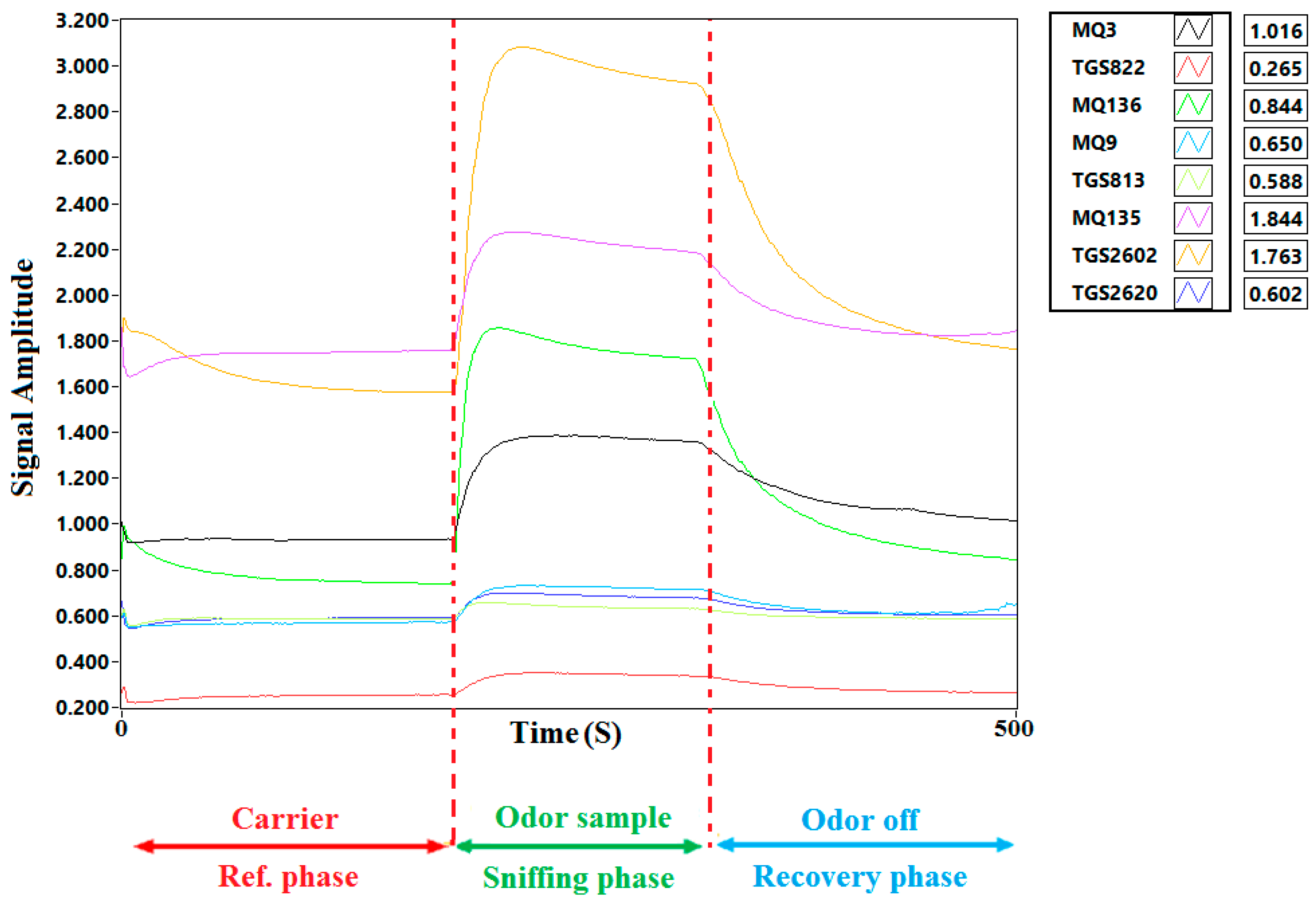
2.2. Electronic Nose
Cucumber Fruit Volatile Analyses
2.3. Determination of Chemical Fertilizer Residues
2.4. Statistical Model Development
3. Results
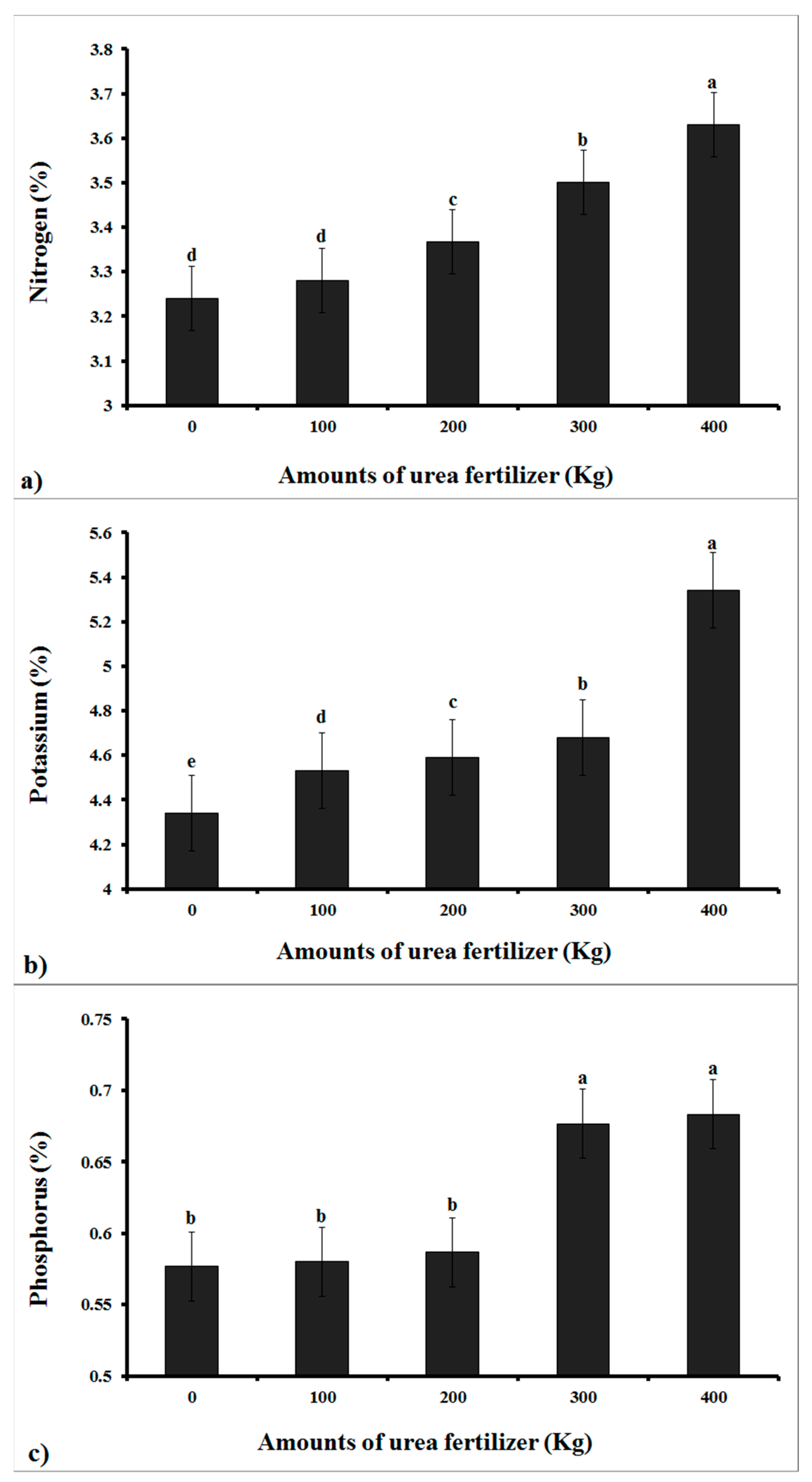
3.1. Results of the Analysis of Variance of the N, P, and K Content of the Cucumber
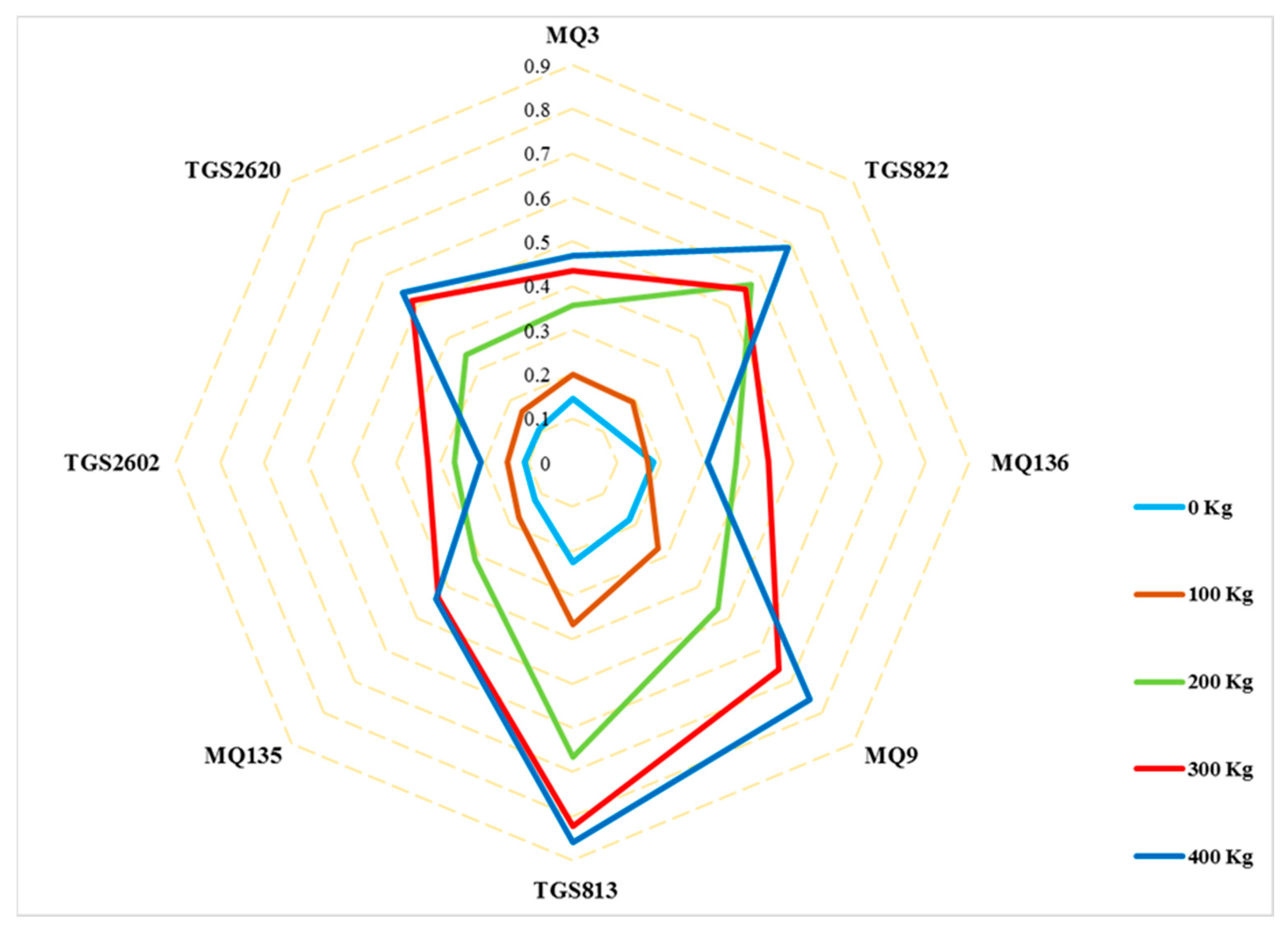
3.2. E-Nose Responses to Cucumber Fruit VOCs
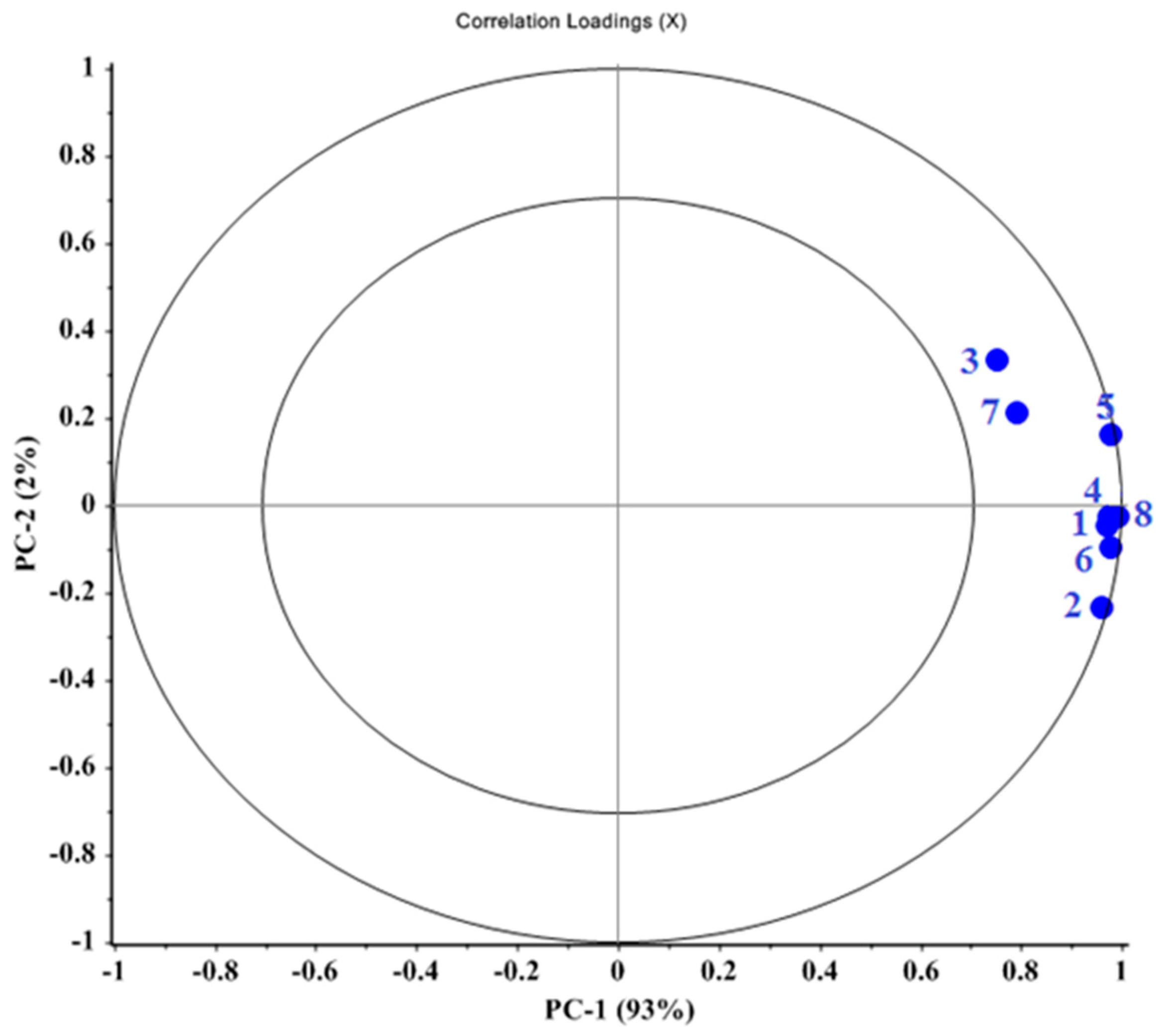
3.3. Prediction of NPK Levels in Fruits Based on PLSR and PCR
3.4. Prediction of NPK Content of Fruits Based on MLR
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Q.; Li, L.; Yang, Z.; Wang, Y.; Li, J.; Chen, W.; Wang, J.; Meng, Z. Transcriptome analysis of genes in response to magnesium nitrate stress on cucumber leaf. Sci. Hortic. 2021, 288, 110391. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Zhu, W.; Qin, X.; Xu, J.; Cheng, C.; Lou, Q.; Li, J.; Chen, J. Complete resistance to powdery mildew and partial resistance to downy mildew in a Cucumis hystrix introgression line of cucumber were controlled by a co-localized locus. Theor. Appl. Genet. 2018, 131, 2229–2243. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Hashemi, M.; Hosseini, S.M. Postharvest treatment of nanochitosan-based coating loaded with Zataria multiflora essential oil improves antioxidant activity and extends shelf-life of cucumber. Innov. Food Sci. Emerg. Technol. 2016, 33, 580–588. [Google Scholar] [CrossRef]
- Gizaw, Z. Public health risks related to food safety issues in the food market: A systematic literature review. Environ. Health Prev. Med. 2019, 24, 68. [Google Scholar] [CrossRef] [Green Version]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant. Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Philippe, V.; Neveen, A.; Marwa, A.; Ahmad Basel, A.-Y. Occurrence of pesticide residues in fruits and vegetables for the Eastern Mediterranean Region and potential impact on public health. Food Control 2021, 119, 107457. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, R.; Zhang, C. Does the adoption of complex fertilizers contribute to fertilizer overuse? Evidence from rice production in China. J. Clean. Prod. 2019, 219, 677–685. [Google Scholar] [CrossRef]
- Sutton, M.A.; Bleeker, A.; Howard, C.; Erisman, J.W.; Abrol, Y.P.; Bekunda, M.; Datta, A.; Davidson, E.; Vries, W.; Oenema, O.; et al. Our nutrient world. In The Challenge to Produce More Food and Energy with Less Pollution; Centre for Ecology & Hydrology on behalf of the Global Partnership on Nutrient Management (GPNM) and the International Nitrogen Initiative (INI): Edinburgh, UK, 2013; ISBN 978-1-906698-40-9. [Google Scholar]
- Al-mansour, B.; Kalaivanan, D.; Suryanarayana, M.A.; Umesha, K.; Nair, A.K. Influence of organic and inorganic fertilizers on yield and quality of sweet basil (Ocimum basilicum L.). J. Spices Aromat. Crops 2018, 27, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Olarewaju, O.A.; Alashi, A.M.; Taiwo, K.A.; Oyedele, D.; Adebooye, O.C.; Aluko, R.E. Influence of nitrogen fertilizer micro-dosing on phenolic content, antioxidant, and anticholinesterase properties of aqueous extracts of three tropical leafy vegetables. J. Food Biochem. 2018, 42, e12566. [Google Scholar] [CrossRef]
- Sabzi, S.; Pourdarbani, R.; Rohban, M.H.; García-Mateos, G.; Arribas, J.I. Estimation of nitrogen content in cucumber plant (Cucumis sativus L.) leaves using hyperspectral imaging data with neural network and partial least squares regressions. Chemometr. Intell. Lab. Syst. 2021, 217, 104404. [Google Scholar] [CrossRef]
- Bryan, N.S.; van Grinsven, H. Chapter Three—The role of nitrate in human health. Adv. Agron. 2013, 119, 153–182. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-M.; Liu, D.-Y.; Zhang, W.; Chen, X.-X.; Zhao, Q.-Y.; Chen, X.-P.; Zou, C.-Q. Health risk assessment of heavy metals (Zn, Cu, Cd, Pb, As and Cr) in wheat grain receiving repeated Zn fertilizers. Environ. Pollut. 2020, 257, 113581. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.D. Diverse applications of electronic-nose technologies in agriculture and forestry. Sensors 2013, 13, 2295–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baietto, M.; Wilson, A.D. Electronic-nose applications for fruit identification, ripeness, and quality grading. Sensors 2015, 15, 899–931. [Google Scholar] [CrossRef]
- Rasekh, M.; Karami, H.; Wilson, A.D.; Gancarz, M. Classification and identification of essential oils from herbs and fruits based on a MOS electronic-nose technology. Chemosensors 2021, 9, 142. [Google Scholar] [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of electronic nose as a non-invasive technique for odor fingerprinting and detection of bacterial foodborne pathogens: A review. J. Food Sci. Technol. 2020, 57, 1977–1990. [Google Scholar] [CrossRef]
- Wilson, A.D. Review of electronic-nose technologies and algorithms to detect hazardous chemicals in the environment. Proc. Technol. 2012, 1, 453–463. [Google Scholar] [CrossRef] [Green Version]
- Binson, V.; Subramoniam, M.; Mathew, L. Discrimination of COPD and lung cancer from controls through breath analysis using a self-developed e-nose. J. Breath Res. 2021, 15, 046003. [Google Scholar] [CrossRef]
- Rasekh, M.; Karami, H.; Wilson, A.D.; Gancarz, M. Performance analysis of MAU-9 electronic-nose MOS sensor array components and ANN classification methods for discrimination of herb and fruit essential oils. Chemosensors 2021, 9, 243. [Google Scholar] [CrossRef]
- Rasekh, M.; Karami, H. E-nose coupled with an artificial neural network to detection of fraud in pure and industrial fruit juices. Int. J. Food Prop. 2021, 24, 592–602. [Google Scholar] [CrossRef]
- Jha, S.K.; Yadava, R.; Hayashi, K.; Patel, N. Recognition and sensing of organic compounds using analytical methods, chemical sensors, and pattern recognition approaches. Chemometr. Intell. Lab. Syst. 2019, 185, 18–31. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, Z.; Zhang, J.; Wang, J.; Zhong, J.; Wang, Y. Discrimination of wood-boring beetles infested Platycladus orientalis plants by using gas chromatography-ion mobility spectrometry. Comput. Electron. Agric. 2021, 180, 105896. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, W.; Gu, S.; Wang, Y.; Wang, J. Evaluation of trunk borer infestation duration using MOS E-nose combined with different feature extraction methods and GS-SVM. Comput. Electron. Agric. 2020, 170, 105293. [Google Scholar] [CrossRef]
- Junaedi, E.C.; Lestari, K.; Muchtaridi, M. Infrared spectroscopy technique for quantification of compounds in plant-based medicine and supplement. J. Adv. Pharm. Technol. Res. 2021, 12, 1. [Google Scholar] [CrossRef]
- Seesaard, T.; Goel, N.; Kumar, M.; Wongchoosuk, C. Advances in gas sensors and electronic nose technologies for agricultural cycle applications. Comput. Electron. Agric. 2022, 193, 106673. [Google Scholar] [CrossRef]
- Khorramifar, A.; Karami, H.; Wilson, A.D.; Sayyah, A.H.A.; Shuba, A.; Lozano, J. Grape cultivar identification and classification by machine olfaction analysis of leaf volatiles. Chemosensors 2022, 10, 125. [Google Scholar] [CrossRef]
- Khorramifar, A.; Rasekh, M.; Karami, H.; Malaga-Toboła, U.; Gancarz, M. A machine learning method for classification and identification of potato cultivars based on the reaction of MOS type sensor-array. Sensors 2021, 21, 5836. [Google Scholar] [CrossRef]
- Tatli, S.; Mirzaee-Ghaleh, E.; Rabbani, H.; Karami, H.; Wilson, A.D. Rapid detection of urea fertilizer effects on VOC emissions from cucumber fruits using a MOS e-nose sensor array. Agronomy 2022, 12, 35. [Google Scholar] [CrossRef]
- Beljkaš, B.; Matić, J.; Milovanović, I.; Jovanov, P.; Mišan, A.; Šarić, L. Rapid method for determination of protein content in cereals and oilseeds: Validation, measurement uncertainty and comparison with the Kjeldahl method. Accredit. Qual. Assur. 2010, 15, 555–561. [Google Scholar] [CrossRef]
- Adenipekun, C.O.; Oyetunji, O.J. Nutritional values of.f some tropical vegetables. J. Appl. Biosci. 2010, 35, 2294–2300. [Google Scholar]
- Kumar, N.; Bansal, A.; Sarma, G.; Rawal, R.K. Chemometrics tools used in analytical chemistry: An overview. Talanta 2014, 123, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Geladi, P. Chemometrics in spectroscopy. Part 1. Classical chemometrics. Spectrochim. Acta Part B At. Spectrosc. 2003, 58, 767–782. [Google Scholar] [CrossRef]
- Malley, D.F.; Yesmin, L.; Wray, D.; Edwards, S. Application of near-infrared spectroscopy in analysis of soil mineral nutrients. Commun. Soil Sci. Plant Anal. 1999, 30, 999–1012. [Google Scholar] [CrossRef]
- Choe, E.; Kim, K.-W.; Bang, S.; Yoon, I.-H.; Lee, K.-Y. Qualitative analysis and mapping of heavy metals in an abandoned Au–Ag mine area using NIR spectroscopy. Environ. Geol. 2009, 58, 477–482. [Google Scholar] [CrossRef]
- Chang, C.-W.; Laird, D.; Mausbach, M.J.; Hurburgh, C.R., Jr. Near-infrared reflectance spectroscopy–principal components regression analyses of soil properties. Soil Sci. Soc. Am. J. 2001, 65, 480. [Google Scholar] [CrossRef] [Green Version]
- Freedman, D.A. Statistical Models: Theory and Practice; Cambridge University Press: Cambridge, UK, 2009; ISBN 1139477315. [Google Scholar]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- He, H.-J.; Wu, D.; Sun, D.-W. Non-destructive and rapid analysis of moisture distribution in farmed Atlantic salmon (Salmo salar) fillets using visible and near-infrared hyperspectral imaging. Innov. Food Sci. Emerg. Technol. 2013, 18, 237–245. [Google Scholar] [CrossRef]
- Rossel, R.A.V. ParLeS: Software for chemometric analysis of spectroscopic data. Chemometr. Intell. Lab. Syst. 2008, 90, 72–83. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, J. Approach to the quantification of milk mixtures by partial least-squares, principal component and multiple linear regression techniques. Food Chem. 2006, 98, 782–789. [Google Scholar] [CrossRef]
- Bautista, R.; Aberasturi, F.; Jimenez, A.; Jimenez, F. Simultaneous spectrophotometric determination of drugs in pharmaceutical preparations using multiple linear regression and partial least-squares regression, calibration and prediction methods. Talanta 1996, 43, 2107–2115. [Google Scholar] [CrossRef]
- Mahesh, S.; Jayas, D.; Paliwal, J.; White, N. Comparison of partial least squares regression (PLSR) and principal components regression (PCR) methods for protein and hardness predictions using the near-infrared (NIR) hyperspectral images of bulk samples of Canadian wheat. Food Bioproc. Technol. 2015, 8, 31–40. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, P.; Chen, J.; Lei, Y. A rapid analytical method for the quantitative determination of the sugar in acarbose fermentation by infrared spectroscopy and chemometrics. Spectrochim. Acta A Mol. Biomol. 2020, 240, 118571. [Google Scholar] [CrossRef] [PubMed]
- Rossel, R.V.; Walvoort, D.; McBratney, A.; Janik, L.J.; Skjemstad, J. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Jin, H.; Li, L.; Cheng, J. Rapid and non-destructive determination of moisture content of peanut kernels using hyperspectral imaging technique. Food Anal. Methods 2015, 8, 2524–2532. [Google Scholar] [CrossRef]
- Abu-Khalaf, N. Identification and quantification of olive oil quality parameters using an electronic nose. Agriculture 2021, 11, 674. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.; Cheng, Z.; Ma, L.; Zhao, L.; Li, J. A comparison of electronic nose and gas chromatography–mass spectrometry on discrimination and prediction of ochratoxin A content in Aspergillus carbonarius cultured grape-based medium. Food Chem. 2019, 297, 124850. [Google Scholar] [CrossRef]
- Zhou, Z.; Zeng, S.; Li, X.; Zheng, J. Nondestructive detection of blackheart in potato by visible/near infrared transmittance spectroscopy. J. Spectrosc. 2015, 2015, 786709. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Wang, J.; Zhang, W. Detecting internal quality of peanuts during storage using electronic nose responses combined with physicochemical methods. Food Chem. 2015, 177, 89–96. [Google Scholar] [CrossRef]
- Khorramifar, A.; Rasekh, M.; Karami, H.; Covington, J.A.; Derakhshani, S.M.; Ramos, J.; Gancarz, M. Application of MOS gas sensors coupled with chemometrics methods to predict the amount of sugar and carbohydrates in potatoes. Molecules 2022, 27, 3508. [Google Scholar] [CrossRef]
- Zaki Dizaji, H.; Adibzadeh, A.; Aghili Nategh, N. Application of e-nose technique to predict sugarcane syrup quality based on purity and refined sugar percentage. J. Food Sci. Technol. 2021, 58, 4149–4156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Fan, J.; Tan, R.; Peng, Q.; Cai, J.; Zhang, W. Prediction of linalool content in Osmanthus fragrans using e-nose technology. J. Sens. 2022, 2022, 7349030. [Google Scholar] [CrossRef]
- Rasekh, M.; Karami, H.; Fuentes, S.; Kaveh, M.; Rusinek, R.; Gancarz, M. Preliminary study non-destructive sorting techniques for pepper (Capsicum annuum L.) using odor parameter. LWT 2022, 164, 113667. [Google Scholar] [CrossRef]
- Biondi, E.; Blasioli, S.; Galeone, A.; Spinelli, F.; Cellini, A.; Lucchese, C.; Braschi, I. Detection of potato brown rot and ring rot by electronic nose: From laboratory to real scale. Talanta 2014, 129, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Rady, A.M.; Guyer, D.E. Evaluation of sugar content in potatoes using NIR reflectance and wavelength selection techniques. Postharvest Biol. Technol. 2015, 103, 17–26. [Google Scholar] [CrossRef]
- Gancarz, M.; Wawrzyniak, J.; Gawrysiak-Witulska, M.; Wiącek, D.; Nawrocka, A.; Tadla, M.; Rusinek, R. Application of electronic nose with MOS sensors to prediction of rapeseed quality. Measurement 2017, 103, 227–234. [Google Scholar] [CrossRef]
- Rusinek, R.; Gancarz, M.; Nawrocka, A. Application of an electronic nose with novel method for generation of smellprints for testing the suitability for consumption of wheat bread during 4-day storage. LWT 2020, 117, 108665. [Google Scholar] [CrossRef]
- Karami, H.; Rasekh, M.; Mirzaee-Ghaleh, E. Qualitative analysis of edible oil oxidation using an olfactory machine. J. Food Meas. Charact. 2020, 14, 2600–2610. [Google Scholar] [CrossRef]
- Gancarz, M.; Dobrzański, B.; Malaga-Toboła, U.; Tabor, S.; Combrzyński, M.; Ćwikła, D.; Strobel, W.R.; Oniszczuk, A.; Karami, H.; Darvishi, Y.; et al. Impact of coffee bean roasting on the content of pyridines determined by analysis of volatile organic compounds. Molecules 2022, 27, 1559. [Google Scholar] [CrossRef]
- Rusinek, R.; Gawrysiak-Witulska, M.; Siger, A.; Oniszczuk, A.; Ptaszyńska, A.A.; Knaga, J.; Malaga-Toboła, U.; Gancarz, M. Effect of supplementation of flour with fruit fiber on the volatile compound profile in bread. Sensors 2021, 21, 2812. [Google Scholar] [CrossRef]
- Raigar, R.K.; Upadhyay, R.; Mishra, H.N. Storage quality assessment of shelled peanuts using non-destructive electronic nose combined with fuzzy logic approach. Postharvest Biol. Technol. 2017, 132, 43–50. [Google Scholar] [CrossRef]
- Wilson, A.D. Future applications of electronic-nose technologies in healthcare and biomedicine. In Wide Spectra of Quality Control; Işin, A., Ed.; InTech Publishing: Rijeka, Croatia, 2011; pp. 267–290. [Google Scholar]
- Kerdcharoen, T.; Pobkrut, T. Soil sensing survey robots based on electronic nose. In Proceedings of the 14th Annual International Conference on Control, Automation, and Systems, Seoul, Korea, 22–25 October 2014; pp. 1604–1609. [Google Scholar] [CrossRef]

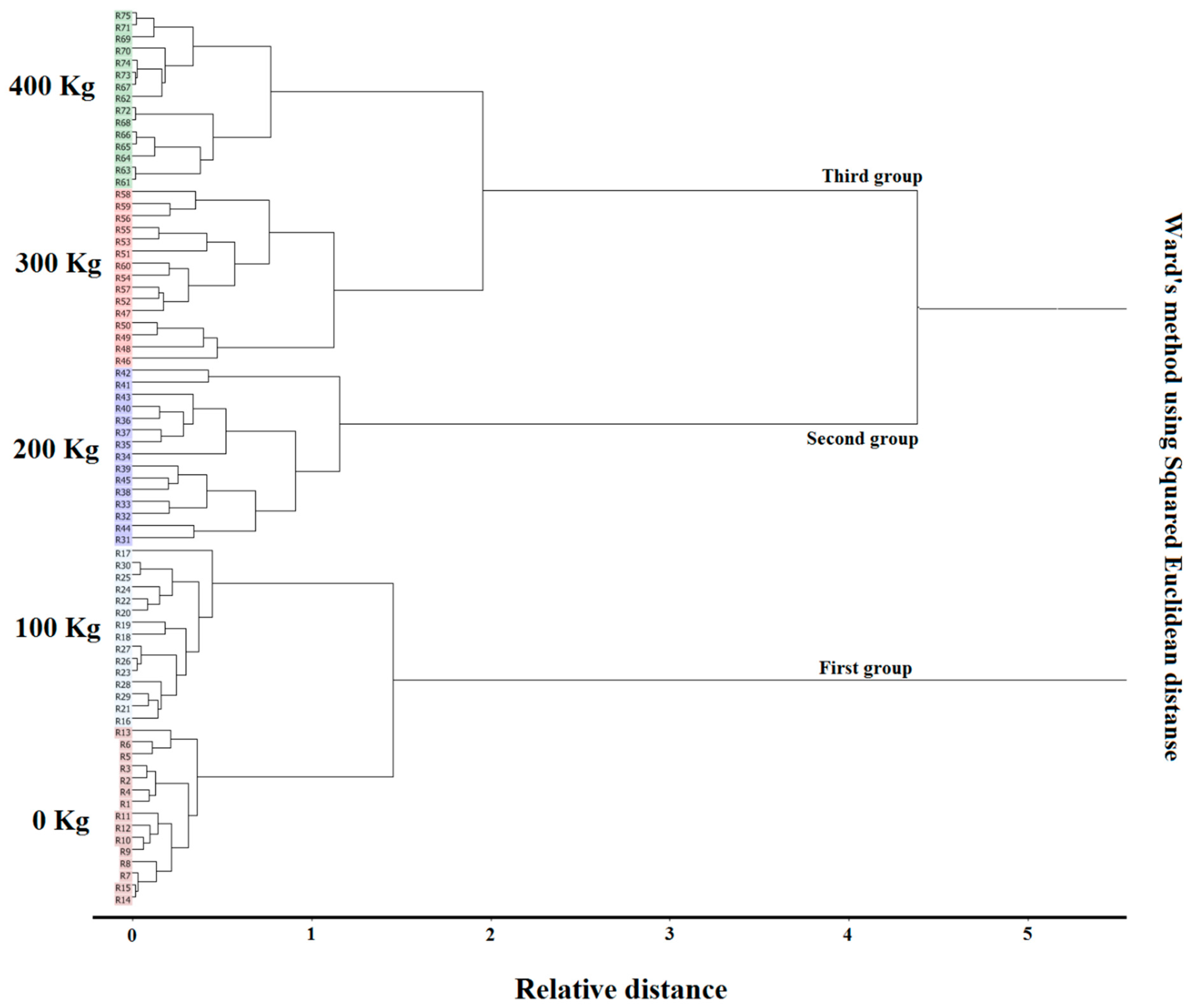

| Soil Sample Chemical and Structural Composition | Greenhouse Soil Parameters |
|---|---|
| Depth (cm) | 0–30 |
| Electrical Conductivity (EC × ) | 1.550 |
| Total Saturated Acidity (pH of paste) | 7.600 |
| Percentage Neutralizing Substances (T.N.V) | 25.000 |
| Permeability (cm/ha) | 1.000 |
| % FC | 37.000 |
| % PWP | 19.000 |
| Bulk density (g/) | 1.280 |
| % Sand | 25.000 |
| % Silt | 33.400 |
| % Clay | 41.600 |
| % Organic Carbon (O.C.) | 1.670 |
| Nitrogen (N mg/kg) | 0.167 |
| Phosphorus (P mg/kg) | 67.400 |
| Potassium (K mg/kg) | 1140.000 |
| Manganese (Mn mg/kg) | 7.200 |
| Iron (Fe mg/kg) | 8.980 |
| Zinc (Zn mg/kg) | 5.550 |
| Copper (Cu mg/kg) | 1.900 |
| Sources | Degrees of Freedom | Mean Square |
|---|---|---|
| Nitrogen | 4 | 0.078 ** |
| Error | 10 | 0.001 |
| Total | 14 | |
| Potassium | 4 | 0.435 ** |
| Error | 10 | 0.001 |
| Total | 14 | |
| Phosphorus | 4 | 0.035 ** |
| Error | 10 | 6.678 × 10−5 |
| Total | 14 |
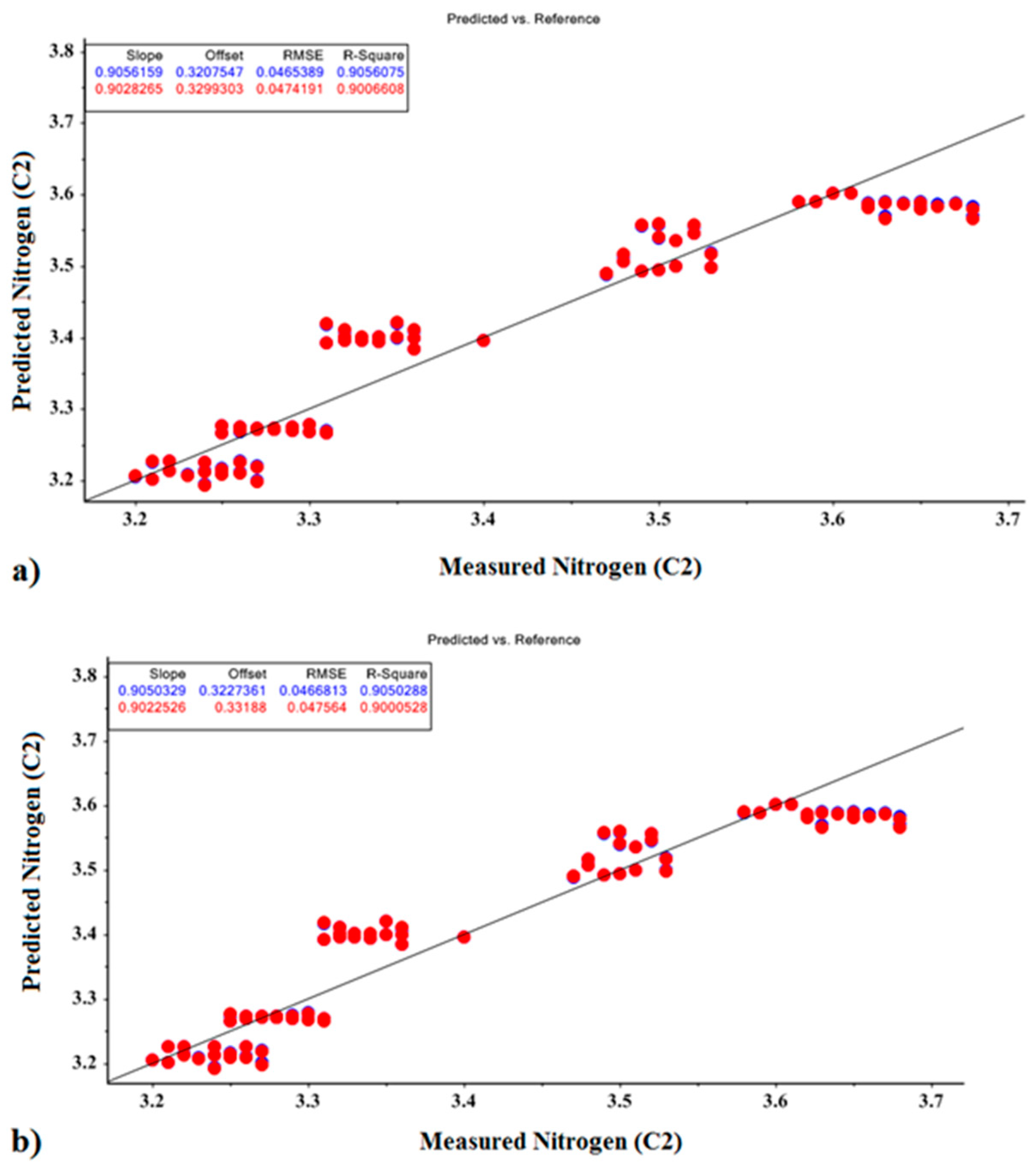
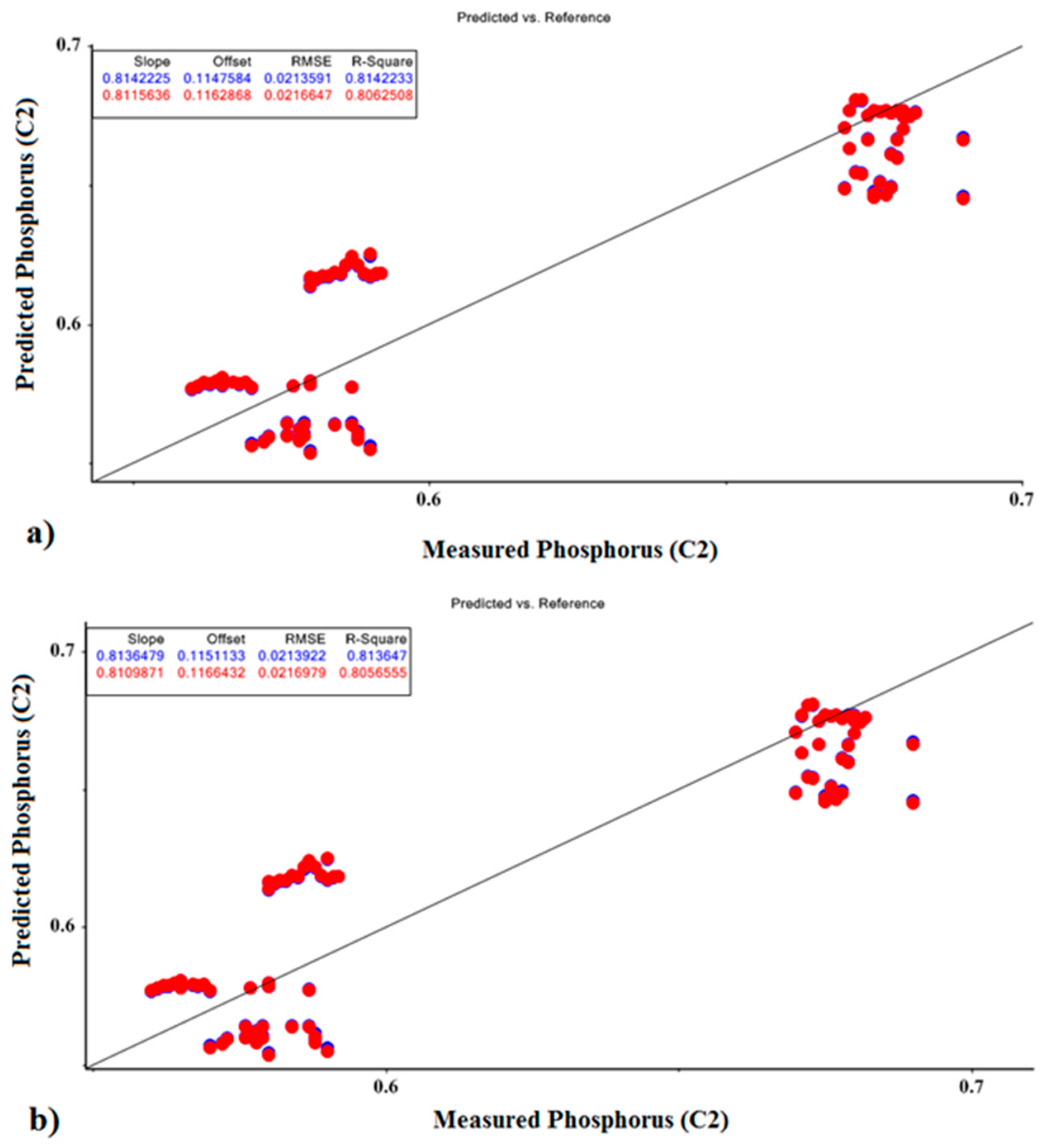
| Model | R2val | R2cal | RMSE-Val | RMSE-Cal | Offsetval | Offsetcal | Optimal Factor | |
|---|---|---|---|---|---|---|---|---|
| Nitrogen | PCR | 0.899 | 0.905 | 0.047 | 0.046 | 0.328 | 0.323 | 1 |
| PLSR | 0.905 | 0.906 | 0.047 | 0.046 | 0.334 | 0.322 | 1 | |
| Potassium | PCR | 0.832 | 0.850 | 0.142 | 0.132 | 0.780 | 0.706 | 4 |
| PLSR | 0.831 | 0.848 | 0.144 | 0.133 | 0.789 | 0.713 | 2 | |
| Phosphorus | PCR | 0.808 | 0.814 | 0.216 | 0.211 | 0.117 | 0.115 | 1 |
| PLSR | 0.806 | 0.814 | 0.217 | 0.210 | 0.119 | 0.115 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tatli, S.; Mirzaee-Ghaleh, E.; Rabbani, H.; Karami, H.; Wilson, A.D. Prediction of Residual NPK Levels in Crop Fruits by Electronic-Nose VOC Analysis following Application of Multiple Fertilizer Rates. Appl. Sci. 2022, 12, 11263. https://doi.org/10.3390/app122111263
Tatli S, Mirzaee-Ghaleh E, Rabbani H, Karami H, Wilson AD. Prediction of Residual NPK Levels in Crop Fruits by Electronic-Nose VOC Analysis following Application of Multiple Fertilizer Rates. Applied Sciences. 2022; 12(21):11263. https://doi.org/10.3390/app122111263
Chicago/Turabian StyleTatli, Sana, Esmaeil Mirzaee-Ghaleh, Hekmat Rabbani, Hamed Karami, and Alphus Dan Wilson. 2022. "Prediction of Residual NPK Levels in Crop Fruits by Electronic-Nose VOC Analysis following Application of Multiple Fertilizer Rates" Applied Sciences 12, no. 21: 11263. https://doi.org/10.3390/app122111263









