Skin Byproducts of Reinhardtius hippoglossoides (Greenland Halibut) as Ecosustainable Source of Marine Collagen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Marine Byproducts
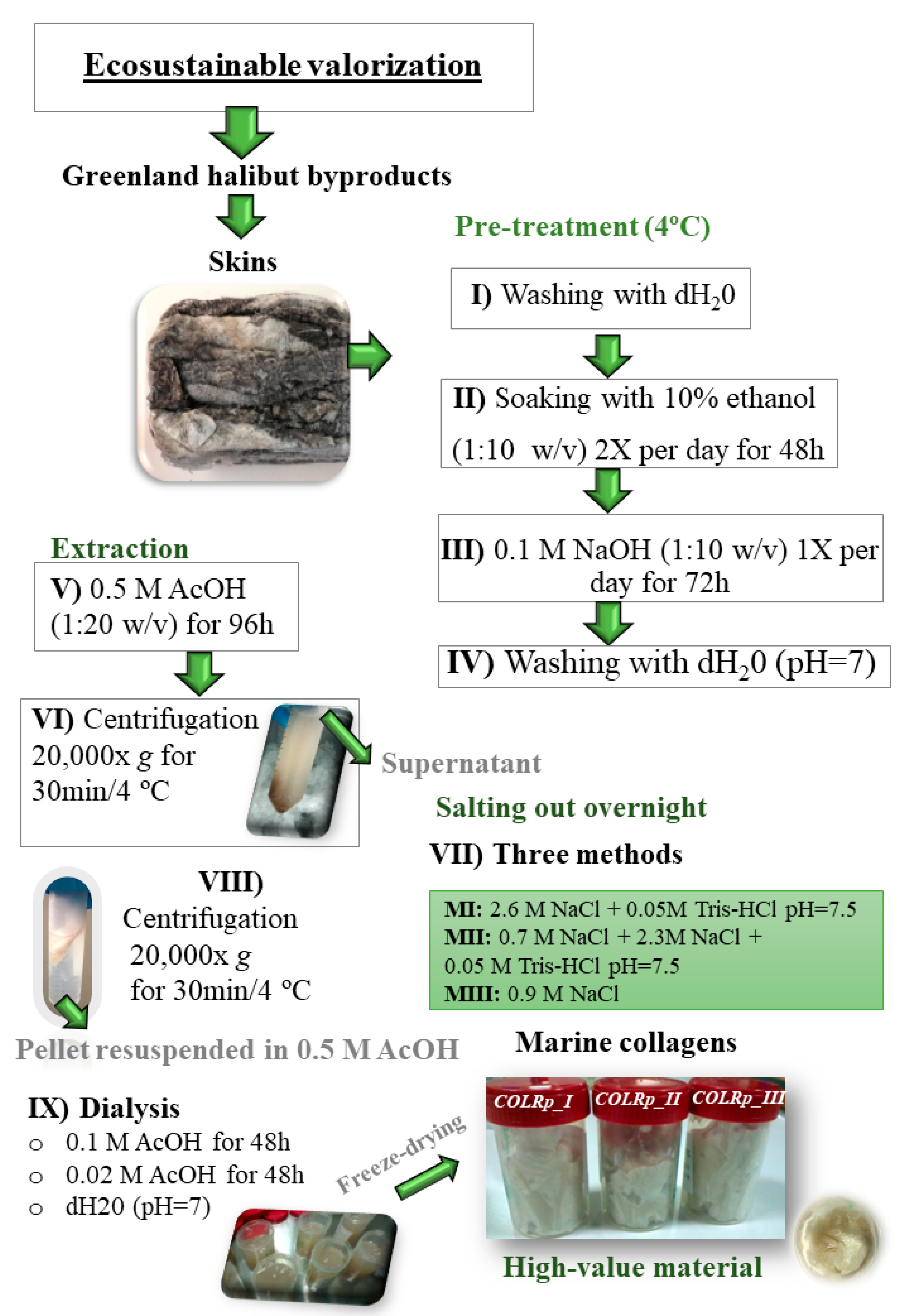
2.2. Collagen Extraction from Fish Skins
2.3. Characterization of Collagen Protein
2.3.1. SDS-PAGE gel Electrophoresis
2.3.2. Circular Dichroism
2.3.3. FTIR Measurement
2.3.4. Rheology
2.3.5. Amino Acid Analysis
2.3.6. Micro-Differential Scanning Calorimetry
2.3.7. Moisture Regain
2.3.8. SEM and EDS Analyses
2.3.9. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Yield and SDS-PAGE Pattern
3.2. Circular Dichroism
3.3. FTIR Measurement
3.4. Rheology
3.5. Amino Acid Analysis
3.6. Micro-Differential Scanning Calorimetry
3.7. Moisture Regain
3.8. SEM and EDS Analyses
3.9. Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sipilä, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Käpylä, J.; Myllyharju, J.; Heino, J. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drugs 2019, 17, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratzl, P. Collagen: Structure and mechanics, an introduction. In Collagen; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1–13. [Google Scholar]
- Sorushanova, A.; Skoufos, I.; Tzora, A.; Mullen, A.M.; Zeugolis, D.I. The influence of animal species, gender and tissue on the structural, biophysical, biochemical and biological properties of collagen sponges. J. Mater. Sci. Mater. Med. 2021, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Heino, J. The collagen family members as cell adhesion proteins. Bioessays 2007, 29, 1001–1010. [Google Scholar] [CrossRef]
- Haug, I.J.; Draget, K.I.; Smidsrød, O. Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll. 2004, 18, 203–213. [Google Scholar] [CrossRef]
- Liu, D.; Liang, L.; Regenstein, J.M.; Zhou, P. Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem. 2012, 133, 1441–1448. [Google Scholar] [CrossRef]
- Ehrlich, H.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T.J. Collagens of poriferan origin. Mar. Drugs 2018, 16, 79. [Google Scholar] [CrossRef] [Green Version]
- Uzel, S.G.; Buehler, M.J. Nanomechanical sequencing of collagen: Tropocollagen features heterogeneous elastic properties at the nanoscale. Integr. Biol. 2009, 1, 452–459. [Google Scholar] [CrossRef]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E.J.; et al. Natural collagenic skeleton of marine sponges in pharmaceutics: Innovative biomaterial for topical drug delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 710–720. [Google Scholar] [CrossRef]
- Le, T.M.T.; Nguyen, V.M.; Tran, T.T.; Takahashi, K.; Osako, K.J. Comparison of acid-soluble collagen characteristic from three important freshwater fish skins in Mekong Delta Region, Vietnam. J. Food Biochem. 2020, 44, e13397. [Google Scholar] [CrossRef]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H.J.C. Biodiversity. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M.J. Production, characterization and biocompatibility evaluation of collagen membranes derived from marine sponge Chondrosia reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine collagen from alternative and sustainable sources: Extraction, processing and applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R.J. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 5183. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Nirmal, N.P.; Chuprom, J. Molecular characteristics of collagen extracted from the starry triggerfish skin and its potential in the development of biodegradable packaging film. Rsc. Adv. 2016, 6, 33868–33879. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic potential of marine fish skin collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Polymer Edition. Acid and enzymatic extraction of collagen from Atlantic cod (Gadus Morhua) swim bladders envisaging health-related applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef]
- Kaewdang, O.; Benjakul, S.; Kaewmanee, T.; Kishimura, H.J. Characteristics of collagens from the swim bladders of yellowfin tuna (Thunnus albacares). Food Chem. 2014, 155, 264–270. [Google Scholar] [CrossRef]
- Nagai, T.; Nagamori, K.; Yamashita, E.; Suzuki, N. Collagen of octopus Callistoctopus arakawai arm. Int. J. Food Sci. Technol. 2002, 37, 285–289. [Google Scholar] [CrossRef]
- Seixas, M.J.; Martins, E.; Reis, R.L.; Silva, T.H. Extraction and Characterization of Collagen from Elasmobranch Byproducts for Potential Biomaterial Use. Mar. Drugs 2020, 18, 617. [Google Scholar] [CrossRef] [PubMed]
- Khong, N.M.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Armania, N.; Nishikawa, J. Improved collagen extraction from jellyfish (Acromitus hardenbergi) with increased physical-induced solubilization processes. Food Chem. 2018, 251, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Belouafa, S.; Bourja, L.; Villain, S.; Tayane, S.; Bennamara, A.; Abourriche, A.J.; Cities, D.A. Biocomposite Based on Collagen/Calcium Salts Extraction from Sardine Scales. In Proceedings of the 2nd International Conference on Smart Applications and Data Analysis for Smart Cities, Casablanca, Morocco, 27–28 February 2018. [Google Scholar]
- Wang, L.; Liang, Q.; Chen, T.; Wang, Z.; Xu, J.; Ma, H. Characterization of collagen from the skin of Amur sturgeon (Acipenser schrenckii). Food Hydrocoll. 2014, 38, 104–109. [Google Scholar] [CrossRef]
- Bae, I.; Osatomi, K.; Yoshida, A.; Osako, K.; Yamaguchi, A.; Hara, K.J. Biochemical properties of acid-soluble collagens extracted from the skins of underutilised fishes. J. Food Biochem. 2008, 108, 49–54. [Google Scholar] [CrossRef]
- Ben Slimane, E.; Sadok, S. Collagen from cartilaginous fish by-products for a potential application in bioactive film composite. Mar. Drugs 2018, 16, 211. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Kim, S.Y.; Chun, T.; Byun, H.-J.; Lee, Y.M. Collagen scaffolds derived from a marine source and their biocompatibility. Biomaterials 2006, 27, 2951–2961. [Google Scholar] [CrossRef]
- Zhang, J.; Elango, J.; Wang, S.; Hou, C.; Miao, M.; Li, J.; Na, L.; Wu, W. Characterization of Immunogenicity Associated with the Biocompatibility of Type I Collagen from Tilapia Fish Skin. Polymers 2022, 14, 2300. [Google Scholar] [CrossRef]
- Gharehgheshlagh, S.N.; Fatemi, M.J.; Jamili, S.; Nourani, M.R.; Sharifi, A.M.; Saberi, M.; Amini, N.; Ganji, F.J. Therapeutics. A Dermal Gel Made of Rutilus Kutum Skin Collagen-Chitosan for Deep Burn Healing. Int. J. Pept. Res. Ther. 2020, 27, 317–328. [Google Scholar] [CrossRef]
- Avila Rodríguez, M.I.; Rodríguez Barroso, L.G.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2018, 17, 20–26. [Google Scholar] [CrossRef]
- Silvipriya, K.; Kumar, K.K.; Bhat, A.; Kumar, B.D.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. App. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Stoichevska, V.; Vashi, A.; Howell, L.; Fehr, F.; Dumsday, G.; Werkmeister, J.; Ramshaw, J. Non–animal collagens as new options for cosmetic formulation. Int. J. Cosmet. Sci. 2015, 37, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.; Paul, B.; Gelinsky, M.J. Biphasic scaffolds from marine collagens for regeneration of osteochondral defects. Mar. Drugs 2018, 16, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, biomedical and pharmaceutical applications of fish gelatin/hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, S.; Nadda, A.K.; Husain, M.S.B.; Gupta, A. Biopolymers from waste biomass and its applications in the cosmetic industry: A review. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Senaratne, L.; Park, P.-J.; Kim, S.-K. Isolation and characterization of collagen from brown backed toadfish (Lagocephalus gloveri) skin. Bioresour. Technol. 2006, 97, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive assessment of Nile tilapia skin (Oreochromis niloticus) collagen hydrogels for wound dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geahchan, S.; Baharlouei, P.; Rahman, A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar. Drugs 2022, 20, 61. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B.J. Effects of collagen and collagen hydrolysate from jellyfish (Rhopilema esculentum) on mice skin photoaging induced by UV irradiation. J. Food Sci. 2009, 74, H183–H188. [Google Scholar] [CrossRef]
- Shibuya, S.; Ozawa, Y.; Toda, T.; Watanabe, K.; Tometsuka, C.; Ogura, T.; Koyama, Y.; Shimizu, T.J.B. Biotechnology Biochemistry. Collagen peptide and vitamin C additively attenuate age-related skin atrophy in Sod1-deficient mice. Biosci. Biotechnol. Biochem. 2014, 78, 1212–1220. [Google Scholar] [CrossRef]
- Heidari, M.G.; Rezaei, M. Extracted pepsin of trout waste and ultrasound-promoted method for green recovery of fish collagen. Sustain. Chem. Pharm. 2022, 30, 100854. [Google Scholar] [CrossRef]
- Xhauflaire-Uhoda, E.; Fontaine, K.; Pierard, G.E. Kinetics of moisturizing and firming effects of cosmetic formulations. Int. J. Cosmet. Sci. 2008, 30, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M.S. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine collagen and its derivatives: Versatile and sustainable bio-resources for healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Lin, P.; Alexander, R.A.; Liang, C.H.; Liu, C.; Lin, Y.H.; Lin, Y.H.; Chan, L.P.; Kuan, C.M. Collagen formula with Djulis for improvement of skin hydration, brightness, texture, crow’s feet, and collagen content: A double-blind, randomized, placebo-controlled trial. J. Cosmet. Dermatol. 2020, 20, 188–194. [Google Scholar] [CrossRef]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Collagen extraction optimization from the skin of the small-spotted catshark (S. canicula) by response surface methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef] [Green Version]
- Zaelani, B.; Safithri, M.; Tarman, K.; Setyaningsih, I. Collagen isolation with acid soluble method from the skin of Red Snapper (lutjanus sp.). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012033. [Google Scholar]
- Ferrario, C.; Rusconi, F.; Pulaj, A.; Macchi, R.; Landini, P.; Paroni, M.; Colombo, G.; Martinello, T.; Melotti, L.; Gomiero, C.; et al. From Food Waste to Innovative Biomaterial: Sea Urchin-Derived Collagen for Applications in Skin Regenerative Medicine. Mar. Drugs 2020, 18, 414. [Google Scholar] [CrossRef]
- Liao, W.; Guanghua, X.; Li, Y.; Shen, X.R.; Li, C. Comparison of characteristics and fibril-forming ability of skin collagen from barramundi (Lates calcarifer) and tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2018, 107, 549–559. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Qiao, Y.; Tian, Y.; Liu, J.; Qin, S.; Wu, W. Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H.J.F. Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Drzewiecki, K.E.; Grisham, D.R.; Parmar, A.S.; Nanda, V.; Shreiber, D.I. Circular dichroism spectroscopy of collagen fibrillogenesis: A new use for an old technique. Biophys. J. 2016, 111, 2377–2386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menezes, M.d.L.L.R.; Ribeiro, H.L.; de Oliveira, M.F.; de Andrade Feitosa, J.P.; Biointerfaces, S.B. Optimization of the collagen extraction from Nile tilapia skin (Oreochromis niloticus) and its hydrogel with hyaluronic acid. Colloids Surf. B Biointerfaces 2020, 189, 110852. [Google Scholar] [CrossRef]
- Kozlowska, J.; Sionkowska, A.; Skopinska-Wisniewska, J.; Piechowicz, K. Northern pike (Esox lucius) collagen: Extraction, characterization and potential application. Int. J. Biol. Macromol. 2015, 81, 220–227. [Google Scholar] [CrossRef]
- Atef, M.; Ojagh, S.M.; Latifi, A.M.; Esmaeili, M.; Udenigwe, C.C. Biochemical and structural characterization of sturgeon fish skin collagen (Huso huso). J. Food Biochem. 2020, 44, e13256. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Li, Y.; Yang, Z.; Jin, H. Physicochemical Properties of Collagen from Acaudina Molpadioides and Its Protective Effects against H2O2-Induced Injury in RAW264. 7 Cells. Mar. Drugs 2020, 18, 370. [Google Scholar] [CrossRef] [PubMed]
- Veeruraj, A.; Arumugam, M.; Ajithkumar, T.; Balasubramanian, T. Isolation and characterization of collagen from the outer skin of squid (Doryteuthis singhalensis). Food Hydrocoll. 2015, 43, 708–716. [Google Scholar] [CrossRef]
- Zanaboni, G.; Rossi, A.; Onana, A.M.T.; Tenni, R. Stability and networks of hydrogen bonds of the collagen triple helical structure: Influence of pH and chaotropic nature of three anions. Matrix Biol. 2000, 19, 511–520. [Google Scholar] [CrossRef]
- Wang, L.; An, X.; Xin, Z.; Zhao, L.; Hu, Q. Isolation and characterization of collagen from the skin of deep-sea redfish (Sebastes mentella). J. Food Sci. 2007, 72, E450–E455. [Google Scholar] [CrossRef]
- Wu, J.; Kong, L.; Zhang, J.; Chen, W. Extraction and properties of acid-soluble collagen and pepsin-soluble collagen from silver carp (Hypophthalmichthys molitrix) scales: Prerequisite information for fishery processing waste reuse. Int. J. Biol. Macromol. 2019, 28, 2923–2930. [Google Scholar] [CrossRef]
- Leuenberger, B.H. Investigation of viscosity and gelation properties of different mammalian and fish gelatins. Food Hydrocoll. 1991, 5, 353–361. [Google Scholar] [CrossRef]
- Sousa, R.O.; Martins, E.; Carvalho, D.N.; Alves, A.L.; Oliveira, C.; Duarte, A.R.C.; Silva, T.H.; Reis, R.L. Collagen from Atlantic cod (Gadus morhua) skins extracted using CO2 acidified water with potential application in healthcare. J. Polym. Res. 2020, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K.J. Properties of collagen from skin, scale and bone of carp (Cyprinus carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Liu, W.; Tian, Z.; Li, C.; Li, G. Thermal denaturation of fish collagen in solution: A calorimetric and kinetic analysis. Thermochim. Acta 2014, 581, 32–40. [Google Scholar] [CrossRef]
- Tan, Z.-J.; Chen, S.-J. Nucleic acid helix stability: Effects of salt concentration, cation valence and size, and chain length. Biophys. J. 2006, 90, 1175–1190. [Google Scholar] [CrossRef] [Green Version]
- Komsa-Penkova, R.; Koynova, R.; Kostov, G.; Tenchov, B.G. Thermal stability of calf skin collagen type I in salt solutions. Biochim. Et Biophys. Acta (BBA)—Protein Struct. Mol. Enzymol. 1996, 1297, 171–181. [Google Scholar] [CrossRef]
- Bianchi, E.; Conio, G.; Ciferri, A.; Puett, D.; Rajagh, L. The role of pH, temperature, salt type, and salt concentration on the stability of the crystalline, helical, and randomly coiled forms of collagen. J. Biol. Chem. 1967, 242, 1361–1369. [Google Scholar] [CrossRef]
- Duan, L.; Li, J.; Li, C.; Li, G. Effects of NaCl on the rheological behavior of collagen solution. Korea-Aust. Rheol. J. 2013, 25, 137–144. [Google Scholar] [CrossRef]
- Kim, H.; Ro, J.; Barua, S.; Hwang, D.S.; Na, S.-J.; Lee, H.S.; Jeong, J.H.; Woo, S.; Kim, H.; Hong, B.J.; et al. Combined skin moisturization of liposomal serine incorporated in hydrogels prepared with carbopol ETD 2020, rhesperse RM 100 and hyaluronic acid. Korean J. Physiol. Pharmacol. 2015, 19, 543–547. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Park, J.W. Characterization of acid-soluble collagen from pacific whiting surimi processing byproducts. J. Food Sci. 2004, 69, C637–C642. [Google Scholar] [CrossRef]
- Safandowska, M.; Pietrucha, K. Effect of fish collagen modification on its thermal and rheological properties. Int. J. Biol. Macromol. 2013, 53, 32–37. [Google Scholar] [CrossRef]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A.J. Technology. Marine collagen: An emerging player in biomedical applications. J. Food Sci. Technol. 2015, 52, 4703–4707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pallela, R.; Venkatesan, J.; Janapala, V.R.; Kim, S.K. Biophysicochemical evaluation of chitosan-hydroxyapatite-marine sponge collagen composite for bone tissue engineering. J. Biomed. Mater. Res. A 2012, 100, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Kadler, K.E.; Holmes, D.F.; Trotter, J.A.; Chapman, J.A. Collagen fibril formation. Biochem. J. 1996, 316, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pal, G.K.; Suresh, P. Comparative assessment of physico-chemical characteristics and fibril formation capacity of thermostable carp scales collagen. Mater. Sci. Eng. C 2017, 70, 32–40. [Google Scholar] [CrossRef]
- Bae, I.; Osatomi, K.; Yoshida, A.; Yamaguchi, A.; Tachibana, K.; Oda, T.; Hara, K. Characteristics of a self-assembled fibrillar gel prepared from red stingray collagen. Fish Sci. 2009, 75, 765–770. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Glattauer, V.; Werkmeister, J.A.; Ramshaw, J.A. Evaluation for collagen products for cosmetic application. Int. J. Cosmet. Sci. 2004, 26, 313. [Google Scholar] [CrossRef]



| Chemical Elements (ppm) | COLRp_I | COLRp_II | COLRp_III | Reference Value |
|---|---|---|---|---|
| Ag | 0.01 | 0.1 | 0.12 | 15 |
| Al | 0.04 | 0 | 0.01 | 500 |
| As | 0.60 | 1.2 | 0.72 | 1.5 |
| Au | 0.07 | 0 | 0.08 | 10 |
| Ca | 0 | 0 | 0 | - |
| Cd | 0.02 | 0 | 0 | 0.5 |
| Fe | 0 | 0 | 0 | 150 |
| K | 0 | 0 | 0 | - |
| Mg | 0 | 0 | 0 | under deliberation |
| Mn | 0 | 0 | 0 | 70 |
| Na | 0 | 0 | 0 | - |
| P | 0.29 | 0.37 | 0.49 | - |
| Pb | 0.08 | 0 | 0.32 | 0.5 |
| Sr | 0 | 0 | 0 | 300 |
| Zn | 0 | 0 | 0 | 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, E.; Fernandes, R.; Alves, A.L.; Sousa, R.O.; Reis, R.L.; Silva, T.H. Skin Byproducts of Reinhardtius hippoglossoides (Greenland Halibut) as Ecosustainable Source of Marine Collagen. Appl. Sci. 2022, 12, 11282. https://doi.org/10.3390/app122111282
Martins E, Fernandes R, Alves AL, Sousa RO, Reis RL, Silva TH. Skin Byproducts of Reinhardtius hippoglossoides (Greenland Halibut) as Ecosustainable Source of Marine Collagen. Applied Sciences. 2022; 12(21):11282. https://doi.org/10.3390/app122111282
Chicago/Turabian StyleMartins, Eva, Rita Fernandes, Ana L. Alves, Rita O. Sousa, Rui L. Reis, and Tiago H. Silva. 2022. "Skin Byproducts of Reinhardtius hippoglossoides (Greenland Halibut) as Ecosustainable Source of Marine Collagen" Applied Sciences 12, no. 21: 11282. https://doi.org/10.3390/app122111282
APA StyleMartins, E., Fernandes, R., Alves, A. L., Sousa, R. O., Reis, R. L., & Silva, T. H. (2022). Skin Byproducts of Reinhardtius hippoglossoides (Greenland Halibut) as Ecosustainable Source of Marine Collagen. Applied Sciences, 12(21), 11282. https://doi.org/10.3390/app122111282








