Effects of Chitosan Coatings on Controlling Listeria monocytogenes and Methicillin-Resistant Staphylococcus aureus in Beef and Mutton Cuts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Meat Samples
2.2. Preparation of the Inoculums
2.3. Application of the Chitosan Solution and the Inocula to the Meat Samples
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ngo, D.-N.; Kang, K.-H.; Je, J.-Y.; Pham, H.N.-D.; Byun, H.-G.; Kim, S.-K. Biological effects of chitosan and its derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef] [Green Version]
- Bennett, S.D.; Walsh, K.A.; Gould, L.H. Foodborne disease outbreaks caused by Bacillus cereus, Clostridium perfringens, and Staphylococcus aureus--United States, 1998-2008. Clin. Infect. Dis. 2013, 57, 425–433. [Google Scholar] [CrossRef]
- Fluit, A.C. Livestock-associated Staphylococcus aureus. Clin. Microbiol. Infect. 2012, 18, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Kadariya, J.; Smith, T.C.; Thapaliya, D. Staphylococcus aureus and staphylococcal food-borne disease: An ongoing challenge in public health. BioMed Res. Int. 2014, 2014, 827965. [Google Scholar] [CrossRef] [Green Version]
- Argudín, M.A.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef]
- Hennekinne, J.; De Buyser, M.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [Green Version]
- Omer, M.K.; Álvarez-Ordoñez, A.; Prieto, M.; Skjerve, E.; Asehun, T.; Alvseike, O.A. A systematic review of bacterial foodborne outbreaks related to red meat and meat products. Foodborne Pathog. Dis. 2018, 15, 598–611. [Google Scholar] [CrossRef]
- Rocourt, J.; Buchrieser, C. The genus Listeria and Listeria monocytogenes: Phylogenetic position, taxonomy, and identification. In Listeria, Listeriosis, and Food Safety, 3rd ed.; Ryser, E.T., Ryser, E.T., Marth, E.M., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–20. [Google Scholar]
- Jemmi, T.; Stephan, R. Listeria monocytogenes: Food-borne pathogen and hygiene indicator. OIE Rev. Sci. Tech. 2006, 25, 571–580. [Google Scholar] [CrossRef]
- Allerberger, F.; Wagner, M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010, 16, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenco, A.; Linke, K.; Wagner, M.; Stessl, B. The saprophytic lifestyle of Listeria monocytogenes and entry into the food-processing environment. Front. Microbiol. 2022, 13, 789801. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Manigandan, V.; Karthik, R.; Ramachandran, S.; Rajagopal, S. Chitosan applications in food industry. In Biopolymers for Food Design; Grumezescu, A.M., Holbanand, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 469–491. [Google Scholar] [CrossRef]
- Tsitsos, A.; Economou, V.; Chouliara, E.; Theodoridis, A.; Arsenos, G.; Amvrosiadis, I. Effects of chitosan and alginate-based membranes with oregano essential oil and olive oil in the microbiota of sheep meat. In Proceedings of the 41st International Congress of the Society for Microbial Ecology in Health and Disease (SOMED), Alexandroupolis, Greece, 14–16 June 2022. [Google Scholar]
- Paparella, A.; Mazzarrino, G.; Chaves-López, C.; Rossi, C.; Sacchetti, G.; Guerrieri, O.; Serio, A. Chitosan boosts the antimicrobial activity of Origanum vulgare essential oil in modified atmosphere packaged pork. Food Microbiol. 2016, 59, 23–31. [Google Scholar] [CrossRef]
- ISO 11290-2; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria monocytogenes and of Listeria spp.—Part 2: Enumeration Method. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6888-1; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Parker Agar Medium. International Organization for Standardization: Geneva, Switzerland, 2021.
- Raafat, D.; Sahl, H. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Zhu, J. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Khanjari, A.; Karabagias, I.K.; Kontominas, M.G. Combined effect of N,O-carboxymethyl chitosan and oregano essential oil to extend shelf life and control Listeria monocytogenes in raw chicken meat fillets. LWT 2013, 53, 94–99. [Google Scholar] [CrossRef]
- Lekjing, S. A chitosan-based coating with or without clove oil extends the shelf life of cooked pork sausages in refrigerated storage. Meat Sci. 2016, 111, 192–197. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, J.; Wu, S. Chitosan based coatings extend the shelf-life of beef slices during refrigerated storage. LWT 2021, 138, 110694. [Google Scholar] [CrossRef]
- Chamanara, V.; Shabanpour, B.; Gorgin, S.; Khomeiri, M. An investigation on characteristics of rainbow trout coated using chitosan assisted with thyme essential oil. Int. J. Biol. Macromol. 2012, 50, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.Y.; Balay, D.; Hu, Y.; McMullen, L.M.; Gänzle, M.G. Effect of chitosan, and bacteriocin—Producing Carnobacterium maltaromaticum on survival of Escherichia coli and Salmonella Typhimurium on beef. Int. J. Food Microbiol. 2019, 290, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.D.; Bratcher, C.L.; Jin, T.Z.; Bilgili, S.F.; Owsley, W.F.; Wang, L. Evaluation of a novel antimicrobial solution and its potential for control Escherichia coli O157: H7, non-O157: H7 shiga toxin-producing E. coli, Salmonella spp.; and Listeria monocytogenes on beef. Food Control 2016, 64, 196–201. [Google Scholar] [CrossRef]
- Hadian, M.; Rajaei, A.; Mohsenifar, A.; Tabatabaei, M. Encapsulation of Rosmarinus officinalis essential oils in chitosan-benzoic acid nanogel with enhanced antibacterial activity in beef cutlet against Salmonella Typhimurium during refrigerated storage. LWT 2017, 84, 394–401. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef]
- Juneja, V.K.; Thippareddi, H.; Bari, L.; Inatsu, Y.; Kawamoto, S.; Friedman, M. Chitosan protects cooked ground beef and turkey against Clostridium perfringens spores during chilling. J. Food Sci. 2006, 71, M236–M240. [Google Scholar] [CrossRef] [Green Version]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT 2013, 53, 321–326. [Google Scholar] [CrossRef]
- He, L.; Zou, L.; Yang, Q.; Xia, J.; Zhou, K.; Zhu, Y.; Han, X.; Pu, B.; Hu, B.; Deng, W.; et al. Antimicrobial activities of nisin, tea polyphenols, and chitosan and their combinations in chilled mutton. J. Food Sci. 2016, 81, M1466–M1471. [Google Scholar] [CrossRef]
- Duran, A.; Kahve, H.I. The effect of chitosan coating and vacuum packaging on the microbiological and chemical properties of beef. Meat Sci. 2020, 162, 107961. [Google Scholar] [CrossRef]
- Silva, A.S.; Sampaio, A.P.; Santos, M.S.; de Souza, B.W.S.; Evangelista-Barreto, N.S. Effect of chitosan coating on contamination of fresh bovine meat sold in the open market. Rev. Cienc. Agron. 2019, 50, 38–43. [Google Scholar] [CrossRef]
- Ashrafi, A.; Jokar, M.; Mohammadi Nafchi, A. Preparation and characterization of biocomposite film based on chitosan and kombucha tea as active food packaging. Int. J. Biol. Macromol. 2018, 108, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Fik, M.; Leszczynska-Fik, A. Microbiological and sensory changes in minced beef treated with potassium lactate and sodium diacetate during refrigerated storage. Int. J. Food Prop. 2007, 10, 589–598. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan glucose complex—A novel food preservative. Food Chem. 2008, 106, 521–528. [Google Scholar] [CrossRef]
- Antoniadou, D.; Govaris, A.; Ambrosiadis, I.; Sergelidis, D. Effect of chitosan coating on the shelf life of ready-to-eat bovine meatballs and the control of Listeria monocytogenes growth on their surface during refrigeration storage. J. Hell. Vet. Med. Soc. 2019, 70, 1495–1502. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Ren, C.W.; Cai, J.H.; Zhang, C.Y.; Li, Y.T.; Xu, B.; Farooq, M.A. The synergistic inhibition and mechanism of epicatechin gallate and chitosan against methicillin-resistant Staphylococcus aureus and the application in pork preservation. LWT 2022, 163, 113575. [Google Scholar] [CrossRef]
- Rubini, D.; Farisa Banu, S.; Veda Hari, B.N.; Ramya Devi, D.; Gowrishankar, S.; Karutha Pandian, S.; Nithyanand, P. Chitosan extracted from marine biowaste mitigates staphyloxanthin production and biofilms of methicillin- resistant Staphylococcus aureus. Food. Chem. Toxicol. 2018, 118, 733–744. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M.M. Insights into chitosan antibiofilm activity against methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2017, 122, 1547–1557. [Google Scholar] [CrossRef]
- Bento, R.A.; Stamford, T.L.M.; Stamford, T.C.M.; de Andrade, S.A.C.; de Souza, E.L. Sensory evaluation and inhibition of Listeria monocytogenes in bovine pâté added of chitosan from Mucor rouxii. LWT 2011, 44, 588–591. [Google Scholar] [CrossRef]
- Mojsova, S.; Angelovski, L.; Jankuloski, D.; Simonovska, J.; Velickova, E. Antimicrobial effect of oregano-chitosan double coatings on Listeria monocytogenes in meat products. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012082. [Google Scholar] [CrossRef]
- Wang, D.; Dong, Y.; Chen, X.; Liu, Y.; Wang, J.; Wang, X.; Wang, C.; Song, H. Incorporation of apricot (Prunus armeniaca) kernel essential oil into chitosan films displaying antimicrobial effect against Listeria monocytogenes and improving quality indices of spiced beef. Int. J. Biol. Macromol. 2020, 162, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Beverlya, R.L.; Janes, M.E.; Prinyawiwatkula, W.; No, H.K. Edible chitosan films on ready-to-eat roast beef for the control of Listeria monocytogenes. Food Microbiol. 2008, 25, 534–537. [Google Scholar] [CrossRef] [PubMed]
- Shekarforoush, S.S.; Basiri, S.; Ebrahimnejad, H.; Hosseinzadeh, S. Effect of chitosan on spoilage bacteria, Escherichia coli and Listeria monocytogenes in cured chicken meat. Int. J. Biol. Macromol. 2015, 76, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Li, N.; Li, Z.; He, H.; Zhao, Y.; Zhu, M.; Wang, Z.; Kang, Z.; Ma, H. Shelf life of fresh chilled pork as affected by antimicrobial intervention with nisin, tea polyphenols, chitosan, and their combination. Int. J. Food Prop. 2019, 22, 1047–1063. [Google Scholar] [CrossRef]
- Jaja, I.F.; Green, E.; Muchenje, V. Aerobic Mesophilic, Coliform, Escherichia coli, and Staphylococcus aureus counts of raw meat from the formal and informal meat sectors in South Africa. Int. J. Environ. Res. Public Health 2018, 15, 819. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Williams, M.; Bissett, A.; Ross, T.; Bowman, J.P. Effect of abattoir, livestock species and storage temperature on bacterial community dynamics and sensory properties of vacuum packaged red meat. Food Microbiol. 2021, 94, 103648. [Google Scholar] [CrossRef]
- Iyer, V.; Raut, J.; Dasgupta, A. Impact of pH on growth of Staphylococcus epidermidis and Staphylococcus aureus in vitro. J. Med. Microbiol. 2021, 70, 001421. [Google Scholar] [CrossRef]
- Koutsoumanis, K.P.; Kendall, P.A.; Sofos, J.N. Effect of food processing-related stresses on acid tolerance of Listeria monocytogenes. Appl. Environ. Microbiol. 2003, 69, 7514–7516. [Google Scholar] [CrossRef] [Green Version]
- Assanti, E.; Karabagias, V.K.; Karabagias, I.K.; Badeka, A.; Kontominas, M.G. Shelf life evaluation of fresh chicken burgers based on the combination of chitosan dip and vacuum packaging under refrigerated storage. J. Food Sci. Technol. 2021, 58, 870–883. [Google Scholar] [CrossRef]
- Karami, N.; Kamkar, A.; Shahbazi, Y.; Misaghi, A. Effects of active chitosan-flaxseed mucilage-based films on the preservation of minced trout fillets: A comparison among aerobic, vacuum, and modified atmosphere packaging. Packag. Technol. Sci. 2020, 33, 469–484. [Google Scholar] [CrossRef]
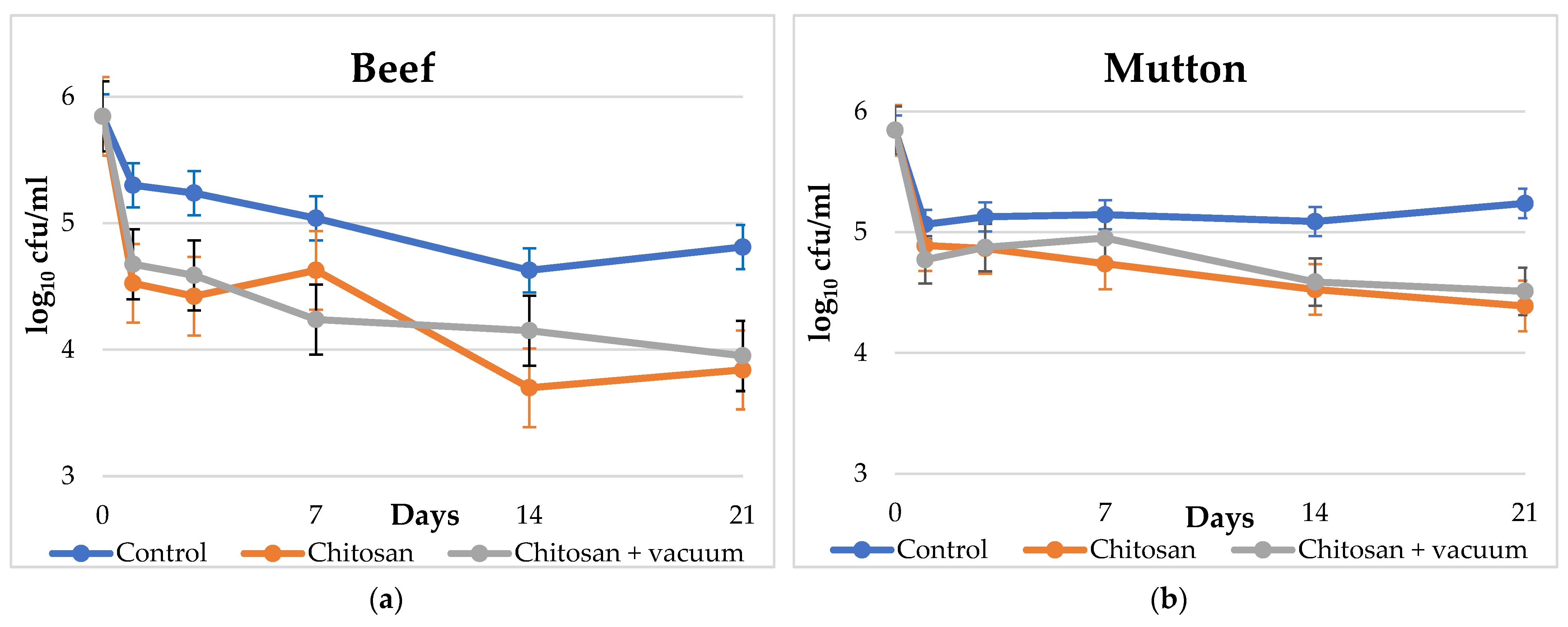
| Day | Beef | Mutton | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 18) | Chitosan (n = 18) | Chitosan + VP (n = 18) | Control (n = 18) | Chitosan (n = 18) | Chitosan + VP (n = 18) | |||||||
| 0 | 5.85 | (0.00) | 5.85 | (0.00) | 5.85 | (0.00) | 5.85 | (0.00) | 5.85 | (0.00) | 5.85 | (0.00) |
| 1 | 5.30 | (0.43) | 4.53 | (0.18) | 4.68 | (0.28) | 5.07 | (0.16) | 4.89 | (0.02) | 4.77 | (0.10) |
| 3 | 5.24 | (0.09) | 4.42 | (0.17) | 4.59 | (0.16) | 5.13 | (0.07) | 4.87 | (0.12) | 4.87 | (0.04) |
| 7 | 5.04 | (0.06) | 4.63 | (0.04) | 4.24 | (0.09) | 5.15 | (0.04) | 4.74 | (0.06) | 4.95 | (0.07) |
| 14 | 4.63 | (0.21) | 3.70 | (0.43) | 4.15 | (0.21) | 5.09 | (0.12) | 4.53 | (0.18) | 4.59 | (0.16) |
| 21 | 4.81 | (0.05) | 3.84 | (0.09) | 3.95 | (0.07) | 5.24 | (0.09) | 4.39 | (0.12) | 4.51 | (0.05) |
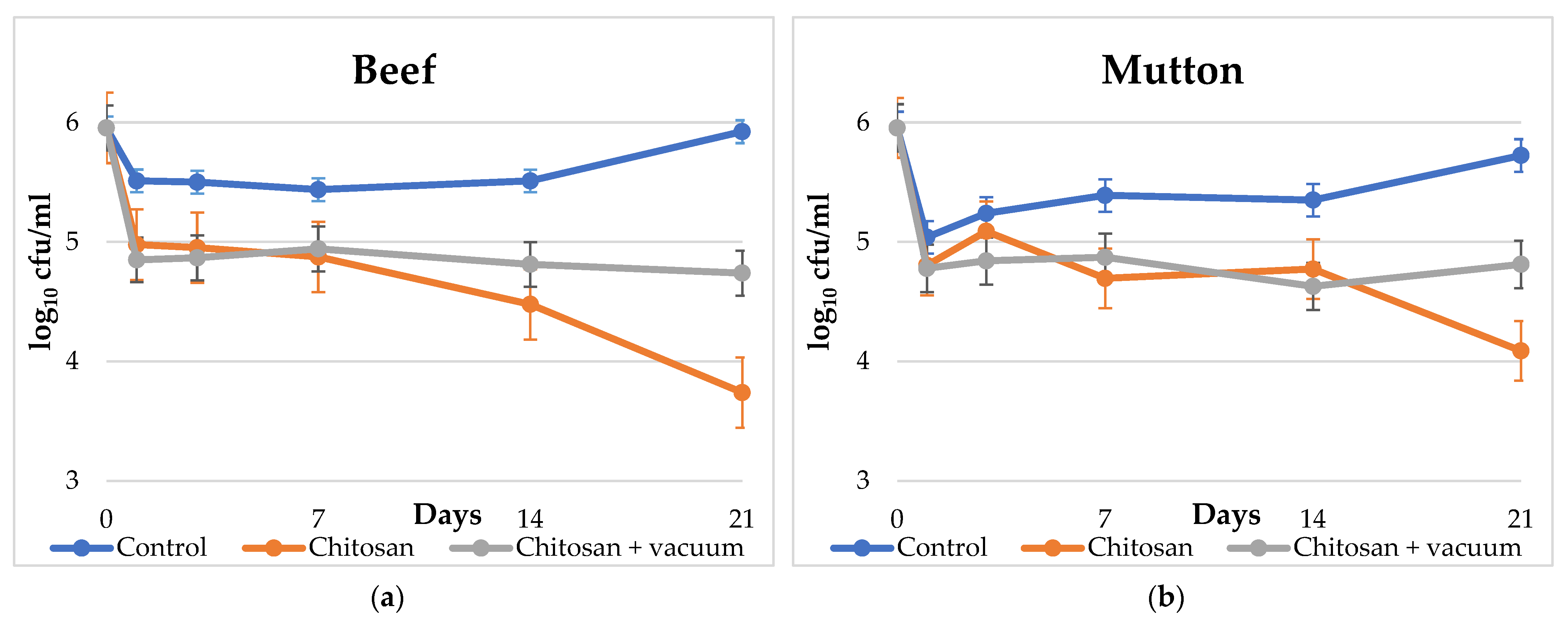
| Day | Beef | Mutton | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 18) | Chitosan (n = 18) | Chitosan + VP (n = 18) | Control (n = 18) | Chitosan (n = 18) | Chitosan + VP (n = 18) | |||||||
| 0 | 5.95 | (0.00) | 5.95 | (0.00) | 5.95 | (0.00) | 5.95 | (0.00) | 5.95 | (0.00) | 5.95 | (0.00) |
| 1 | 5.51 | (0.05) | 4.98 | (0.28) | 4.85 | (0.21) | 5.04 | (0.62) | 4.80 | (0.21) | 4.78 | (0.25) |
| 3 | 5.50 | (0.28) | 4.95 | (0.07) | 4.87 | (0.12) | 5.24 | (0.34) | 5.09 | (0.12) | 4.84 | (0.09) |
| 7 | 5.44 | (0.37) | 4.87 | (0.04) | 4.94 | (0.02) | 5.39 | (0.12) | 4.69 | (0.21) | 4.87 | (0.08) |
| 14 | 5.51 | (0.05) | 4.48 | (0.25) | 4.81 | (0.05) | 5.35 | (0.07) | 4.77 | (0.10) | 4.63 | (0.21) |
| 21 | 5.92 | (0.11) | 3.74 | (0.62) | 4.74 | (0.06) | 5.72 | (0.17) | 4.09 | (0.12) | 4.81 | (0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Economou, V.; Tsitsos, A.; Theodoridis, A.; Ambrosiadis, I.; Arsenos, G. Effects of Chitosan Coatings on Controlling Listeria monocytogenes and Methicillin-Resistant Staphylococcus aureus in Beef and Mutton Cuts. Appl. Sci. 2022, 12, 11345. https://doi.org/10.3390/app122211345
Economou V, Tsitsos A, Theodoridis A, Ambrosiadis I, Arsenos G. Effects of Chitosan Coatings on Controlling Listeria monocytogenes and Methicillin-Resistant Staphylococcus aureus in Beef and Mutton Cuts. Applied Sciences. 2022; 12(22):11345. https://doi.org/10.3390/app122211345
Chicago/Turabian StyleEconomou, Vangelis, Anestis Tsitsos, Alexandros Theodoridis, Ioannis Ambrosiadis, and Georgios Arsenos. 2022. "Effects of Chitosan Coatings on Controlling Listeria monocytogenes and Methicillin-Resistant Staphylococcus aureus in Beef and Mutton Cuts" Applied Sciences 12, no. 22: 11345. https://doi.org/10.3390/app122211345






