Microstructure, Chemistry and Mineralogy Approach for the Diagnostics of Metallic Finds of the Tomba della Biga (Adria, Italy)
Abstract
:1. Introduction
2. Materials and Methods
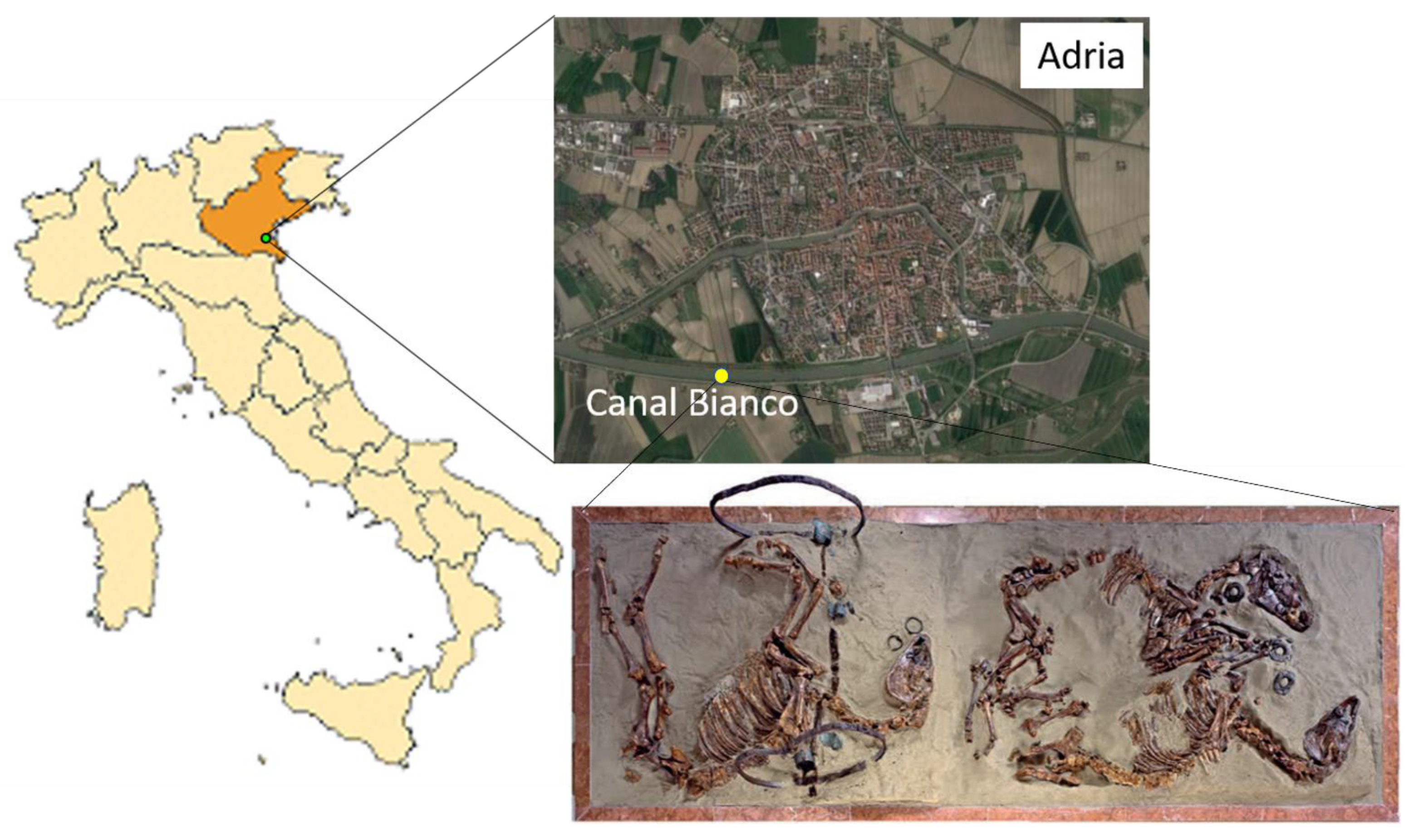
2.1. Sampling Site Description
2.2. Archaeological Context
2.3. Samples Description
2.4. Analytical Techniques
3. Results and Discussion
3.1. Macroscopic Characterization
3.2. Microscopic Characterization
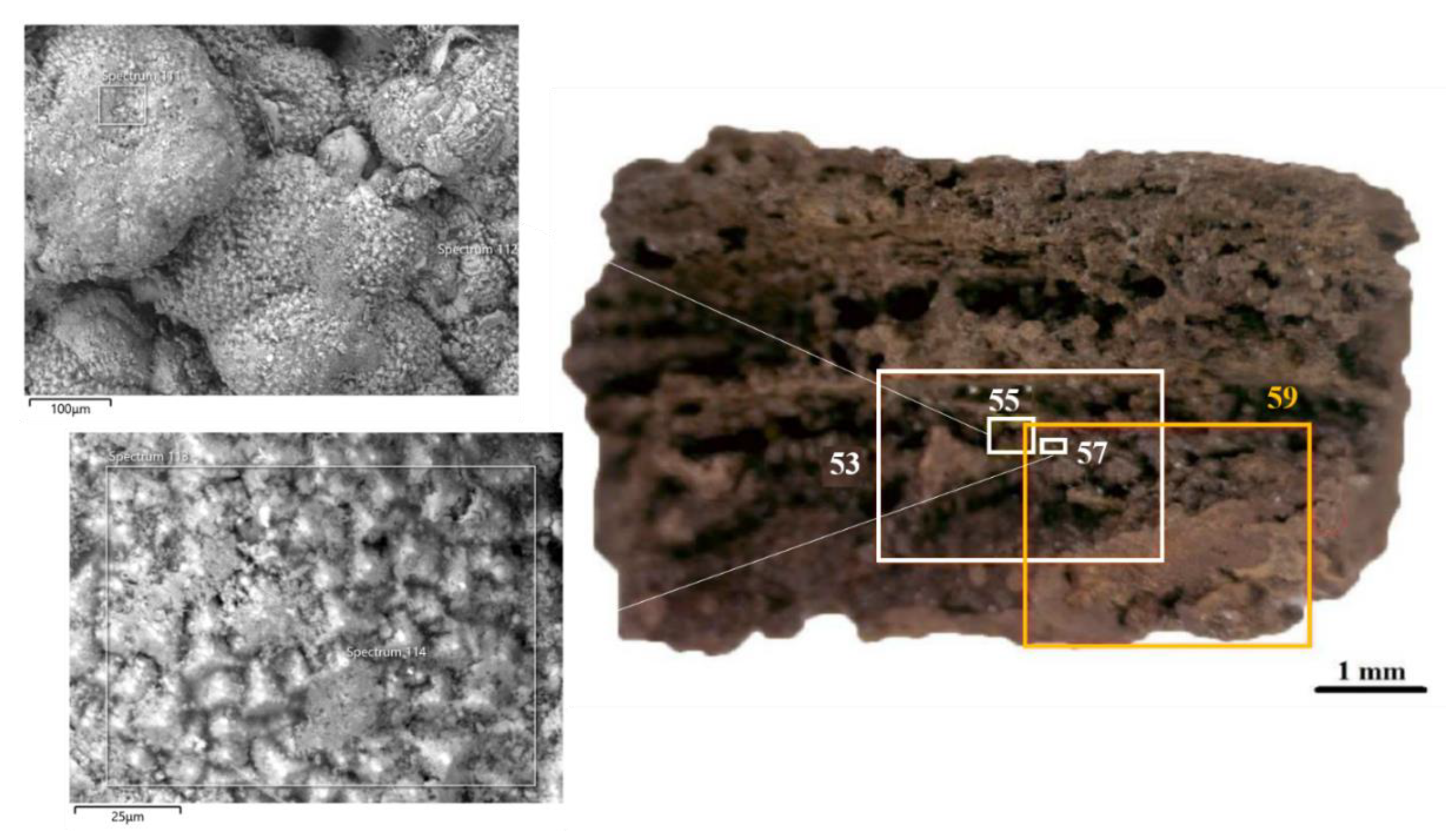
3.2.1. Iron Samples
3.2.2. Bronze Samples
3.2.3. Optical Transmitted Polarized Light Both on Iron and Bronze Samples
3.3. SEM-EDS Analysis
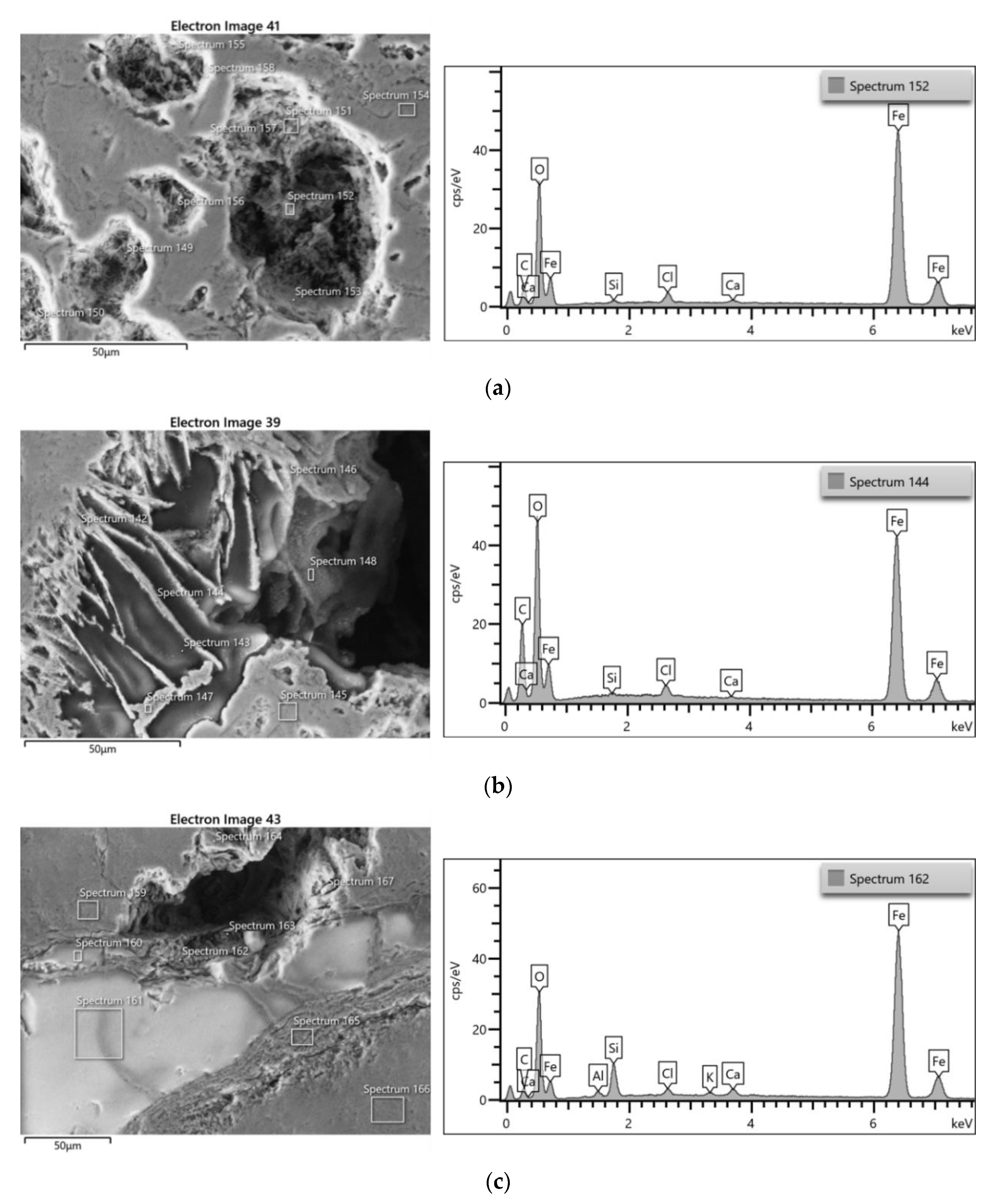
3.3.1. Iron Samples
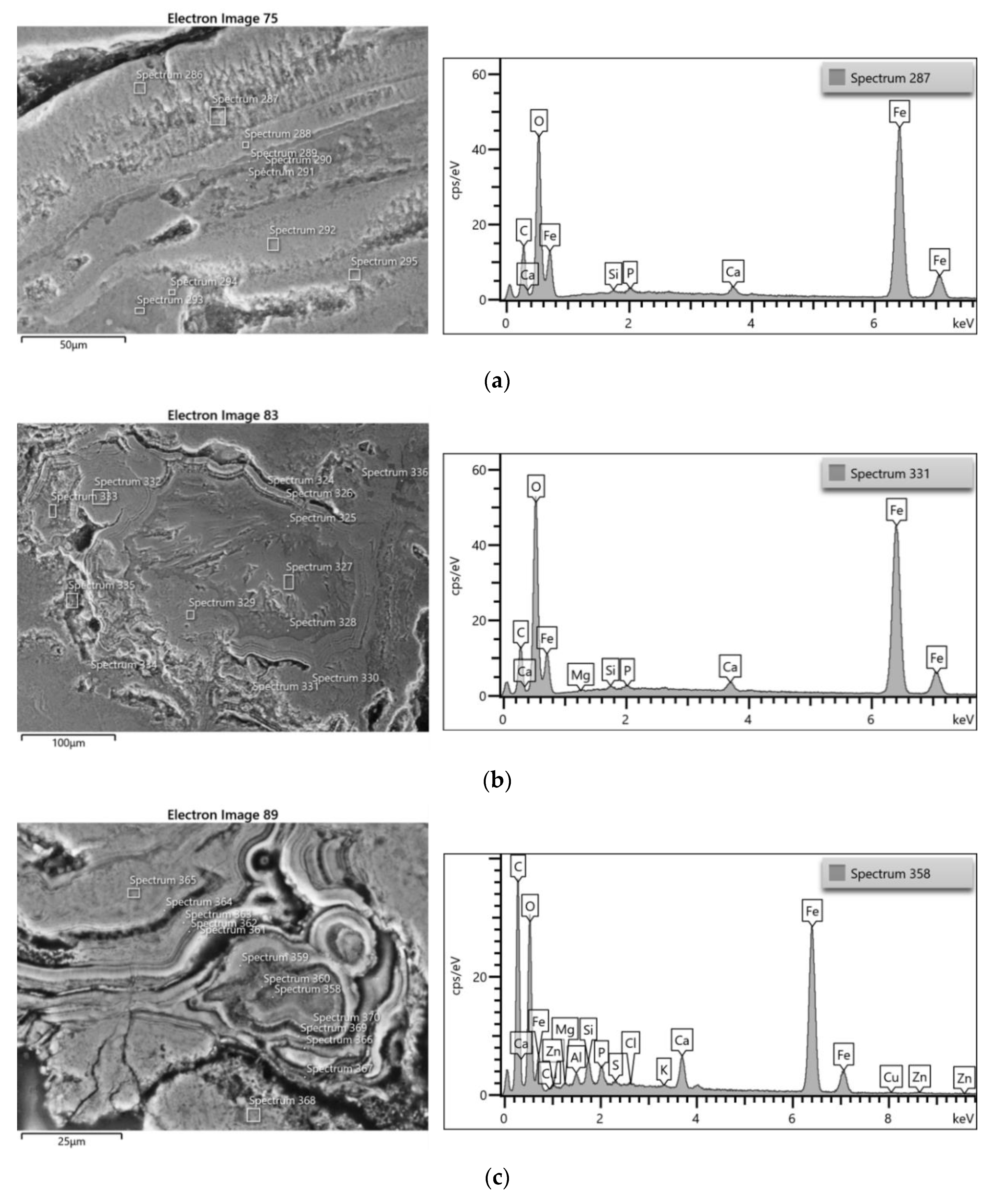
3.3.2. Bronze Samples
3.4. Micro-Raman Spectroscopy
3.4.1. Iron Samples
3.4.2. Bronze Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giblin, J.I.; Knudson, K.J.; Bereczki, Z.; Palfi, G.; Pap, I. Strontium isotope analysis and human mobility during the Neolithic and Copper Age: A case study from the Great Hungarian Plain. J. Archaeol. Sci. 2013, 40, 227–239. [Google Scholar] [CrossRef]
- Greenfield, H.; Marciniak, A. Retention of old technologies following the end of the Neolithic: Microscopic analysis of the butchering marks on animal bones from Çatalhöyük East. World Archaeol. 2019, 51, 76–103. [Google Scholar] [CrossRef]
- Dolfini, A. From the Neolithic to the Bronze Age in Central Italy: Settlement, Burial, and Social Change at the Dawn of Metal Production. J. Archaeol. Res. 2020, 28, 503–556. [Google Scholar] [CrossRef] [Green Version]
- Pearce, M. The ‘Copper Age’—A History of the Concept. J. World Prehistory 2019, 32, 229–250. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Muta, H.; Kurosaki, K.; Ohishi, Y. Thermal and Electrical Conductivity of Liquid Al-Si Alloys. Int. J. Thermophys. 2019, 40, 31. [Google Scholar] [CrossRef]
- Huang, M.; Xu, C.; Fan, G.; Maawad, E.; Gan, W.; Geng, L.; Lin, F.; Tang, G.; Wu, H.; Du, Y.; et al. Role of layered structure in ductility improvement of layered Ti-Al metal composite. Acta Mater. 2018, 153, 235–249. [Google Scholar] [CrossRef]
- Ayedun, F.; Adebambo, P.O.; Adetunji, B.I.; Ozebo, V.C.; Oguntuase, J.A.; Adebayo, G.A. Increased Malleability in Tetragonal Zrx Ti1−x O2 Ternary Alloys: First-Principles Approach. Z. Nat. A 2017, 72, 567–572. [Google Scholar] [CrossRef]
- Hauptmann, A. Making Metals: Ancient Metallurgical Processes. In Archaeometallurgy—Materials Science Aspects; Natural Science in Archaeology; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Artioli, G.; Canovaro, C.; Nimis, P.; Angelini, I. LIA of Prehistoric Metals in the Central Mediterranean Area: A Review. Archaeometry 2020, 62, 53–85. Available online: https://publons.com/publon/10.1111/arcm.12542. (accessed on 7 November 2022).
- Liss, B.; Levy, T.E.; Day, J.M.D. Origin of iron production in the Eastern Mediterranean: Osmium isotope and highly siderophile element evidence from Iron Age Jordan. J. Archaeol. Sci. 2020, 122, 105227. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Pascucci, M.; Messina, E.; Giuliani, C.; Fierro, G.; Di Carlo, G. Integrated analytical methodologies for the study of the corrosion products naturally grown on Roman Ag-based artefacts. Appl. Surf. Sci. 2018, 446, 279–286. [Google Scholar] [CrossRef]
- Gambacurta, G.; Tirelli, M. Le Sepolture di Cavallo Nella Necropoli “Le Brustolade”. La Protostoria tra Sile e Tagliamento. Antiche Genti tra Veneto e Friuli, Catalogo Della Mostra; Esedra: Padova, Italy, 1996; pp. 71–74. [Google Scholar]
- Robbiola, L.; Vilbert, D.; Lejars, T.; Bourgarit, D.; Mille, B. Characterization of a buried archaeological bronze from the Celtic tomb n°1002 of La Fosse Cotheret (Roissy-en-France). Metal 2001, 237–242. [Google Scholar]
- Gerwin, W.; Baumhauer, R. Effect of soil parameters on the corrosion of archaeological metal finds. Geoderma 2000, 96, 63–80. [Google Scholar] [CrossRef]
- Bernabale, M.; Nigro, L.; Vaccaro, C.; Nicoli, M.; Montanari, D.; Bigini, P.; De Vito, C. Micro-Raman spectroscopy and complementary techniques for the study of iron weapons from Motya and Lilybaeum (Sicily, Italy): Corrosion patterns in lagoon-like and calcarenitic hypogea environments. J. Raman Spectrosc. 2022, 53, 272. [Google Scholar] [CrossRef]
- Jia, M.; Hu, P.; Hu, G. Corrosion Layers on Archaeological Cast Iron from Nanhai I. Materials 2022, 15, 4980. [Google Scholar] [CrossRef]
- Ashkenazi, D. How can fracture mechanics and failure analysis assist in solving mysteries of ancient metal artifacts? Archaeol. Anthropol. Sci. 2020, 12, 34. [Google Scholar] [CrossRef]
- Vietti, A.; Angelini, E.; Grassini, S.; Donato, N. Raman spectroscopic characterization of corrosion products of archaeological iron. J. Phys. Conf. Ser. 2022, 2204, 012066. [Google Scholar] [CrossRef]
- Liang, Z.; Jiang, K.; Zhang, T. Electrochemical and passive behaviour of Cu–Sn bronze in simulated archaeological soil media. Mater. Corros. 2021, 72, 743–756. [Google Scholar] [CrossRef]
- Liang, Z.; Jiang, K.; Zhang, T. Corrosion behaviour of lead bronze from the Western Zhou Dynasty in an archaeological-soil medium. Corros. Sci. 2021, 191, 109721. [Google Scholar] [CrossRef]
- Neff, D.; Bellot-Gurlet, L.; Dillmann, P.; Reguer, S.; Legrand, L. Raman imaging of ancient rust scales on archaeological iron artefacts for long-term atmospheric corrosion mechanisms study. J. Raman Spectrosc. 2006, 37, 1228–1237. [Google Scholar] [CrossRef]
- Mercier-Bion, F.; Li, J.; Lotz, H.; Tortech, L.; Neff, D.; Dillmann, P. Electrical properties of iron corrosion layers formed in anoxic environments at the nanometer scale. Corros. Sci. 2018, 137, 98–110. [Google Scholar] [CrossRef]
- Oudbashi, O. A methodological approach to estimate soil corrosivity for archaeological copper alloy artefacts. Herit. Sci. 2018, 6, 2. [Google Scholar] [CrossRef]
- Ingo, G.M.; Riccucci, C.; Guida, G.; Pascucci, M.; Giuliani, C.; Messina, E.; Fierro, G.; Di Carlo, G. Micro-chemical investigation of corrosion products naturally grown on archaeological Cu-based artefacts retrieved from the Mediterranean sea. Appl. Surf. Sci. 2019, 470, 695–706. [Google Scholar] [CrossRef]
- Amorosi, A.; Maselli, V.; Trincardi, F. Onshore to offshore anatomy of a late Quaternary source-to-sink system (Po Plain-AdriaticJia Sea, Italy). Earth Sci. Rev. 2016, 153, 212–237. [Google Scholar] [CrossRef]
- Stefani, M. The Po Delta Region: Depositional Evolution, Climate Change and Human Intervention through the Last 5000 Years. In Landscapes and Landforms of Italy; World Geomorphological Landscapes; Soldati, M., Marchetti, M., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Stefani, M.; Vincenzi, S. The interplay of eustasy, climate and human activity in the late Quaternary depositional evolution and sedimentary architecture of the Po Delta system. Mar. Geol. 2005, 222–223, 19–48. [Google Scholar] [CrossRef]
- Bruno, L.; Amorosi, A.; Curina, R.; Severi, P.; Bitelli, R. Human–landscape interactions in the Bologna area (northern Italy) during the mid-late Holocene, with focus on the Roman period. Holocene 2013, 23, 1560–1571. [Google Scholar] [CrossRef]
- Gambacurta, G. Adria. Museo Archeologico Nazionale di Adria. Guide Tematiche dei Musei Archeologici del Veneto; Tiné V. La Tipografica srl: Udine, Italy, 2013. [Google Scholar]
- Bonomi, S.; Zega, L. La Sezione Etrusca: Adria e il Basso Polesine tra i Secoli VI e il III a.C.; Ministero per i beni e le Attività Culturali, Soprintendenza per i Beni Archeologici del Veneto, Apogeo Editore: Milano, Italy, 2008. [Google Scholar]
- Reggiani, P.; Rizzi Zorzi, J. I cavalli della Tomba della Biga conservata al Museo archeologico nazionale di Adria (RO). Atti del 4° Convegno Nazionale di Archeozoologia, Quaderni del Museo archeologico del Friuli Occidentale; Malerba, G., Visentini, P., Eds.; Pordenone, Italy, 2003; 6, pp. 315–322. Available online: https://www.researchgate.net/publication/330638801_I_cavalli_della_Tomba_della_Biga_conservata_al_Museo_Archeologico_Nazionale_di_Adria_RO. (accessed on 7 November 2022).
- Habermehl, K.H. Die Altersbestimmungbei Hais-und Labortieren; Parey: Berlin, Germany, 1975. [Google Scholar]
- Chrószcz, A.; Baranowski, P.; Janowski, A.; Poradowski, D.; Janeczek, M.; Onar, V.; Sudoł, B.; Spychalski, P.; Dudek, A.; Sienkiewicz, W.; et al. Withers height estimation in medieval horse samples from Poland: Comparing the internal cranial cavity based modified Wyrost and Kucharczyk method with existing methods. Int. J. Osteoarchaeol. 2022, 32, 378–395. [Google Scholar] [CrossRef]
- Marrocchino, E.; Telloli, C.; Pedrini, M.; Vaccaro, C. Natural stones used in the Orsi-Marconi palace façade (Bologna): A petro-mineralogical characterization. Heritage 2020, 3, 1109–1123. [Google Scholar] [CrossRef]
- Rosina, P.; Collado, H.; Garces, S.; Comes, H.; Eftekhari, N.; Nicoli, M.; Vaccaro, C. Benquerencia (La Serena—Spain) rock art: An integrated spectroscopy analysis with FTIR and Raman. Heliyon 2019, 5, e02561. [Google Scholar] [CrossRef] [Green Version]
- Telloli, C.; Chicca, M.; Pepi, S.; Vaccaro, C. Saharan dust particles in snow samples of Alps and Apennines during an exceptional event of transboundary air pollution. Environ. Monit. Assess. 2018, 109, 37. [Google Scholar] [CrossRef]
- Marrocchino, E.; Telloli, C.; Caraccio, S.; Guarnieri, C.; Vaccaro, C. Medieval Glassworks in the City of Ferrara (North Eastern Italy): The Case Study of Piazza Municipale. Heritage 2020, 3, 819–837. [Google Scholar] [CrossRef]
- Portillo, H.; Zuluaga, M.C.; Ortega, L.A.; Alonso-Olazabal, A.; Murelaga, X.; Martinez-Salcedo, A. XRD, SEM/EDX and micro-Raman spectroscopy for mineralogical and chemical characterization of iron slags from the Roman archaeological site of Forua (Biscay, North Spain). Microchem. J. 2018, 138, 246–254. [Google Scholar] [CrossRef]
- Sharma, A.; Tripathi, A.; Narsimhachary, D.; Mahto, R.P.; Paul, J. Surface alteration of aluminium alloy by an exfoliated graphitic tribolayer during friction surfacing using a consumable graphite rich tool. Surf. Topogr. Metrol. Prop. 2019, 7, 045015. [Google Scholar] [CrossRef]
- Casaletto, M.P.; Ingo, G.M.; Riccucci, C.; Faraldi, F. Production of reference alloys for the conservation of archaeological silver-based artifacts. Appl. Phys. A 2010, 100, 937–944. [Google Scholar] [CrossRef]
- Cerri, G.; Brundu, A. Solid-state transformations of Zn-clinoptilolite through heating. J. Solid State Chem. 2020, 283, 121165. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Li, Z.; Zhang, P.; Li, L. Microtexture and Rolling Deformation Behavior Analysis of the Formation Mechanism Fe3O4 at the Interface Formed on Hot-Rolled High-Strength Steel. Metals 2021, 11, 312. [Google Scholar] [CrossRef]
- Veneranda, M.; Aramendia, J.; Bellot-Gurlet, L.; Colomban, P.; Castro, K.; Madariaga, J.M. FTIR spectroscopic semi-quantification of iron phases: A new method to evaluate the protection ability index (PAI) of archaeological artefacts corrosion systems. Corros. Sci. 2018, 133, 68–77. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Honeychurch, W.; Chunag, A. Novel Micro-Scale Steel-Making from Molten Cast Iron Practised in Medieval Nomadic Communities of East Mongolia. Archaeometry 2019, 61, 83–98. [Google Scholar] [CrossRef]
- Velmurugan, C.; Senthilkumar, V.; Dinesh, S.; Arulkirubakaran, D. Review on phase transformation behavior of NiTi shape memory alloys. Mater. Today Proc. 2018, 5, 14597–14606. [Google Scholar] [CrossRef]
- Tasew, F.; Thothadri, G. Barrier Corrosion Protection Properties of Metakaolin Clay-Kadilux Epoxy Coatings on Galvanized Steel. Int. J. Corros. 2021, 2021, 1049021. [Google Scholar] [CrossRef]
- Marshall, C.P.; Dufresne, W.J.B.; Rufledt, C.J. Polarized Raman spectra of hematite and assignment of external modes. J. Raman Spectrosc. 2020, 51, 1522–1529. [Google Scholar] [CrossRef]
- Fornasini, L.; Raneri, S.; Bersani, D.; Mantovani, L.; Scognamiglio, V.; Di Giuseppe, D.; Gualtieri, A.F. Identification of iron compounds in chrysotile from the Balangero mine (Turin, Italy) by micro-Raman spectroscopy. J. Raman Spectrosc. 2022, 1, 1–11. [Google Scholar] [CrossRef]
- Bernabale, M.; Montanari, D.; Nigro, L.; Spagnoli, F.; Vaccaro, C.; Eftekhari, N.; Nicoli, M.; De Vito, C. Raman spectroscopy and complementary techniques applied for the study of copper and iron wastes from Motya (Italy). J. Raman Spectrosc. 2022, 1. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Bodappa, N.; Smith, R.D.L. Mechanistic insights into lepidocrocite conversion to hematite from variable temperature Raman microscopy. J. Phys. Energy 2021, 3, 044002. [Google Scholar] [CrossRef]
- Al Shenawa, A.; Argade, G.; Chilukuri, A.; D’Souza, N.A.; Nasrazadani, S.; Scharf, T.; Banerjee, R. Effect of supercritical CO2 on saltwater corrosion and wear resistance of bismaleimide coating filled with organophilic montmorillonite clay. J. Adhes. Sci. Technol. 2021, 35, 2301–2318. [Google Scholar] [CrossRef]
- Fan, X.; Wang, Q.; Wang, Y. Non-destructive in situ Raman spectroscopic investigation of corrosion products on the bronze dagger-axes from Yujiaba site in Chongqing, China. Archaeol. Anthropol. Sci. 2020, 12, 90. [Google Scholar] [CrossRef]
- Privitera, A.; Corbascio, A.; Calcani, G.; Della Ventura, G.; Ricci, M.A.; Sodo, A. Raman approach to the forensic study of bronze patinas. J. Archaeol. Sci. Rep. 2021, 39, 103115. [Google Scholar] [CrossRef]
- Doménech-Carbó, A.; Mödlinger, M.; Doménech-Carbó, M.T. Multiple-scan voltammetry and OCP: Archaeometric tools for dating archaeological bronzes. J. Electroanal. Chem. 2021, 893, 115336. [Google Scholar] [CrossRef]




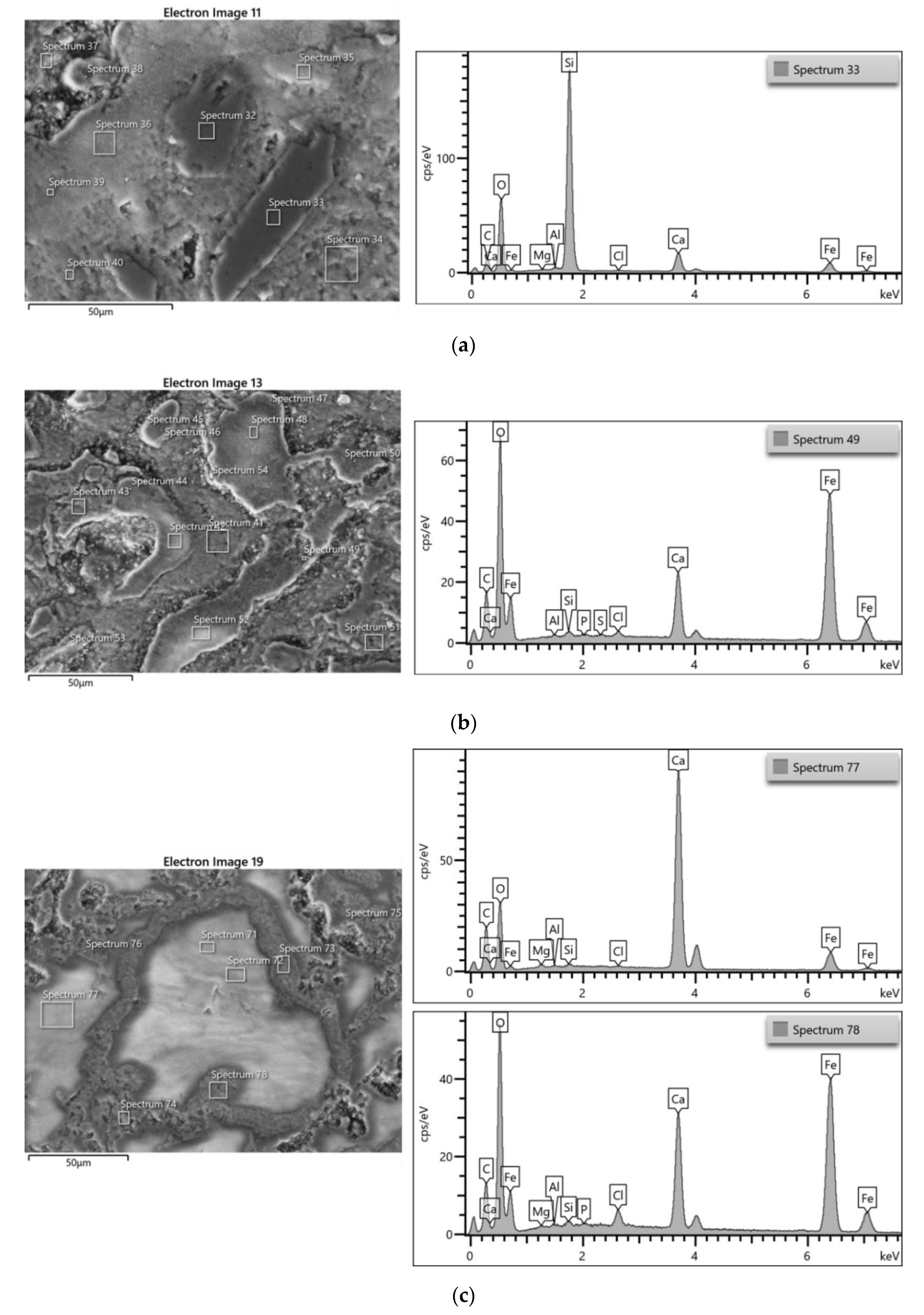

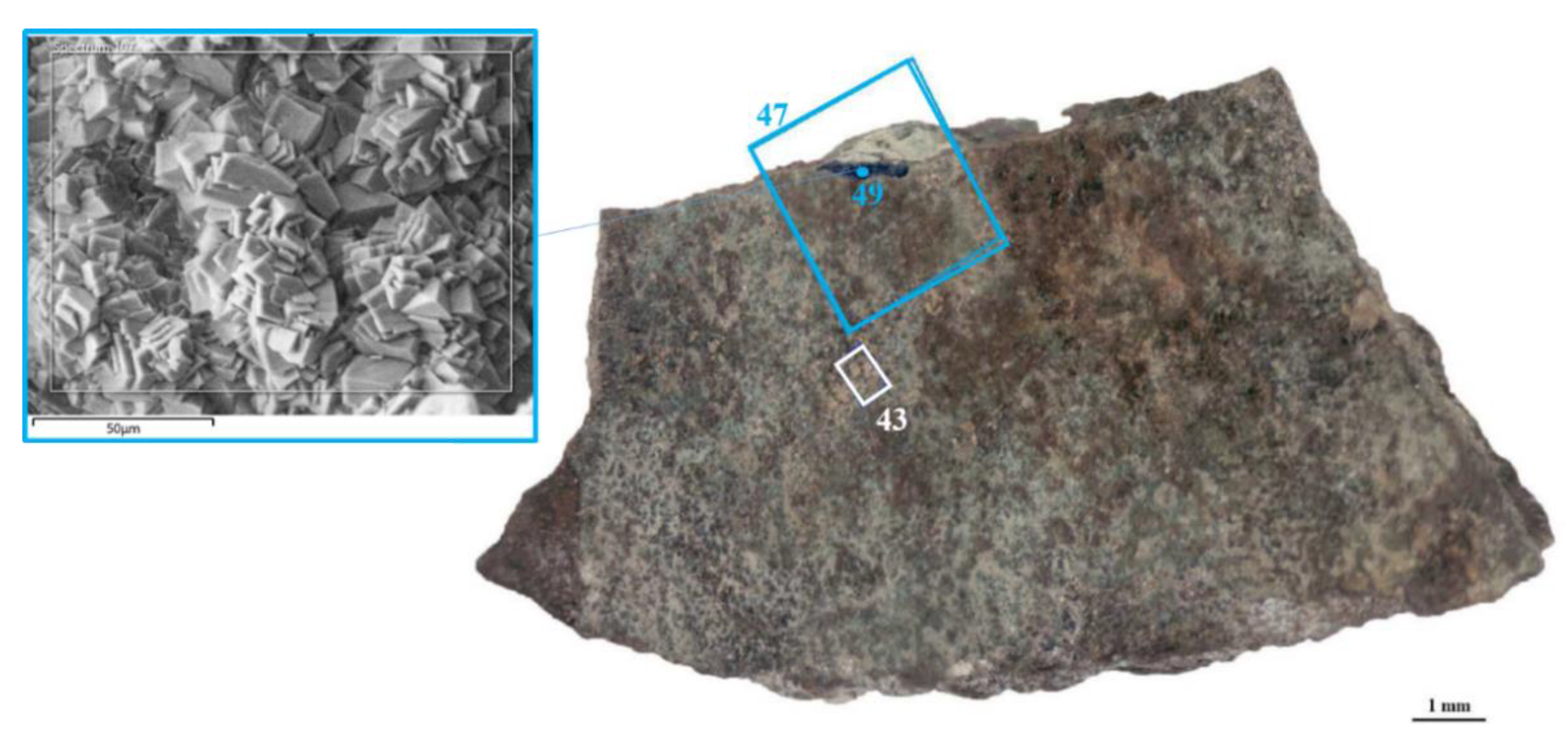

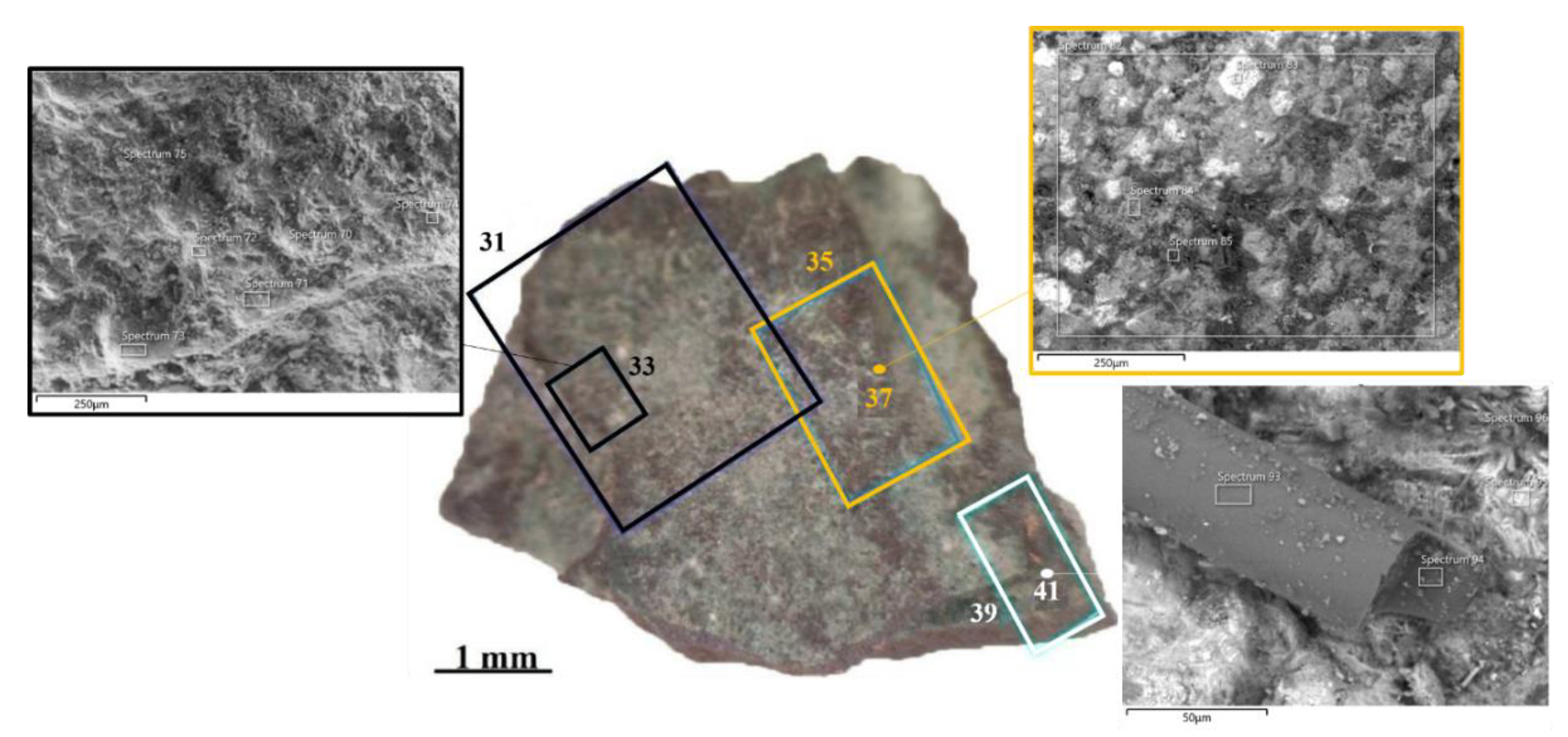
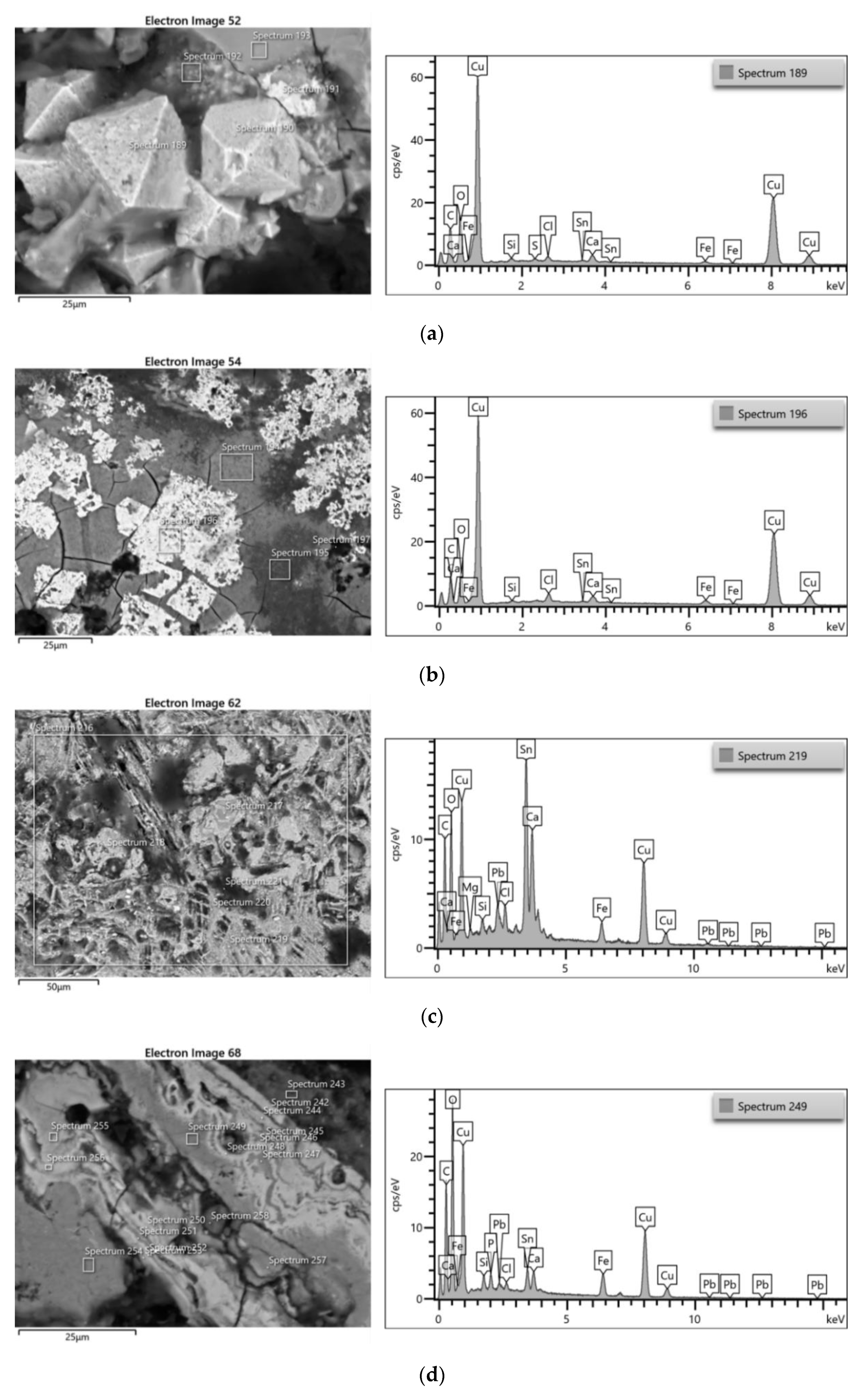
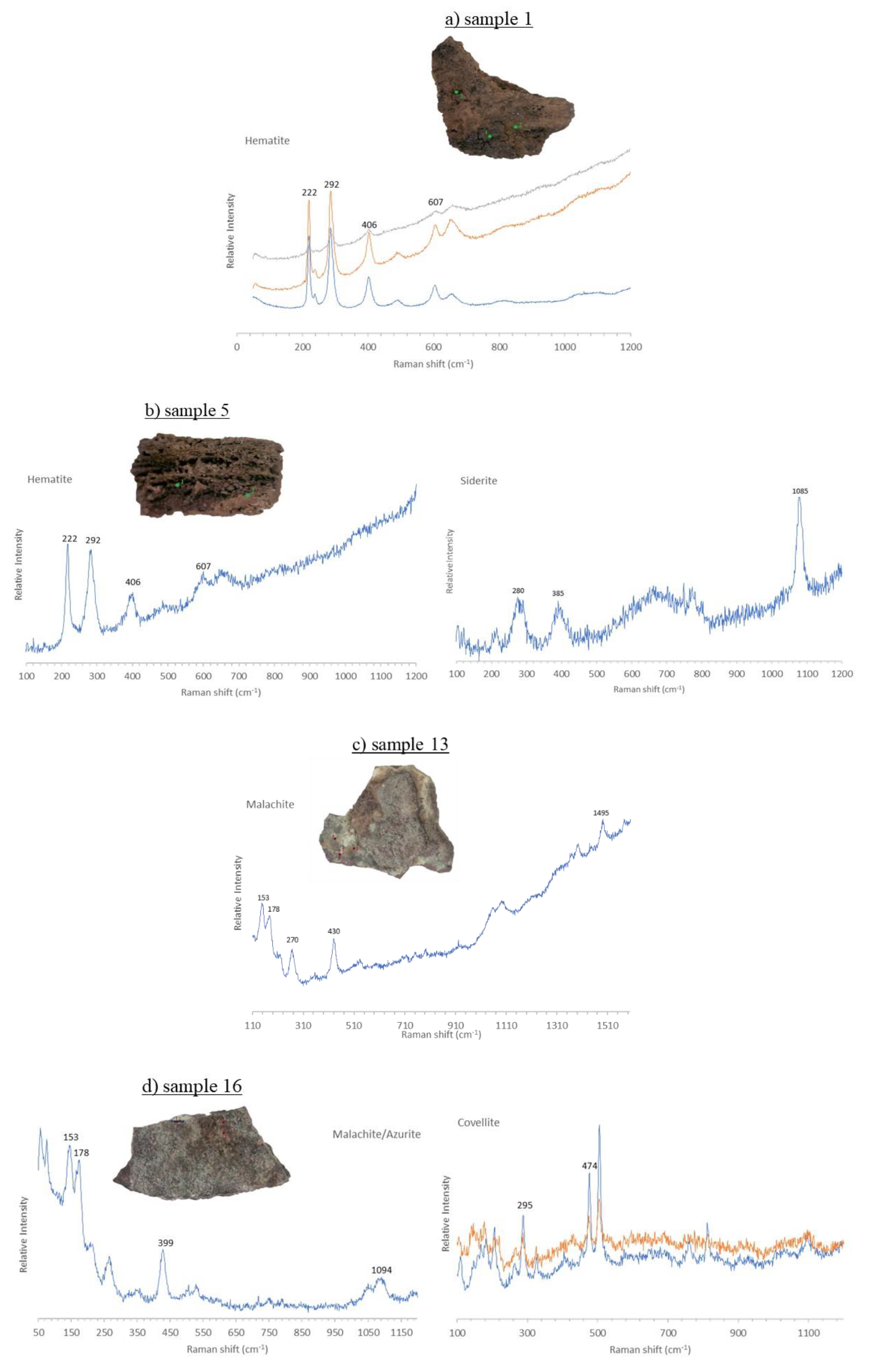
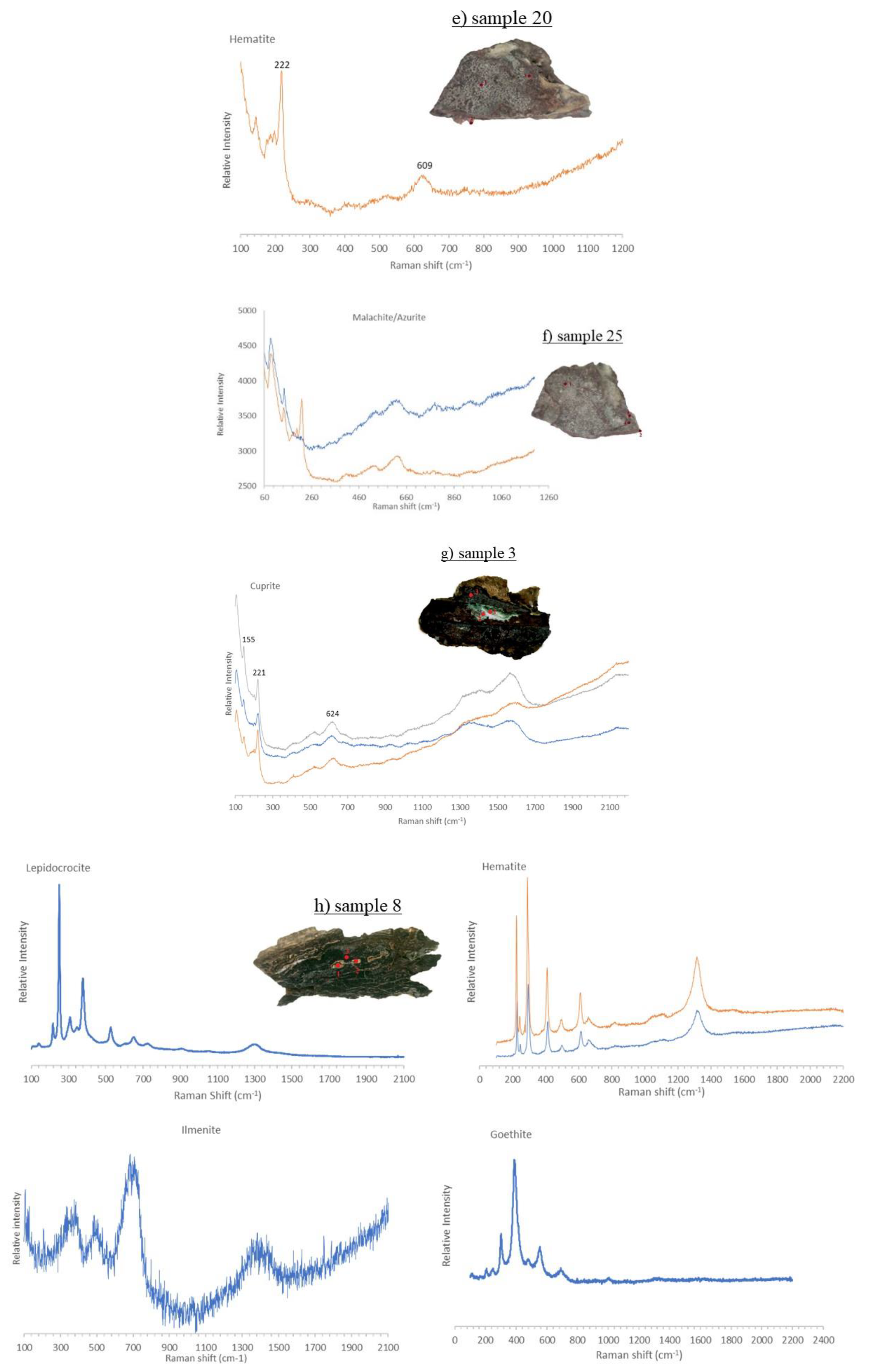
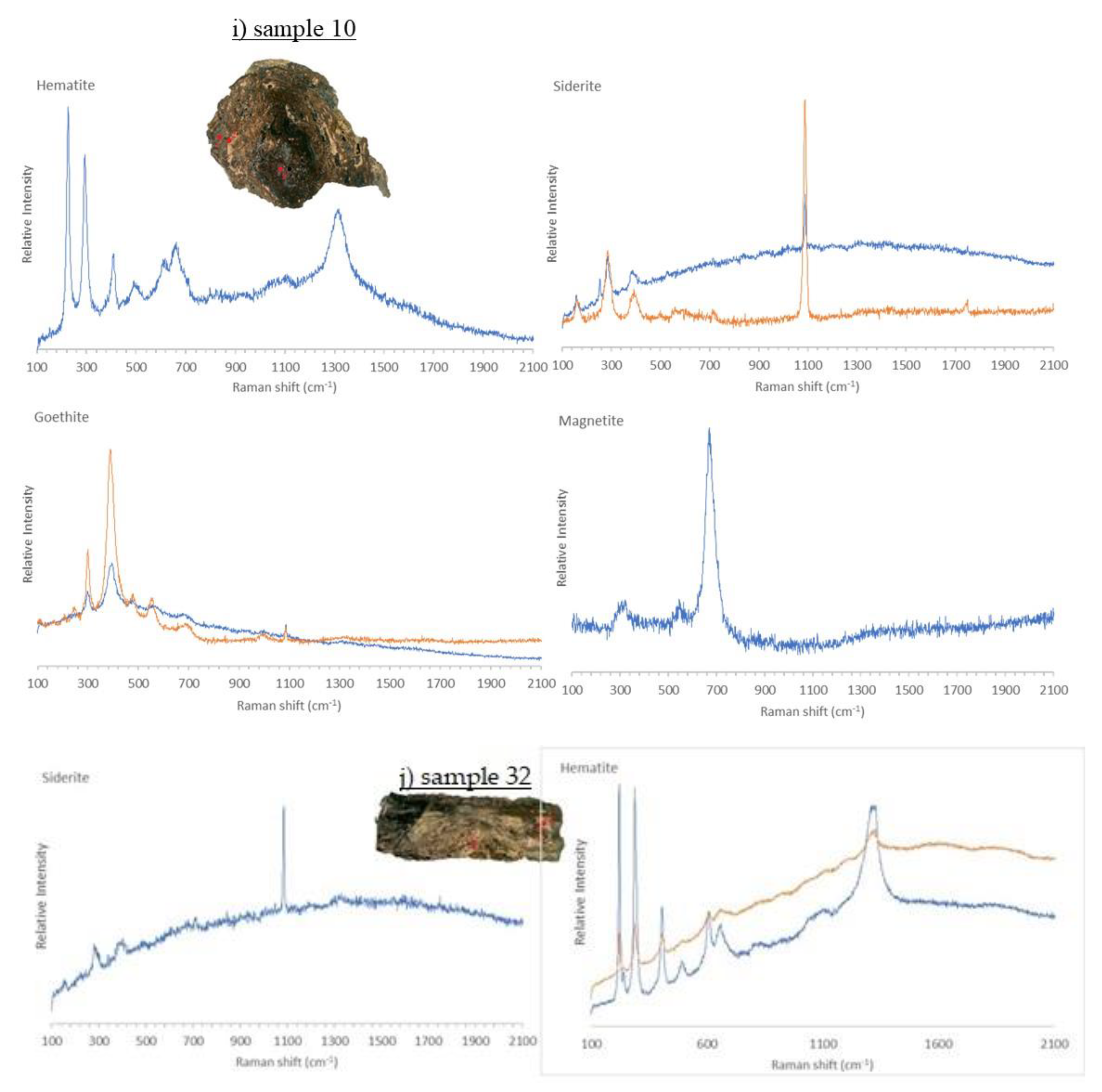
| ID sample | Length | Width | Thickness | Material | Origin |
|---|---|---|---|---|---|
| 1 | 8 | 7 | 2 | Iron | Wheels |
| 3 | 8 | 4 | 1 | Bronze | Pair ring |
| 5 | 5 | 8 | 2 | Iron | Axle |
| 8 | 12 | 9 | 3 | Iron | Wheels |
| 10 | 9 | 8 | 6 | Iron | Axle |
| 13 | 9 | 11 | 1 | Bronze | Hubcaps |
| 16 | 7 | 15 | 1 | Bronze | Hubcaps |
| 20 | 5 | 10 | 1 | Bronze | Hubcaps |
| 25 | 6 | 6 | 1 | Bronze | Hubcaps |
| 32 | 26 | 8 | 2 | Iron | Wheels |
| Iron | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | SO2 | Cl | ZnO | CuO | P2O5 | Tot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sample 1 | 1.46 | 4.02 | 81.54 | 0.48 | 1.92 | 1.12 | 0.33 | 0.44 | 0.21 | 1.91 | 0.39 | 6.19 | 100 |
| 0.64 | 2.52 | 82.71 | 0.48 | 1.65 | 0.97 | 0.22 | 0.25 | 0.23 | 2.07 | 0.42 | 7.84 | 100 | |
| 0.63 | 2.09 | 87.79 | 0.39 | 1.70 | 0.57 | 0.21 | 0.25 | n.d. | 1.57 | 0.30 | 4.52 | 100 | |
| 0.48 | 1.39 | 90.50 | 0.24 | 1.33 | 0.78 | 0.23 | 0.21 | n.d. | 1.12 | n.d. | 3.73 | 100 | |
| 0.46 | 0.63 | 93.49 | 0.24 | 0.92 | 0.39 | 0.12 | 0.17 | n.d. | 1.28 | n.d. | 2.31 | 100 | |
| 3.72 | 0.52 | 84.70 | 0.52 | 2.02 | 0.72 | 0.18 | 0.46 | n.d. | 1.77 | n.d. | 5.38 | 100 | |
| sample 5 | 1.79 | 1.70 | 87.54 | 0.30 | 1.07 | 0.77 | n.d. | 0.17 | 0.17 | 1.58 | 0.88 | 4.02 | 100 |
| 0.99 | 1.63 | 90.94 | 0.25 | 0.67 | 0.61 | n.d. | 0.11 | n.d. | 1.09 | 0.78 | 2.93 | 100 | |
| 1.04 | 1.07 | 90.22 | 0.34 | 1.04 | 0.73 | n.d. | n.d. | 0.22 | 1.14 | 0.84 | 3.37 | 100 | |
| 0.60 | 7.22 | 89.27 | 0.53 | 0.77 | n.d. | n.d. | 0.13 | 0.13 | n.d. | 0.52 | 0.84 | 100 | |
| sample 8 | 0.20 | n.d. | 94.38 | n.d. | 0.29 | n.d. | n.d. | n.d. | 4.61 | n.d. | n.d. | 0.51 | 100 |
| 0.18 | n.d. | 98.45 | n.d. | 0.17 | n.d. | n.d. | n.d. | 1.20 | n.d. | n.d. | n.d. | 100 | |
| 0.31 | n.d. | 98.37 | n.d. | 0.25 | n.d. | n.d. | n.d. | 1.07 | n.d. | n.d. | n.d. | 100 | |
| 3.81 | 1.27 | 93.15 | n.d. | 0.63 | n.d. | 0.47 | n.d. | 0.67 | n.d. | n.d. | n.d. | 100 | |
| sample 10 | 0.27 | n.d. | 98.02 | n.d. | 0.75 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.97 | 100 |
| 0.22 | n.d. | 96.49 | n.d. | 1.97 | n.d. | 0.00 | 0.25 | 0.22 | n.d. | n.d. | 0.84 | 100 | |
| 0.34 | n.d. | 97.91 | 0.16 | 0.81 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.77 | 100 | |
| 2.09 | 1.99 | 87.49 | 0.33 | 2.87 | n.d. | 0.30 | 0.35 | 0.20 | 0.44 | 0.28 | 3.67 | 100 | |
| sample 32 | 70.40 | 1.19 | 20.47 | 0.20 | 7.50 | n.d. | n.d. | n.d. | 0.24 | n.d. | n.d. | n.d. | 100 |
| 0.49 | 0.46 | 90.96 | n.d. | 6.87 | n.d. | n.d. | 0.20 | 0.56 | n.d. | n.d. | 0.46 | 100 | |
| 0.73 | 0.61 | 33.81 | 0.98 | 63.47 | n.d. | n.d. | n.d. | 0.39 | n.d. | n.d. | n.d. | 100 | |
| 0.46 | 0.41 | 85.93 | 0.19 | 10.97 | n.d. | n.d. | n.d. | 1.59 | n.d. | n.d. | 0.45 | 100 | |
| 0.56 | n.d. | 41.31 | 0.22 | 57.91 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 100 | |
| 0.19 | n.d. | 96.33 | 0.00 | 0.67 | n.d. | n.d. | n.d. | 0.20 | 0.37 | n.d. | 2.24 | 100 |
| Bronze | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | SO2 | Cl | CuO | SnO | PbO | P2O5 | Tot |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sample 13 | 0.57 | 3.60 | 1.48 | 0.22 | 0.40 | n.d. | n.d. | n.d. | 0.51 | 89.63 | 3.12 | 0.47 | n.d. | 100 |
| 0.31 | 2.21 | 0.93 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.28 | 94.53 | 1.73 | n.d. | n.d. | 100 | |
| 0.21 | 2.97 | 1.57 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.17 | 90.17 | 4.91 | n.d. | n.d. | 100 | |
| 0.51 | 2.87 | 3.71 | n.d. | n.d. | n.d. | n.d. | n.d. | 0.24 | 76.76 | 14.34 | 1.10 | 0.47 | 100 | |
| 0.87 | 2.62 | 2.34 | 0.71 | 2.51 | n.d. | n.d. | 0.19 | 1.65 | 85.26 | 2.95 | 0.48 | 0.43 | 100 | |
| 1.18 | 0.62 | 14.51 | 0.35 | n.d. | n.d. | n.d. | n.d. | 1.32 | 21.64 | 55.02 | 3.66 | 1.70 | 100 | |
| 1.67 | 1.74 | 23.04 | 0.76 | 1.00 | n.d. | n.d. | n.d. | n.d. | 20.59 | 44.98 | 3.54 | 2.67 | 100 | |
| sample 16 | 3.58 | 2.29 | 21.70 | 0.85 | 1.29 | 1.47 | n.d. | 0.87 | 1.45 | 26.42 | 33.58 | 3.66 | 2.82 | 100 |
| 0.61 | 0.61 | 1.39 | n.d. | 0.20 | n.d. | n.d. | n.d. | 0.27 | 93.94 | 2.98 | n.d. | n.d. | 100 | |
| 0.47 | 0.43 | 1.62 | n.d. | 0.17 | n.d. | n.d. | n.d. | 0.26 | 91.08 | 2.52 | 0.41 | 3.04 | 100 | |
| sample 20 | 2.32 | 2.15 | 4.44 | 0.38 | 0.70 | n.d. | 0.38 | 1.09 | 1.08 | 82.39 | 4.47 | n.d. | 0.61 | 100 |
| 1.84 | 1.73 | 4.23 | 0.43 | 0.72 | n.d. | n.d. | 0.37 | 0.60 | 81.84 | 6.79 | 0.81 | 0.64 | 100 | |
| 3.44 | 4.41 | 17.72 | 0.60 | 1.49 | 0.66 | n.d. | 0.75 | 0.29 | 12.53 | 52.08 | 3.65 | 2.38 | 100 | |
| 2.30 | 4.24 | 17.49 | 0.61 | n.d. | n.d. | 1.00 | 0.72 | n.d. | 9.43 | 57.81 | 4.09 | 2.31 | 100 | |
| 1.53 | 1.52 | 10.18 | 0.63 | 0.56 | n.d. | n.d. | n.d. | 0.24 | 47.16 | 33.71 | 2.76 | 1.71 | 100 | |
| 0.22 | 0.37 | 0.46 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 97.67 | 1.28 | n.d. | n.d. | 100 | |
| 0.50 | 0.49 | 3.62 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 75.77 | 18.24 | 1.37 | n.d. | 100 | |
| 12.33 | 10.48 | 6.36 | 1.50 | 1.32 | 0.37 | 1.77 | 0.25 | 0.17 | 64.34 | 0.45 | 0.36 | 0.29 | 100 | |
| sample 25 | 5.67 | 3.85 | 29.79 | 0.77 | 1.62 | n.d. | 0.47 | 0.80 | 0.51 | 25.18 | 27.59 | 2.09 | 1.65 | 100 |
| 7.50 | 5.19 | 12.53 | 1.09 | 9.49 | n.d. | 0.70 | 0.98 | 0.35 | 35.50 | 24.89 | n.d. | 1.76 | 100 | |
| 3.99 | 1.12 | 3.87 | 0.56 | 0.73 | n.d. | 0.00 | 0.47 | 0.55 | 78.74 | 9.02 | 0.95 | n.d. | 100 | |
| 3.46 | 2.41 | 1.69 | 0.52 | 0.49 | n.d. | 0.35 | 0.28 | 0.55 | 89.69 | 0.56 | n.d. | n.d. | 100 | |
| 4.96 | 3.27 | 3.86 | 0.75 | 1.20 | n.d. | 0.46 | 0.80 | 0.42 | 83.56 | 0.72 | n.d. | n.d. | 100 | |
| 44.25 | 7.71 | 2.54 | 2.78 | 5.77 | 20.23 | 1.61 | 0.77 | 0.00 | 11.67 | 2.66 | n.d. | n.d. | 100 | |
| sample 3 | 0.65 | n.d. | 1.81 | n.d. | 1.15 | n.d. | n.d. | 0.40 | 0.92 | 94.54 | 0.53 | n.d. | n.d. | 100 |
| 0.38 | n.d. | 3.06 | n.d. | 0.94 | n.d. | n.d. | n.d. | 1.53 | 93.75 | 0.34 | n.d. | n.d. | 100 | |
| 1.21 | n.d. | 8.12 | 0.81 | 1.37 | n.d. | n.d. | n.d. | 2.30 | 50.08 | 33.14 | 2.97 | n.d. | 100 | |
| 1.95 | n.d. | 15.45 | n.d. | 1.39 | n.d. | n.d. | n.d. | 1.02 | 67.40 | 6.64 | 1.39 | 4.76 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrocchino, E.; Telloli, C.; Finotti, S.; Facchi, A.; Eftekhari, N.; De Vito, C. Microstructure, Chemistry and Mineralogy Approach for the Diagnostics of Metallic Finds of the Tomba della Biga (Adria, Italy). Appl. Sci. 2022, 12, 11365. https://doi.org/10.3390/app122211365
Marrocchino E, Telloli C, Finotti S, Facchi A, Eftekhari N, De Vito C. Microstructure, Chemistry and Mineralogy Approach for the Diagnostics of Metallic Finds of the Tomba della Biga (Adria, Italy). Applied Sciences. 2022; 12(22):11365. https://doi.org/10.3390/app122211365
Chicago/Turabian StyleMarrocchino, Elena, Chiara Telloli, Sara Finotti, Alberta Facchi, Negar Eftekhari, and Caterina De Vito. 2022. "Microstructure, Chemistry and Mineralogy Approach for the Diagnostics of Metallic Finds of the Tomba della Biga (Adria, Italy)" Applied Sciences 12, no. 22: 11365. https://doi.org/10.3390/app122211365
APA StyleMarrocchino, E., Telloli, C., Finotti, S., Facchi, A., Eftekhari, N., & De Vito, C. (2022). Microstructure, Chemistry and Mineralogy Approach for the Diagnostics of Metallic Finds of the Tomba della Biga (Adria, Italy). Applied Sciences, 12(22), 11365. https://doi.org/10.3390/app122211365







