Characterization of Waxes in the Roman Wall Paintings of the Herculaneum Site (Italy)
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Instruments and Methods
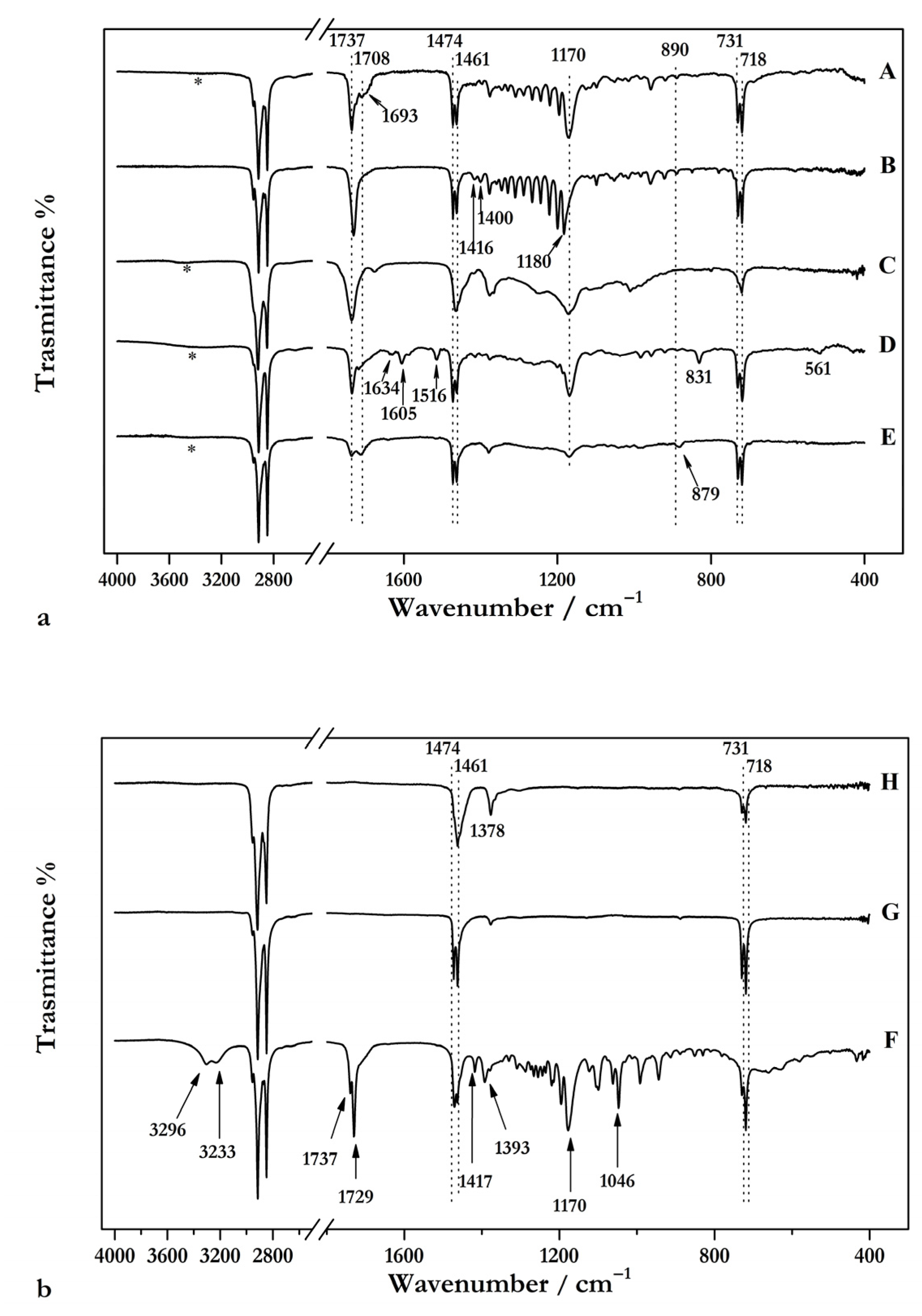
2.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.2. Micro-Raman Spectroscopy
2.2.3. Gas Chromatography–Mass Spectrometry (GC/MS)
3. Results and Discussion
3.1. Analyses of the Reference Wax Samples
3.1.1. FTIR and Raman Spectroscopies
3.1.2. GC/MS
3.2. Analyses of Archeological Samples
3.2.1. FTIR and Raman Spectroscopies
3.2.2. GC/MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pliny the Elder. The Elder Pliny’s Chapters on the History of Art, Eds and Trans; Jex-Blake, K., Sellers, E., Eds.; Ares Publishers: Chicago, IL, USA, 1977. [Google Scholar]
- Vitruvius; Morgan, M.H. (Eds.) De Architectura Libri Decem, II (Materials) and VII (Finishes and Colours); Kessinger Publishing: Whitefish, MT, USA, 2005. [Google Scholar]
- Cuní, J. What do we know of Roman wall painting technique? Potential confounding factors in ancient paint media analysis. Herit. Sci. 2016, 4, 44. [Google Scholar] [CrossRef] [Green Version]
- Mora, P.; Mora, L.; Philippot, P. La Conservazione Delle Pitture Murali; Bresciani, S.r.l., Ed.; Editrice Compositori: Bologna, Italy, 1999. [Google Scholar]
- Casoli, A. Research on the organic binders in archaeological wall paintings. Appl. Sci. 2021, 11, 9179. [Google Scholar] [CrossRef]
- Wagner, B.; Sabbatini, L.; Van der Werf, I.D. (Eds.) Chemical analysis in cultural heritage. Anal. Bioanal. Chem. 2020, 412, 7047–7048. [Google Scholar] [CrossRef]
- Casoli, A.; Musini, P.C.; Palla, G. Gas chromatographic-mass spectrometric approach to the problem of characterizing binding media in paintings. J. Chromatogr. A 1996, 731, 237–246. [Google Scholar] [CrossRef]
- Colombini, M.P.; Andreotti, A.; Bonaduce, I.; Modugno, F.; Ribechini, E. Analytical Strategies for Characterizing Organic Paint Media Using Gas Chromatography/Mass Spectrometry. Acc. Chem. Res. 2010, 43, 715–727. [Google Scholar] [CrossRef]
- Ribechini, E.; Modugno, F.; Colombini, M.P.; Evershed, R.P. Gas chromatographic and mass spectrometric investigations of organic residues from Roman glass unguentarla. J. Chromatog. A 2008, 1183, 158–169. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Bernal, J.L.; del Nozal, M.J.; Martín, M.T.; Bernal, J. Sample preparation methods for beeswax characterization by gaschromatography with flame ionization detection. J. Chromatog. A 2006, 1129, 262–272. [Google Scholar] [CrossRef]
- Tulloch, A.P. Beeswax-composition and analysis. Bee World 1980, 61, 47–62. [Google Scholar] [CrossRef]
- Mills, J.S.; White, R. The Organic Chemistry of Museum Objects; Butterworth Heinemann: Oxford, UK, 1994. [Google Scholar]
- Hunter, J. Observation on bees. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1792, 82, 128–196. [Google Scholar]
- Heron, C.; Nemcek, N.; Bonfield, K.; Dixon, D.; Ottaway, B.S. The chemistry of Neolithic beeswax. Naturwissenschaften 1994, 81, 266. [Google Scholar] [CrossRef]
- Evershed, R.P.; Vaughan, S.J.; Dudd, S.N.; Soles, J.S. Fuel for thought? Beeswax in lamps and conical cups from late Minoan Crete. Antiquity 1997, 71, 979–985. [Google Scholar] [CrossRef]
- Kakoulli, I. Late Classical and Hellenistic painting techniques and materials: A review of the technical literature. Stud. Conserv. 2002, 47, 56–67. [Google Scholar] [CrossRef]
- Buckley, S.A.; Clark, K.A.; Evershed, R.P. Complex organic chemical balms of Pharaonic animal mummies. Nature 2004, 431, 294. [Google Scholar] [CrossRef]
- Colombini, M.P.; Giachi, G.; Modugno, F.; Pallecchi, P.; Ribechini, E. The Characterization of paints and waterproofing materials from the shipwrecks found at the archeological site of the Etruscan and roman harbour of Pisa. Archaeometry 2003, 45, 659–674. [Google Scholar] [CrossRef]
- Pliny the Elder, Naturalis Historia, Liber XXXV, 122–150. Available online: https://la.wikisource.org/wiki/Naturalis_Historia/Liber_XXXV (accessed on 15 March 2022).
- Languri, G.M.; Boon, J. Between Myth and Reality: Mummy Pigment from The Hafkenscheid Collection. Stud. Conserv. 2005, 50, 161–178. [Google Scholar] [CrossRef]
- Miliani, C.; Daveri, A.; Spaabaek, L.; Romani, A.; Manuali, V.; Sgamellotti, A.; Brunetti, B.G. Bleaching of red lake paints in encaustic mummy portraits. Appl. Phys. A 2010, 100, 703–711. [Google Scholar] [CrossRef]
- Salvant, J.; Williams, J.; Ganio, M.; Casadio, F.; Daher, C.; Sutherland, K.; Monico, L.; Vanmeert, F.; De Meyer, S.; Janssens, K.; et al. A roman egyptian painting workshop: Technical investigation of the portraits from Tebtunis, Egypt. Archaeometry 2018, 60, 815–833. [Google Scholar] [CrossRef]
- Cuní, J.; Cuní, P.; Eisen, B.; Savizky, R.; Bové, J. Characterization of the binding medium used in Roman encaustic paintings on wall and wood. Anal. Methods 2012, 4, 659–669. [Google Scholar] [CrossRef]
- Casoli, A.; Santoro, S. Organic materials in the wall paintings in Pompei: A case study of Insula del Centenario. Chem. Cent. J. 2012, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- D′Alconzo, P. Picturae Excisae: Conservazione e Restauro Dei Dipinti ercolanesi e Pompeiani Tra il 18° e 19° Secolo; L’Erma di Bretschneider: Roma, Italy, 2002. [Google Scholar]
- Duran, A.; Jimenez De Haro, M.C.; Perez-Rodriguez, J.L.; Franquelo, M.L.; Herrera, L.K.; Justo, A. Determination of pigments and binders in Pompeian wall paintings using synchrotron Radiation—high-resolution x-ray powder diffraction and conventional spectroscopy—chromatography. Archaeometry 2010, 52, 286–307. [Google Scholar] [CrossRef]
- Amadori, M.L.; Barcelli, S.; Poldi, G.; Ferrucci, F.; Andreotti, A.; Baraldi, P.; Colombini, M.P. Invasive and non-invasive analyses for knowledge and conservation of Roman wall paintings of the Villa of the Papyri in Herculaneum. Microchem. J. 2015, 118, 183–192. [Google Scholar] [CrossRef]
- Chen, J.C.T.; Amar, A.P.; Levy, M.L.; Apuzzo, M.L.J. The development of anatomical art and sciences: The ceruplastica anatomical models of la Specola. Neurosurgery 1999, 45, 883–891. [Google Scholar] [CrossRef]
- Regert, M.; Langlois, J.; Colinart, S. Characterisation of wax works of art by gas chromatographic procedures. J. Chromatog. A 2005, 1091, 124–136. [Google Scholar] [CrossRef]
- Cole, L.J.; Brown, J.B. Chromatographic isolation of the original constituents of natural waxes. The composition of ouricuri wax. J. Am. Oil Chem. Soc. 1960, 37, 359–364. [Google Scholar] [CrossRef]
- Colombini, M.P.; Modugno, F. Organic Materials in Art and Archaeology; John Wiley and Sons, Ltd.: Chichester, UK, 2009; pp. 3–36. [Google Scholar]
- Elder, R.L. Final Report on the Safety Assessment of Candelilla Wax, Carnauba Wax, Japan Wax, and Beeswax. Int. J. Toxicol. 1984, 3, 1–41. [Google Scholar] [CrossRef]
- Horie, C.V. Materials for Conservation. Organic Consolidants, Adhesives and Coatings; Butterworth-Heinemann: London, UK, 1987. [Google Scholar] [CrossRef]
- Hodge, W.H.; Sineath, H.H. The Mexican candelilla plant and its wax. Econ. Bot. 1956, 10, 134–154. [Google Scholar] [CrossRef]
- Rojas-Molina, R.; De León-Zapata, M.A.; Saucedo-Pompa, S.; Aguilar-Gonzalez, M.A.; Aguilar, C.N. Chemical and structural characterization of Candelilla (Euphorbia antisyphilitica Zucc.). J. Med. Plant Res. 2013, 7, 702–705. [Google Scholar] [CrossRef]
- Béarat, H.; Fuchs, M.; Maggetti, M.; Paunier, D. (Eds.) Material, Techniques, Analysis and Conservation. In Proceedings of the International Workshop “Roman Wall Paintings”, Fribourg, Switzerland, 7–9 March 1996; Institute of Mineralogy and Petrography: Fribourg, Switzerland, 1997. [Google Scholar]
- Barbet, A. La Peinture Murale Romaine: Les Styles Décoratifs Pompéiens; Picard édit.: Paris, France, 1985. [Google Scholar]
- Mazurek, J.; Svoboda, M.; Schilling, M. GC/MS Characterization of Beeswax, Protein, Gum, Resin, and Oil in Romano-Egyptian Paintings. Heritage 2019, 2, 1960–1985. [Google Scholar] [CrossRef] [Green Version]
- Pronti, L.; Romani, M.; Viviani, G.; Stani, C.; Gioia, P.; Cestelli-Guidi, M. Advanced methods for the analysis of Roman wall paintings: Elemental and molecular detection by means of synchrotron FT-IR and SEM micro-imaging spectroscopy. Rend. Fis. Acc. Lincei 2020, 31, 485–493. [Google Scholar] [CrossRef]
- Evershed, R.P.; Ciliberto, E.; Spoto, G. (Eds.) Modern Analytical Methods in Art and Archaeology; Wiley-Interscience: New York, NY, USA, 2000; p. 177. [Google Scholar]
- Bergamonti, L.; Graiff, C.; Tegoni, M.; Predieri, G.; Bellot-Gurlet, L.; Lottici, P.P. Raman and NMR kinetics study of the formation of amidoamines containing N-hydroxyethyl groups and investigations on their Cu (II) complexes in water. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 515–524. [Google Scholar] [CrossRef]
- Patnaik, P. Dean’s Analytical Chemistry Handbook; McGraw-Hill Education: New York, NY, USA, 2004. [Google Scholar]
- Bucio, A.; Moreno-Tovar, R.; Bucio, L.; Espinosa-Dávila, J.; Anguebes-Franceschi, F. Characterization of beeswax, candelilla wax and paraffin wax for coating cheeses. Coatings 2021, 11, 261. [Google Scholar] [CrossRef]
- Gorce, J.P.; Spells, S.J. Structural information from progression bands in the FTIR spectra of long chain n-alkanes. Polymer 2002, 43, 4043–4046. [Google Scholar] [CrossRef]
- Stacey, R.J.; Dyer, J.; Mussell, C.; Lluveras-Tenorio, A.; Colombini, M.P.; Duce, C.; La Nasa, J.; Cantisani, E.; Prati, S.; Sciutto, G.; et al. Ancient encaustic: An experimental exploration of technology, ageing behaviour and approaches to analytical investigation. Microchem. J. 2018, 138, 472–487. [Google Scholar] [CrossRef]
- Invernizzi, C.; Rovetta, T.; Licchelli, M.; Malagodi, M. Mid and near-infrared reflection spectral database of natural organic materials in the cultural heritage field. Int. J. Anal. Chem. 2018, 16, 7823248. [Google Scholar] [CrossRef]
- Regert, M.; Colinart, S.; Degrand, L.; Decavallas, O. Chemical alteration and use of beeswax through time: Accelerated ageing tests and analysis of archaeological samples from various environmental contexts. Archaeometry 2001, 43, 549–569. [Google Scholar] [CrossRef]
- Lattuati-Derieux, A.; Egasse, C.; Regert, M.; Chung, Y.J.; Lavédrine, B. Characterization and degradation pathways of ancient Korean waxed papers. J. Cult. Herit. 2009, 10, 422–427. [Google Scholar] [CrossRef]
- Edwards, H.G.; Farwell, D.W.; Daffner, L. Fourier-transform Raman spectroscopic study of natural waxes and resins. I. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1996, 52, 1639–1648. [Google Scholar] [CrossRef]
- Nyquist, R.A. Interpreting Infrared, Raman, and Nuclear Magnetic Resonance Spectra; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Vandenabeele, P.; Wehling, B.; Moens, L.; Edwards, H.; De Reu, M.; Van Hooydonk, G. Analysis with micro-Raman spectroscopy of natural organic binding media and varnishes used in art. Anal. Chim. Acta 2000, 407, 261–274. [Google Scholar] [CrossRef]
- Asperger, A.; Engewald, W.; Fabian, G. Analytical characterization of natural waxes employing pyrolysis–gas chromatography–mass spectrometry. J. Anal. Appl. Pyrolysis 1999, 50, 103–115. [Google Scholar] [CrossRef]
- Andreotti, A.; Bonaduce, I.; Colombini, M.P.; Gautier, G.; Modugno, F.; Ribechini, E. Combined GC/MS Analytical Procedure for the Characterization of Glycerolipid, Waxy, Resinous, and Proteinaceous Materials in a Unique Paint Microsample. Anal. Chem. 2006, 78, 4490–4500. [Google Scholar] [CrossRef]
- Asperger, A.; Engewald, W.; Fabian, G. Thermally assisted hydrolysis and methylation–a simple and rapid online derivatization method for the gas chromatographic analysis of natural waxes. J. Anal. Appl. Pyrolysis 2001, 61, 91–109. [Google Scholar] [CrossRef]
- Was, E.; Szczesna, T.; Rybak-Chmielewska, H. Efficiency of GC-MS method in detection of beeswax adulterated with paraffin. J. Apic. Sci. 2016, 60, 145. [Google Scholar] [CrossRef] [Green Version]
- Špaldoňová, A.; Havelcová, M.; Lapčák, L.; Machovič, V.; Titěra, D. Analysis of beeswax adulteration with paraffin using GC/MS, FTIR-ATR and Raman spectroscopy. J. Apic. Res. 2021, 60, 73–83. [Google Scholar] [CrossRef]
- Bell, I.M.; Clark, R.J.H.; Gibbs, P.J.C. Raman spectroscopic library of natural and synthetic pigments (pre- ≈ 1850 AD). Spectrochim. Acta A Mol. Biomol. Spectrosc. 1997, 53, 2159–2179. [Google Scholar] [CrossRef]
- Price, B.; Pretzel, B.; Quillen Lomax, S. (Eds.) Infrared and Raman Users Group Spectral Database, 2007; IRUG: Philadelphia, PA, USA, 2009; Available online: www.irug.org (accessed on 20 June 2022).
- Bersani, D.; Lottici, P.P. Raman spectroscopy of minerals and mineral pigments in archaeometry. J. Raman Spectrosc. 2016, 47, 499–530. [Google Scholar] [CrossRef]
- Salvadori, B.; Errico, V.; Mauro, M.; Melnik, E.; Dei, L. Evaluation of gypsum and calcium oxalates in deteriorated mural paintings by quantitative FTIR spectroscopy. Spectrosc. Lett. 2003, 36, 501–513. [Google Scholar] [CrossRef]
- Fikri, I.; El Amraoui, M.; Haddad, M.; Ettahiri, A.S.; Falguères, C.; Bellot-Gurlet, L.; Lamhasni, T.; Lyazidi, S.A.; Bejjit, L. Raman and ATR-FTIR analyses of medieval wall paintings from al-Qarawiyyin in Fez (Morocco). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121557. [Google Scholar] [CrossRef]
- Mazzeo, R.; Prati, S.; Quaranta, M.; Joseph, E.; Kendix, E.; Galeotti, M. Attenuated total reflection micro FTIR characterisation of pigment–binder interaction in reconstructed paint films. Anal. Bioanal. Chem. 2008, 392, 65–76. [Google Scholar] [CrossRef]
- Bruni, S.; Cariati, F.; Casadio, F.; Toniolo, L. Spectrochemical characterization by micro-FTIR spectroscopy of blue pigments in different polychrome works of art. Vib. Spectrosc. 1999, 20, 15–25. [Google Scholar] [CrossRef]
- Brøns, C.; Rasmussen, K.L.; Melchiorre Di Crescenzo, M.; Stacey, R.; Lluveras-Tenorio, A. Painting the Palace of Apries I: Ancient binding media and coatings of the reliefs from the Palace of Apries, Lower Egypt. Herit. Sci. 2018, 6, 6. [Google Scholar] [CrossRef]
- Maia, M.; Barros, A.I.; Nunes, F.M. A novel, direct, reagent-free method for the detection of beeswax adulteration by single-reflection attenuated total reflectance mid-infrared spectroscopy. Talanta 2013, 107, 74–80. [Google Scholar] [CrossRef]
- Aliatis, I.; Bersani, D.; Campani, E.; Casoli, A.; Lottici, P.P.; Mantovan, S.; Marino, I.G. Pigments Used in Roman Wall Paintings in the Vesuvian Area. J. Raman Spectrosc. 2010, 41, 1537–1542. [Google Scholar] [CrossRef]
- Skovmøller, A.; Brøns, C.; Sargent, M.L. Egyptian blue: Modern myths, ancient realities. J. Rom. Archaeol. 2016, 29, 371–387. [Google Scholar] [CrossRef]
- Mazzocchin, G.A.; Minghelli, S.; Rudello, D. Pitture Parietali Romane ad Ercolano.Analisi Dei Pigmenti; Coralini, A., Ed.; Vesuviana. Archeologie a Confronto; Atti del Convegno Internazionale; Ante Quem Editoria: Bologna, Italy, 2009. [Google Scholar]
- Piqué, F.; MacDonald-Korth, E.; Rainer, L. Observations on Materials and Techniques Used in Roman Wall Paintings of the Tablinum, House of the Bicentenary at Herculaneum. In Beyond Iconography: Materials, Methods, and Meaning in Ancient Surface Decoration: Selected Papers on Ancient Art and Architecture; Lepinski, S., McFadden, S., Eds.; Archaeological Institute of America: Boston, MA, USA, 2015; Volume 1, pp. 57–76. [Google Scholar]
- Rainer, L.H.; Graves, K.; Maekawa, S.; Gittins, M.; Piqué, F. Conservation of the Architectural Surfaces in the Tablinum of the House of the Bicentenary, Herculaneum: Phase 1, Examination, Investigations, and Condition Assessment; Getty Conservation Institute: Los Angeles, CA, USA, 2017. [Google Scholar]
- Bersani, D.; Lottici, P.P.; Casoli, A. Case study: Micro-Raman and GC/MS of frescoes. In Raman Spectroscopy in Archaeology and Art History; Edwards, H.G.M., Chalmers, J.M., Eds.; RSC—Analytical Spectroscopy Monographs: London, UK, 2005; pp. 130–141. [Google Scholar]
- Sotiropoulou, S.; Sciutto, G.; Tenorio, A.L.; Mazurek, J.; Bonaduce, I.; Prati, S.; Mazzeo, R.; Schilling, M.; Colombini, M.P. Advanced analytical investigation on degradation markers in wall paintings. Microchem. J. 2018, 139, 278–294. [Google Scholar] [CrossRef] [Green Version]
- Cariati, F.; Rampazzi, L.; Toniolo, L.; Pozzi, A. Calcium Oxalate Films on Stone Surfaces: Experimental Assessment of the Chemical Formation. Stud. Conserv. 2000, 45, 180–188. [Google Scholar] [CrossRef]
- Peris-Vicente, J.; Adelantado, J.G.; Carbó, M.D.; Castro, R.M.; Reig, F.B. Characterization of waxes used in pictorial artworks according to their relative amount of fatty acids and hydrocarbons by gas chromatography. J. Chromatog. A 2006, 1101, 254–260. [Google Scholar] [CrossRef]
- Čížová, K.; Vizárová, K.; Ház, A.; Vykydalová, A.; Cibulková, Z.; Šimon, P. Study of the degradation of beeswax taken from a real artefact. J. Cult. Herit. 2019, 37, 103–112. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Bermejo, F.O. Detection of adulterated commercial Spanish beeswax. Food Chem. 2012, 132, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.J.; Bernal, J.L.; del Nozal, M.J.; Toribio, L.; Bernal, J. Detection of beeswax adulterations using concentration guide-values. Eur. J. Lipid Sci. Technol. 2007, 109, 682–690. [Google Scholar] [CrossRef]
- Gehad, B.; Aly, M.F.; Marey, H. Identification of the Byzantine encaustic mural painting in Egypt. Mediterr. Archaeol. Ar. 2015, 15, 243–256. [Google Scholar] [CrossRef]








| Sampling Site | Sampling Point | Sample Name, Description and Analyses |
|---|---|---|
| House of the Skeleton (Insula III) Oecus 10, north wall of triclinium, apse |  | AC 17 Green decoration GC/MS |
| House of the Skeleton (Insula III) Oecus 10, north wall of triclinium, apse |  | AC 18 Blue decoration FTIR and Raman spectroscopies |
| House of the Deer (Insula IV) Oecus 17, East wall, II panel, detail of painted decoration from the column |  | AC 20 White decoration on a red ground GC/MS |
| House of the Deer (Insula IV) Oecus 17, East wall, II-sn panel detail of painted decoration from the column |  | AC 21 Red decoration FTIR and Raman spectroscopies |
| House of the Deer (Insula IV) Oecus 16, East wall, bottom panel |  | AC 22 Blue paint FTIR and Raman spectroscopies |
| House of the Deer (Insula IV) Oecus 7, Tablinum, East wall II-sn, dark vertical band |  | AC 23 Red paint FTIR and Raman spectroscopies |
| House of the Double Atrium (Insula VI) Oecus 7, East wall |  | AC 25 Red decoration GC/MS |
| House of the Double Atrium (Insula VI) Oecus 7, East wall |  | AC 26 Red decoration FTIR and Raman spectroscopies |
| Peak | Identified Compound | Formula | Reference Waxes | Samples |
|---|---|---|---|---|
| 1 | Decanol (capric alcohol) | C10H22O | L | |
| 2 | Decanoic acid (caprylic acid) | C10H20O2 | CN, S, L | AC 17, AC 20, AC 25 |
| 3 | Dodecanol (lauryl alcohol) | C12H26O | S, L | |
| 4 | Dodecanoic acid (lauric acid) | C12H24O2 | B, L | AC 17, AC 20, AC 25 |
| 5 | Tetradecanol (myristyl alcohol) | C14H30O | B, S, L | AC 17, AC 20, AC 25 |
| 6 | Tetradecanoic acid (myristic acid) | C14H28O2 | B, L | AC 25 |
| 7 | Nonadecane | C19H40 | B | AC 17, AC 20 |
| 8 | Methyl hexadecanoate (methyl palmitate) | C17H34O2 | B, S, FC | AC 17, AC 20, AC 25 |
| 9 | Hexadecanol (cetyl alcohol, palmityl alcohol) | C16H34O | S, L, FC | |
| IS | Eicosane (internal standard) | C20H42 | ||
| 10 | Hexadecanoic acid (palmitic acid) | C16H34O2 | B, S, LCN, CB, FC | AC 20, AC 25 |
| 11 | Heneicosane | C21H44 | B, FC, MC, P | AC 17, AC 25 |
| 12 | Methyl octadecanoate (methyl stearate) | C19H38O2 | B, FC | AC 17, AC 25 |
| 13 | Octadecanol (stearyl alcohol) | C18H38O | B, S, L, FC | |
| 14 | Docosane | C22H46 | CN, FC, MC, P | AC 17, AC 20, AC 25 |
| 15 | Octadecenoic acid (oleic acid) | C18H34O2 | B, L | |
| 16 | Octadecanoic acid (stearic acid) | C18H36O2 | B, L, CN, CB, FC | AC 20, AC 25 |
| 17 | Tricosane | C23H48 | B, FC, MC, P | AC 20, AC 25 |
| 18 | Eicosanol (arachidyl alcohol) | C20H42O | B, L | AC 20, AC 25 |
| 19 | Tetracosane | C24H50 | FC, MC, P | AC 17, AC 20, AC 25 |
| 20 | Eicosenoic acid | C20H38O2 | B, L | |
| 21 | Eicosanoic acid (arachidic acid) | C20H40O2 | B, CN, CB | AC 17 |
| 22 | Pentacosane | C25H52 | B, FC, MC, P | AC 17, AC 20, AC 25 |
| 23 | Docosanol (behenyl alcohol) | C22H46O | B, L, CB, FC | AC 17, AC 20, AC 25 |
| 24 | Hexacosane | C26H54 | B, FC, MC, P | AC 17, AC 20, AC 25 |
| 25 | Docosanoic acid (behenic acid) | C22H44O2 | B, L, CN, CB | AC 17, AC 25 |
| 26 | Heptacosane | C27H56 | B, CB, FC, MC, P | AC 17, AC 20, AC 25 |
| 27 | Tetracosanol (lignoceryl alcohol) | C24H50O | B, CN, CB | AC 17, AC 20, AC 25 |
| 28 | Octacosane | C28H58 | B, CN, FC, MC, P | AC 17, AC 20, AC 25 |
| 29 | Tetracosanoic acid (lignoceric acid) | C24H48O2 | B, CN, CB | AC 17, AC 20, AC 25 |
| 30 | Nonacosene | C29H58 | B, CB | |
| 31 | Nonacosane | C29H60 | B, CN, CB, FC, MC, P | AC 17, AC 20, AC 25 |
| 32 | Hexacosanol (ceryl alcohol) | C26H54O | B, CN, CB | AC 17, AC 20, AC 25 |
| 33 | Triacontane | C30H62 | CN, FC, MC, P | AC 17, AC 20, AC 25 |
| 34 | Methyl hexacosanoate | C27H54O2 | B | AC 17, AC 20, AC 25 |
| 35 | Hexacosanoic acid (cerotic acid) | C26H52O2 | B, L, CB | |
| 36 | Eptacosanol | C27H56O | B, L | |
| 37 | Hentriacontene | C31H62 | B, CB, FC | |
| 38 | Hentriacontane | C31H64 | B, CN, CB, FC, MC, P | AC 17, AC 20, AC 25 |
| 39 | Octacosanol (montanyl alcohol) | C28H58O | B, L, CN, CB | AC 17, AC 20, AC 25 |
| 40 | Dotriacontane | C32H66 | B, CN, FC, MC, P | AC 17, AC 20, AC 25 |
| 41 | Methyl octacosanoate (cerotic acid methyl Ester) | C29H58O2 | B | AC 17, AC 20, AC 25 |
| 42 | Octacosanoic acid (montanic acid) | C28H56O2 | B, L, CN, CB | |
| 43 | Tritriacontene | C33H66 | B, CN, CB | AC 17, AC 20, AC 25 |
| 44 | Tritriacontane | C33H68 | B, CB, FC, MC, P | AC 17, AC 20, AC 25 |
| 45 | Triacontanol (myricyl alcohol) | C30H62O | B, L, CN, CB, FC | AC 20, AC 25 |
| 46 | Hexadecyl hexadecanoate (cetyl palmitate) | C32H64O2 | S, FC | |
| 47 | Methyl dotriacontanoate | C33H66O2 | CN | |
| 48 | Dotriacontanoic acid (lacceroic acid) | C32H64O2 | B, L | |
| 49 | Pentatriacontene | C35H70 | CN, CB | |
| 50 | Pentatriacontane | C35H72 | CB, FC, MC |
| Sample | Fatty Acids (FA) (wt %) | Alcohols (A) (wt %) | FA/A | Odd Alkanes (OA) (wt %) | Even Alkanes (EA) (wt %) | OA/EA |
|---|---|---|---|---|---|---|
| AC 17 | 2.98 | 8.61 | 0.35 | 30.84 | 57.61 | 0.54 |
| AC 20 | 1.26 | 3.28 | 0.38 | 35.87 | 59.58 | 0.60 |
| AC 25 | 10.01 | 4.65 | 2.16 | 33.47 | 51.89 | 0.65 |
| Beeswax | 10.43 | 25.87 | 0.40 | 62.69 | 1.58 | 60.74 |
| Spermaceti | 21.08 | 79.12 | 0.27 | - | - | |
| Carnauba | 55.51 | 37.54 | 1.48 | 6.62 | 0.33 | 20.06 |
| Candelilla | 15.04 | 14.09 | 1.07 | 70.08 | 0.79 | 88.23 |
| Lanoline | 41.75 | 58.25 | 0.72 | - | - | |
| F-Candelilla | 22.06 | 7.50 | 2.94 | 28.82 | 40.72 | 0.71 |
| Microcrystalline | 14.61 | - | - | 56.44 | 28.95 | 1.95 |
| Paraffin | - | - | - | 48.50 | 51.60 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergamonti, L.; Cirlini, M.; Graiff, C.; Lottici, P.P.; Palla, G.; Casoli, A. Characterization of Waxes in the Roman Wall Paintings of the Herculaneum Site (Italy). Appl. Sci. 2022, 12, 11264. https://doi.org/10.3390/app122111264
Bergamonti L, Cirlini M, Graiff C, Lottici PP, Palla G, Casoli A. Characterization of Waxes in the Roman Wall Paintings of the Herculaneum Site (Italy). Applied Sciences. 2022; 12(21):11264. https://doi.org/10.3390/app122111264
Chicago/Turabian StyleBergamonti, Laura, Martina Cirlini, Claudia Graiff, Pier Paolo Lottici, Gerardo Palla, and Antonella Casoli. 2022. "Characterization of Waxes in the Roman Wall Paintings of the Herculaneum Site (Italy)" Applied Sciences 12, no. 21: 11264. https://doi.org/10.3390/app122111264








