Relationships between Body Weight Support and Gait Speed Parameters and Muscle Activity and Torque during Robot-Assisted Gait Training in Non-Neurological Adults: A Preliminary Investigation
Abstract
:1. Introduction
2. Methods
2.1. Gait Rehabilitation Robot
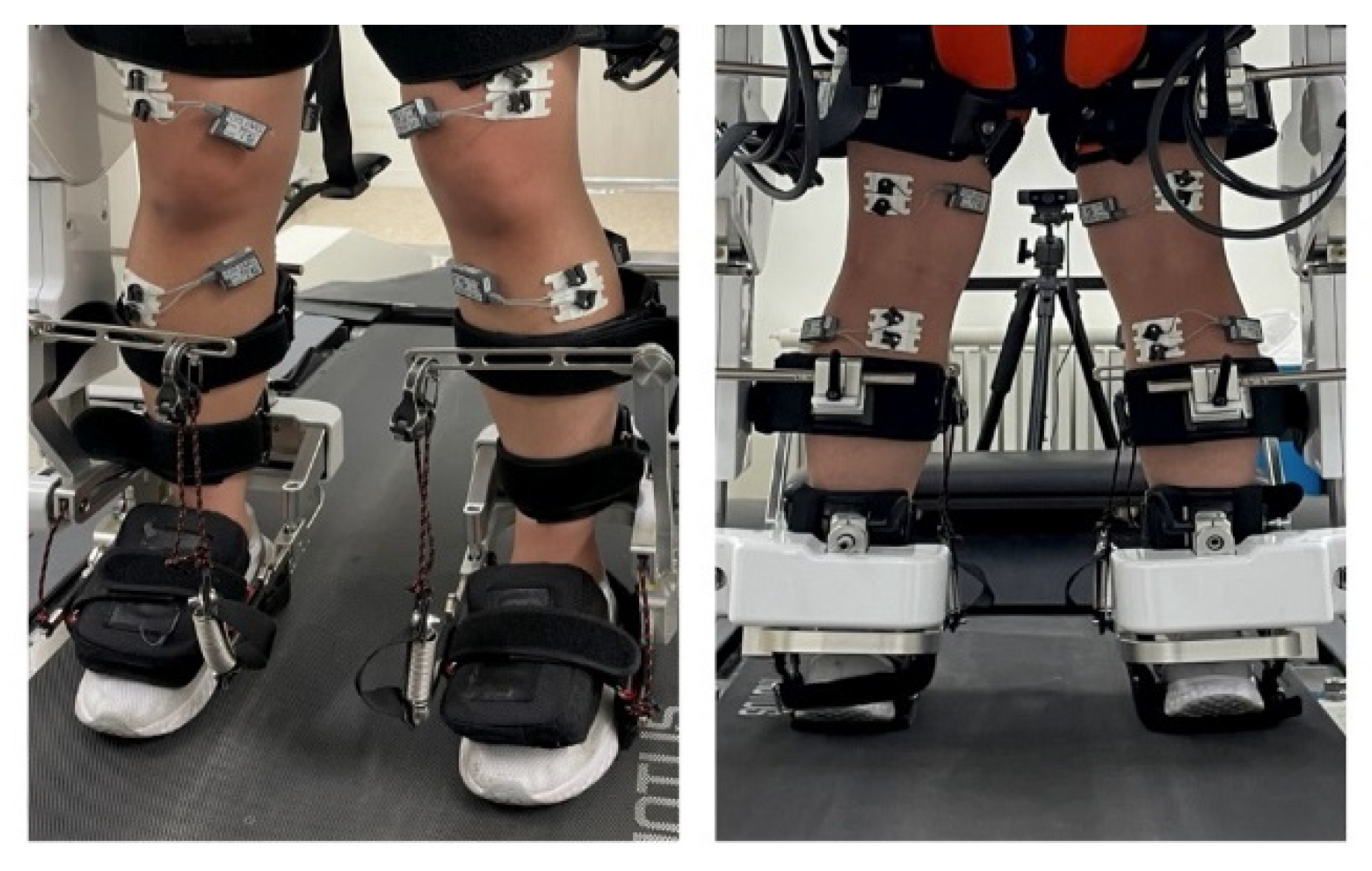
2.2. Surface Electromyography (EMG)
2.3. Statistical Analysis
2.4. Participants
2.5. Experiment Setup
2.6. Experiment Procedure
2.7. Data Collection and Processing
2.8. Correlation Analysis by EMG Window Criteria for Muscle Activity
- Thigh
- WINDOW_VLO: Mean activity of the VLO in the EMG activation window (0–23% and 95–100%)
- WINDOW_BF: Mean activity of BF in the EMG activation window (0–19% and 82–100%)
- Lower leg
- WINDOW_TA: Mean activity of the TA in the EMG activation window (0–16%, 67–85%, and 95–100%)
- WINDOW_GCM: Mean activity of the GCM in the EMG activation window (10–58%)
3. Results
Relationship between EMG and Torque Data during Different RAGT Gait Speed and BWS Conditions
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swinnen, E.; Duerinck, S.; Kerckhofs, E. Effectiveness of robot-assisted gait training in persons with spinal cord injury: A systematic review. In Rehabilitation: Mobility, Exercise and Sports; IOS Press: Amsterdam, The Netherlands, 2010; pp. 279–281. [Google Scholar]
- Morone, G.; Paolucci, S.; Cherubini, A.; De Angelis, D.; Venturiero, V.; Coiro, P.; Iosa, M. Robot-assisted gait training for stroke patients: Current state of the art and perspectives of robotics. Neuropsychiatr. Dis. Treat. 2017, 13, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, I.; Sajin, A.; Moreh, E.; Fisher, I.; Neeb, M.; Forest, A.; Vaknin-Dembinsky, A.; Karusis, D.; Meiner, Z. Robot-assisted gait training in multiple sclerosis patients: A randomized trial. Mult. Scler. J. 2012, 18, 881–890. [Google Scholar] [CrossRef]
- Hilderley, A.J.; Fehlings, D.; Lee, G.W.; Wright, F.V. Comparison of a robotic-assisted gait training program with a program of functional gait training for children with cerebral palsy: Design and methods of a two group randomized controlled cross-over trial. Springerplus 2016, 5, 1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Kammen, K.; Boonstra, A.M.; van der Woude, L.H.; Reinders-Messelink, H.A.; den Otter, R. The combined effects of guidance force, bodyweight support and gait speed on muscle activity during able-bodied walking in the Lokomat. Clin. Biomech. 2016, 36, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Van Kammen, K.; Boonstra, A.; Reinders-Messelink, H.; den Otter, R. The combined effects of body weight support and gait speed on gait related muscle activity: A comparison between walking in the Lokomat exoskeleton and regular treadmill walking. PLoS ONE 2014, 9, e107323. [Google Scholar] [CrossRef]
- Cramer, S.C.; Riley, J.D. Neuroplasticity and brain repair after stroke. Curr. Opin. Neurol. 2008, 21, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Riener, R.; Lunenburger, L.; Jezernik, S.; Anderschitz, M.; Colombo, G.; Dietz, V. Patient-cooperative strategies for robot-aided treadmill training: First experimental results. IEEE Trans. Neural Syst. Rehabil. Eng. 2005, 13, 380–394. [Google Scholar] [CrossRef]
- Riener, R.; Lünenburger, L.; Maier, I.C.; Colombo, G.; Dietz, V. Locomotor training in subjects with sensori-motor deficits: An overview of the robotic gait orthosis lokomat. J. Healthc. Eng. 2010, 1, 197–216. [Google Scholar] [CrossRef] [Green Version]
- Colombo, G.; Joerg, M.; Schreier, R.; Dietz, V. Treadmill training of paraplegic patients using a robotic orthosis. J. Rehabil. Res. Dev. 2000, 37, 693–700. [Google Scholar]
- Wolpert, D.M.; Ghahramani, Z.; Flanagan, J.R. Perspectives and problems in motor learning. Trends Cogn. Sci. 2001, 5, 487–494. [Google Scholar] [CrossRef]
- Van Tran, Q.; Kim, S.; Lee, K.; Kang, S.; Ryu, J. Force/torque sensorless impedance control for indirect driven robot-aided gait rehabilitation system. In Proceedings of the 2015 IEEE International Conference on Advanced Intelligent Mechatronics (AIM), Busan, Korea, 7–11 July 2015; pp. 652–657. [Google Scholar]
- Marchal-Crespo, L.; Reinkensmeyer, D.J. Review of control strategies for robotic movement training after neurologic injury. J. Neuroeng. Rehabil. 2009, 6, 20. [Google Scholar] [CrossRef] [PubMed]
- Higginson, J.; Zajac, F.; Neptune, R.; Kautz, S.; Delp, S. Muscle contributions to support during gait in an individual with post-stroke hemiparesis. J. Biomech. 2006, 39, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.C.; Barroso, F.; Farina, D.; Gizzi, L.; Santos, C.; Molinari, M.; Pons, J.L. Effects of robotic guidance on the coordination of locomotion. J. Neuroeng. Rehabil. 2013, 10, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duschau-Wicke, A.; Caprez, A.; Riener, R. Patient-cooperative control increases active participation of individuals with SCI during robot-aided gait training. J. Neuroeng. Rehabil. 2010, 7, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiguchi, K.; Hayashi, Y. An EMG-based control for an upper-limb power-assist exoskeleton robot. IEEE Trans. Syst. Man Cybern. Part B 2012, 42, 1064–1071. [Google Scholar] [CrossRef]
- Loconsole, C.; Dettori, S.; Frisoli, A.; Avizzano, C.A.; Bergamasco, M. An EMG-based approach for on-line predicted torque control in robotic-assisted rehabilitation. In Proceedings of the 2014 IEEE Haptics Symposium (HAPTICS), Houston, TX, USA, 23–26 February 2014; pp. 181–186. [Google Scholar]
- Song, R.; Tong, K.-y.; Hu, X.; Li, L. Assistive control system using continuous myoelectric signal in robot-aided arm training for patients after stroke. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-Y.; Yang, L.; Park, I.J.; Kim, E.J.; Park, M.S.; You, S.H.; Kim, Y.-H.; Ko, H.-Y.; Shin, Y.-I. Effects of innovative WALKBOT robotic-assisted locomotor training on balance and gait recovery in hemiparetic stroke: A prospective, randomized, experimenter blinded case control study with a four-week follow-up. IEEE Trans. Neural Syst. Rehabil. Eng. 2015, 23, 636–642. [Google Scholar] [CrossRef]
- Yang, H.E.; Kyeong, S.; Lee, S.H.; Lee, W.-J.; Ha, S.W.; Kim, S.M.; Kang, H.; Lee, W.M.; Kang, C.S.; Kim, D.H. Structural and functional improvements due to robot-assisted gait training in the stroke-injured brain. Neurosci. Lett. 2017, 637, 114–119. [Google Scholar] [CrossRef]
- Jung, J.-H.; Lee, N.-G.; You, J.-H.; Lee, D.-C. Validity and feasibility of intelligent Walkbot system. Electron. Lett. 2009, 45, 1016–1017. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Portney, L.G.; Watkins, M.P. Foundations of Clinical Research: Applications to Practice (Vol. 892); ISO 690; Pearson: London, UK; Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Wan, X.; Qu, F.; Garrett, E.W.; Liu, H.; Yu, B. Relationships among hamstring muscle optimal length and hamstring flexibility and strength. J. Sport Health Sci. 2017, 6, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gizzi, L.; Nielsen, J.F.; Felici, F.; Moreno, J.C.; Pons, J.L.; Farina, D. Motor modules in robot-aided walking. J. Neuroeng. Rehabil. 2012, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.; Oh-Park, M.; Bialek, A.; Friel, K.; Edwards, D.; You, J.S.H. Abnormal synergistic gait mitigation in acute stroke using an innovative ankle–knee–hip interlimb humanoid robot: A preliminary randomized controlled trial. Sci. Rep. 2021, 11, 22823. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Hwang, J.; You, J.S.H. Comparative effectiveness of robot-interactive gait training with and without ankle robotic control in patients with brain damage. J. Mech. Med. Biol. 2021, 21, 2140035. [Google Scholar] [CrossRef]
- Ivanenko, Y.P.; Grasso, R.; Macellari, V.; Lacquaniti, F. Control of foot trajectory in human locomotion: Role of ground contact forces in simulated reduced gravity. J. Neurophysiol. 2002, 87, 3070–3089. [Google Scholar] [CrossRef] [Green Version]
- Hof, A.; Elzinga, H.; Grimmius, W.; Halbertsma, J. Speed dependence of averaged EMG profiles in walking. Gait Posture 2002, 16, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Den Otter, A.; Geurts, A.; Mulder, T.; Duysens, J. Speed related changes in muscle activity from normal to very slow walking speeds. Gait Posture 2004, 19, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, J.; Thorstensson, A. Ground reaction forces at different speeds of human walking and running. Acta Physiol. Scand. 1989, 136, 217–227. [Google Scholar] [CrossRef]



| Participant ID | Sex | Age (Years) | Height (m) | Weight (kg) | BMI | Activity Level * | Profession |
|---|---|---|---|---|---|---|---|
| SUB 00 | M | 29 | 1.76 | 86 | 27.8 | Vigorous | student |
| SUB 01 | M | 28 | 1.88 | 88 | 24.9 | Vigorous | student |
| SUB 02 | M | 59 | 1.66 | 60 | 21.8 | Moderate | office worker |
| SUB 03 | M | 52 | 1.83 | 82 | 24.5 | Moderate | office worker |
| SUB 04 | M | 29 | 1.72 | 77 | 26.0 | Vigorous | student |
| SUB 05 | M | 48 | 1.74 | 63 | 20.8 | Moderate | office worker |
| SUB 06 | M | 45 | 1.75 | 65 | 21.2 | Vigorous | office worker |
| SUB 07 | F | 28 | 1.55 | 61 | 25.4 | Vigorous | student |
| SUB 08 | M | 38 | 1.81 | 120 | 36.6 | Vigorous | office worker |
| SUB 09 | M | 37 | 1.71 | 80 | 41.0 | Vigorous | office worker office worker |
| SUB 10 | F | 27 | 1.63 | 60 | 22.6 | Vigorous | student |
| SUB 11 | F | 58 | 1.55 | 62 | 25.8 | Moderate | activity assistant teacher |
| SUB 12 | F | 26 | 1.63 | 54 | 20.3 | Moderate | student |
| SUB 13 | F | 26 | 1.63 | 55 | 20.7 | Moderate | student |
| SUB 14 | F | 27 | 1.63 | 55 | 20.7 | Vigorous | student |
| SUB 15 | F | 20 | 1.7 | 55 | 19.0 | Moderate | student |
| SUB 16 | M | 26 | 1.74 | 61 | 20.2 | Moderate | student |
| SUB 17 | F | 22 | 1.66 | 63 | 22.9 | Moderate | student |
| Experiment Conditions | Body Weight Supports (%) | Gait Speeds (km/h) |
|---|---|---|
| 1 | 0 | 1 |
| 2 | 0 | 1.5 |
| 3 | 0 | 2 |
| 4 | 30 | 1 |
| 5 | 30 | 1.5 |
| 6 | 30 | 2 |
| 7 | 60 | 1 |
| 8 | 60 | 1.5 |
| 9 | 60 | 2 |
| Spearman’s rho (r) | VLO | BF | TA | GCM |
|---|---|---|---|---|
| Experimental conditions | −0.383 ** | −0.190 ** | −0.072 ** | −0.243 ** |
| STANCE hip torque | −0.108 ** | 0.412 ** | −0.168 * | 0.116 ** |
| STANCE knee torque | −0.211 ** | −0.590 ** | −0.335 ** | 0.069 ** |
| SWING hip torque | −0.214 ** | −0.412 ** | −0.240 ** | 0.119 ** |
| SWING knee torque | 0.180 ** | 0.241 ** | 0.136 ** | 0.120 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Cha, B.; Park, C.; Ryu, J.; You, J.H. Relationships between Body Weight Support and Gait Speed Parameters and Muscle Activity and Torque during Robot-Assisted Gait Training in Non-Neurological Adults: A Preliminary Investigation. Appl. Sci. 2022, 12, 11326. https://doi.org/10.3390/app122211326
Park H, Cha B, Park C, Ryu J, You JH. Relationships between Body Weight Support and Gait Speed Parameters and Muscle Activity and Torque during Robot-Assisted Gait Training in Non-Neurological Adults: A Preliminary Investigation. Applied Sciences. 2022; 12(22):11326. https://doi.org/10.3390/app122211326
Chicago/Turabian StylePark, Haeun, Baekdong Cha, Chanhee Park, Jeha Ryu, and Joshua (Sung) H. You. 2022. "Relationships between Body Weight Support and Gait Speed Parameters and Muscle Activity and Torque during Robot-Assisted Gait Training in Non-Neurological Adults: A Preliminary Investigation" Applied Sciences 12, no. 22: 11326. https://doi.org/10.3390/app122211326





