A Review on the Motion of Magnetically Actuated Bio-Inspired Microrobots
Abstract
:1. Introduction
2. Bio-Inspired Mechanisms Used in Locomotion
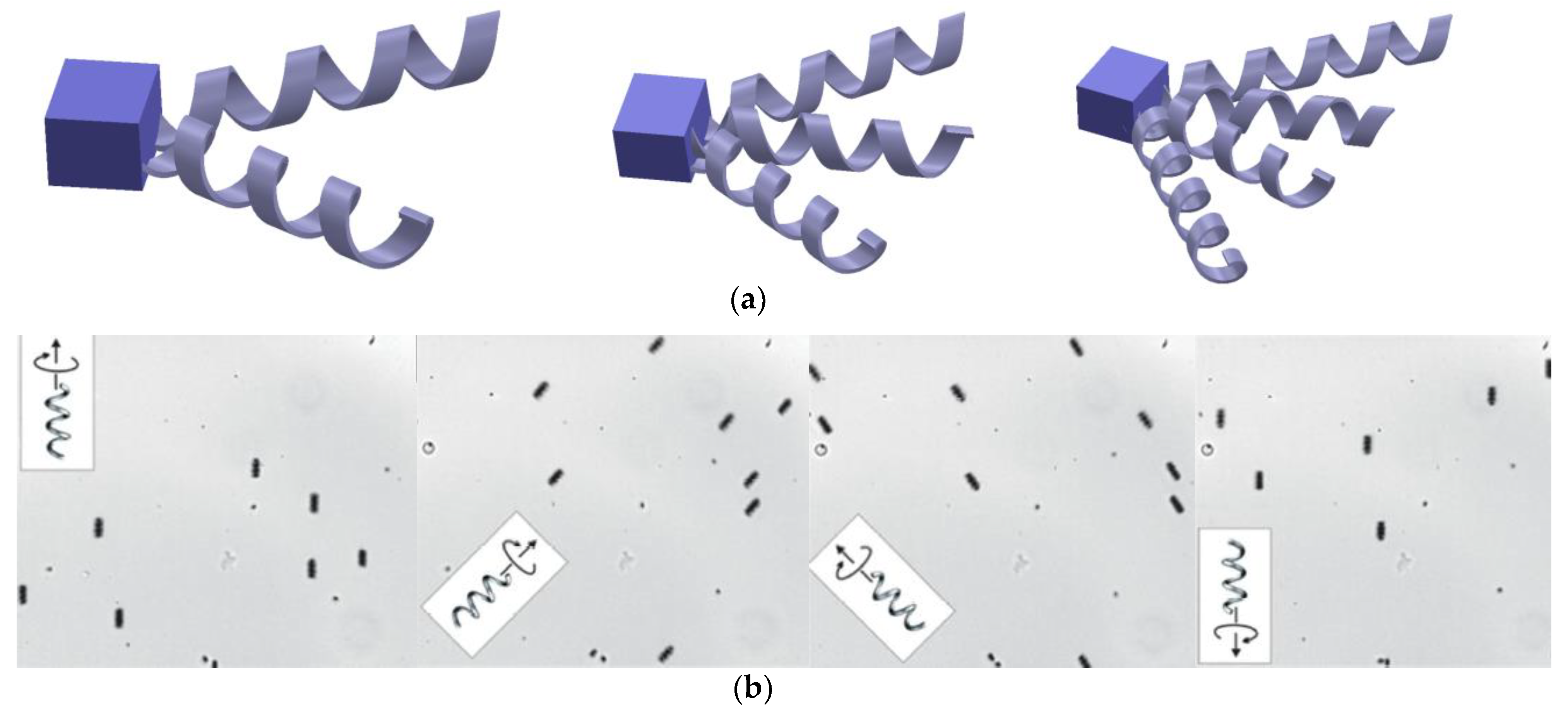
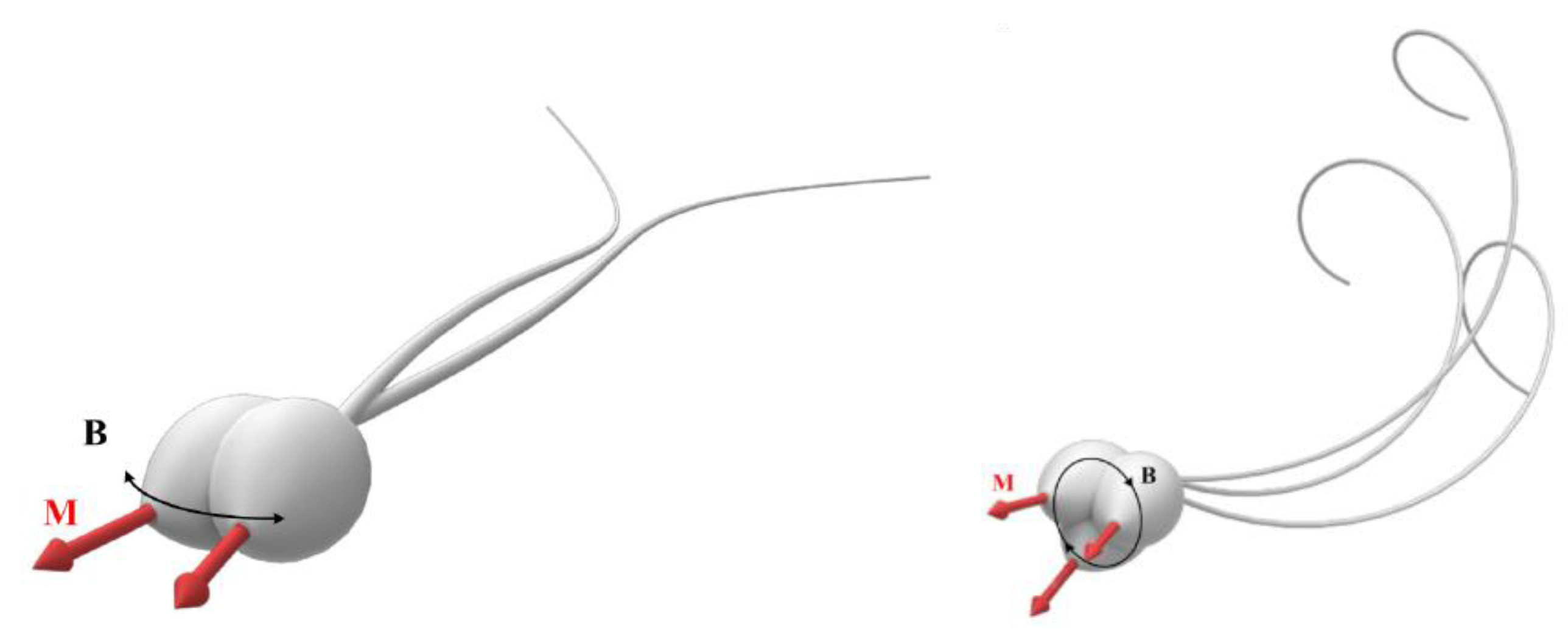
2.1. Bacteria Flagella
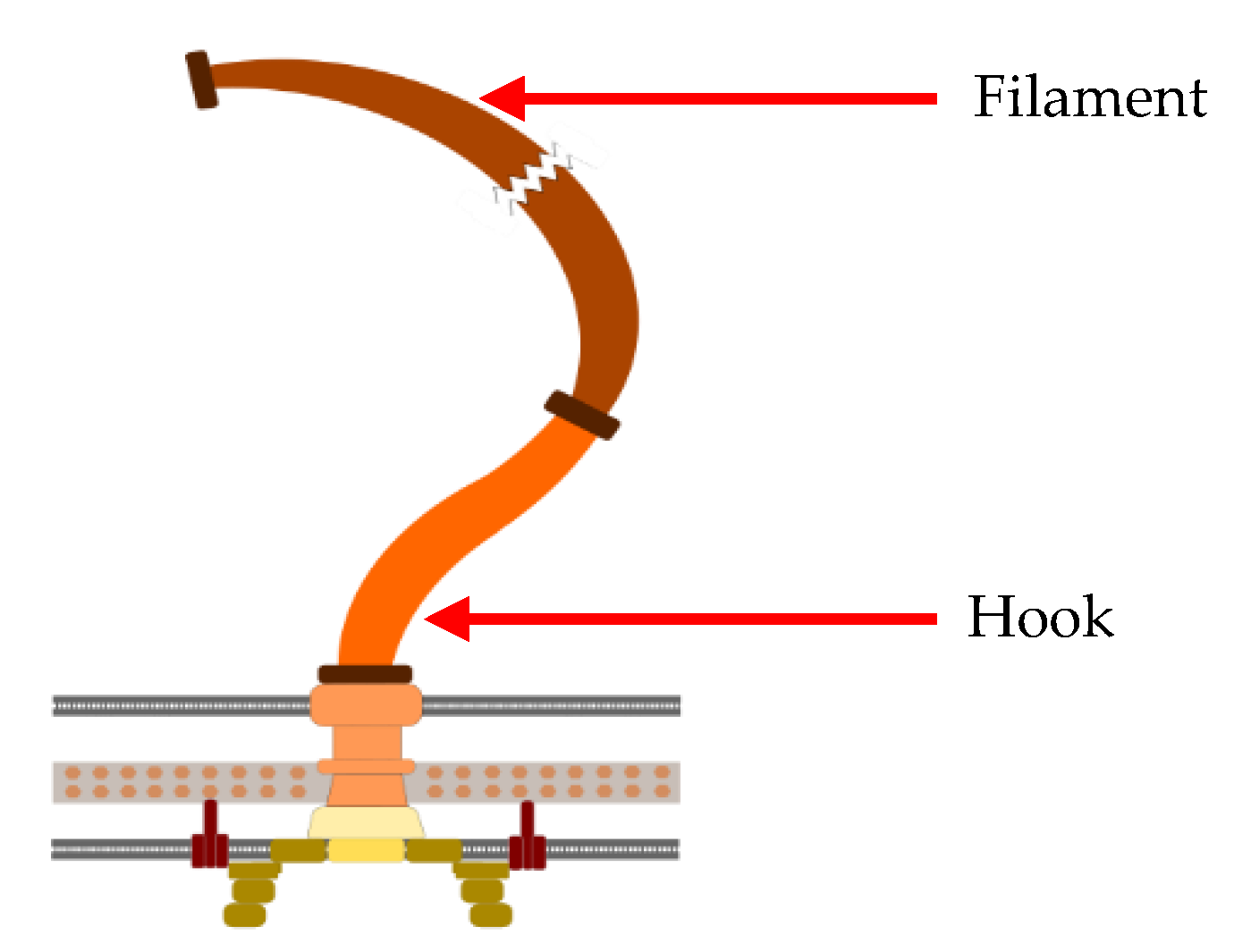
2.2. Sperm Flagella
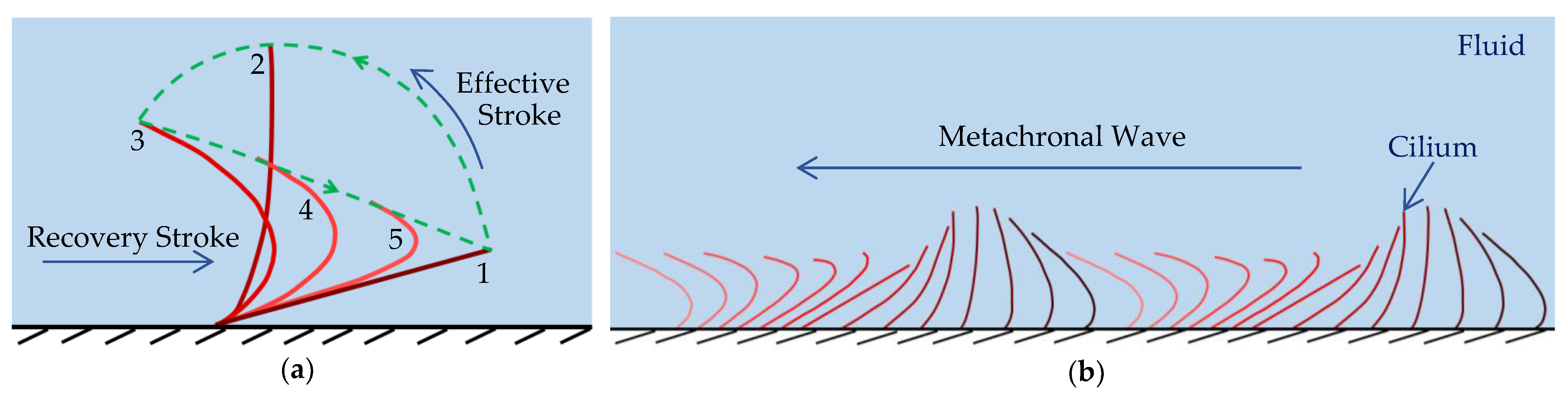
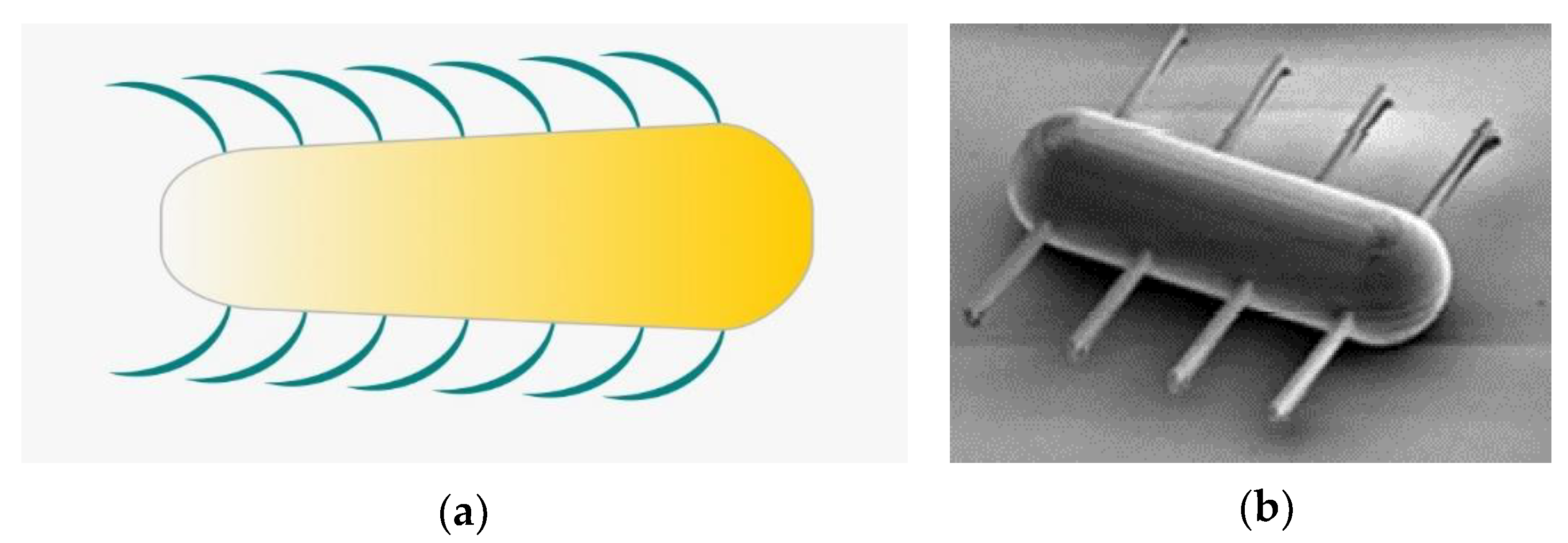
2.3. Cilia
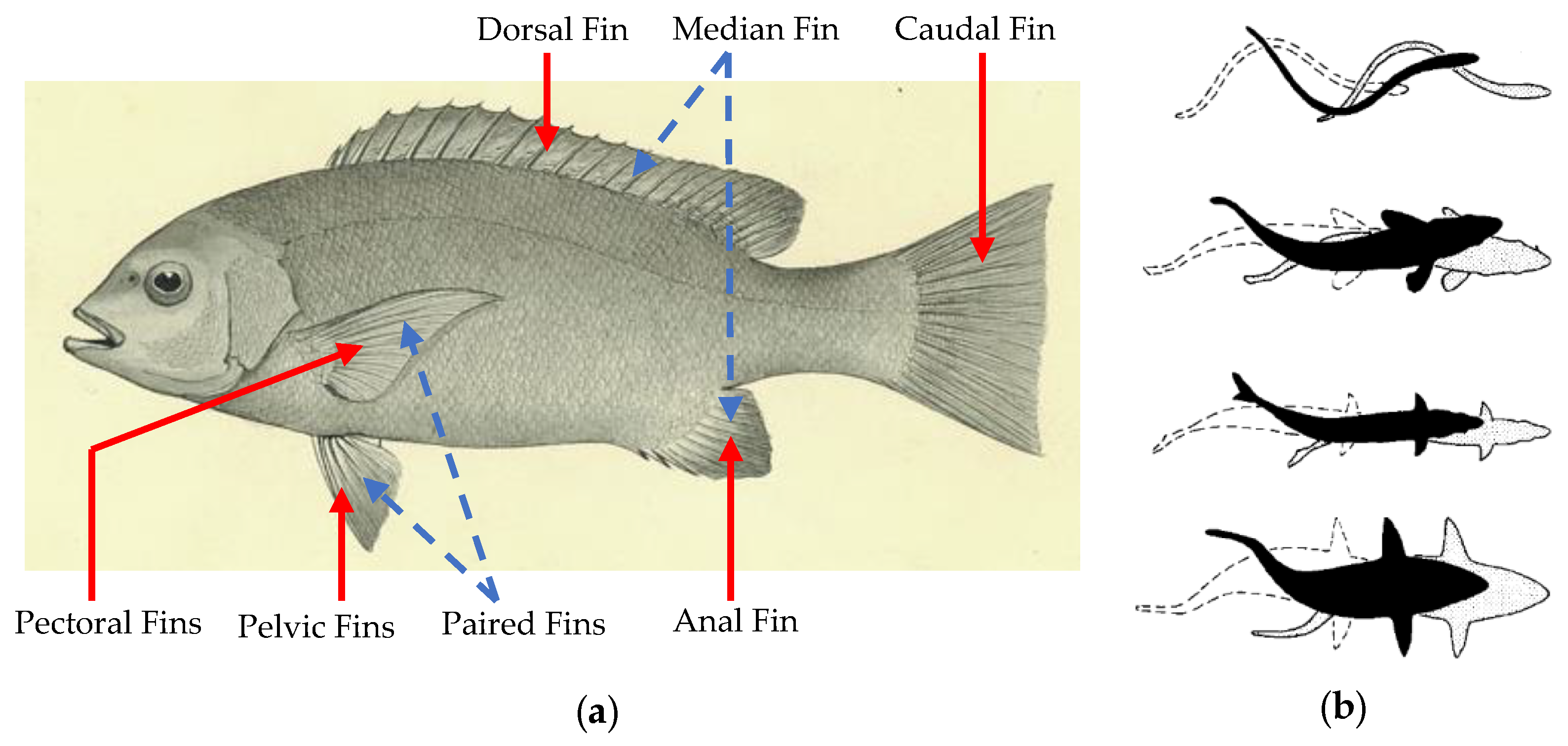
2.4. Fish
3. Parameters Related to the Motion of Microrobots
4. Materials and Fabrication Methods
5. Magnetic Field Generation Techniques
6. Discussion and Future Directions
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hettiarachchi, S.; Melroy, G.; Mudugamuwa, A.; Sampath, P.; Premachandra, C.; Amarasinghe, R.; Dau, V. Design and Development of a Microfluidic Droplet Generator with Vision Sensing for Lab-on-a-Chip Devices. Sens. Actuators A Phys. 2021, 332, 113047. [Google Scholar] [CrossRef]
- Mudugamuwa, A.P.; Hettiarachchi, S.P.; Basnayake, B.A.D.J.C.K.; Melroy, N.H.R.G.; Amarasinghe, Y.W.R. Review on Photomicrography Based Full Blood Count (FBC) Testing and Recent Advancements. Adv. Technol. 2021, 1, 422–453. [Google Scholar] [CrossRef]
- Kankanige, N.M.P.; Hanchapola Appuhamilage, G.C.P.; Dodampegama, S.K.; Yattowita Withanage, R.A. Design and Simulation of a Novel Magnetic Microactuator for Microrobots in Lab-On-a-Chip Applications. Adv. Technol. 2022, 2, 221–232. [Google Scholar]
- Suriyage, M.P.; Asanka, P.V.K.; Cooray, T.M.G.C.S.P.; Liyanage, D.L.F.M.; Pushpakumara, R.A.N.I.; Hendavitharana, D.K.; Fernando, W.W.A.T.I.; Sampath, W.H.P.; Amarasinghe, Y.W.R. Design and Simulation of a Novel MEMS Based Microfluidic Circulating Tumor Cell (CTC) Detection System for a Lab on a Chip Device. Proceedings of the 11th International Conference on Mechatronics and Manufacturing (ICMM 2020) Volume 895, 012016. [CrossRef]
- Mudugamuwa, A.; Hettiarachchi, S.; Melroy, G.; Amarasinghe, R. Deep Learning Based Cell Classification for Future Vision Implemented Lab-on-a-Chip Devices; KDU IRC: Ratmalana, Sri Lanka, 2021. [Google Scholar] [CrossRef]
- Hettiarachchi, S.; Mudugamuwa, A.; Amarasinghe, R. Microfluidic Channel Network Analysis of a Lab-on-a-Chip Device Using Electrical Circuit Analogy. In Proceedings of the 2020 From Innovation to Impact (FITI), Colombo, Sri Lanka, 15 December 2020; pp. 1–4. [Google Scholar]
- Melroy, G.; Mudugamuwa, A.; Hettiarachchi, S.; Amarasinghe, R.; Dau, V.; Kumarage, P.; Jayaweera, N.; Qing-guang, C. PZT Based Active Microfluidic Droplet Generator for Lab-on-a-Chip Devices. In Sustainable Design and Manufacturing; Scholz, S.G., Howlett, R.J., Setchi, R., Eds.; Smart Innovation Systems and Technologies; Springer: Singapore, 2022; Volume 262, pp. 277–289. ISBN 9789811661273. [Google Scholar]
- Mudugamuwa, A.; Hettiarachchi, S.; Melroy, G.; Dodampegama, S.; Konara, M.; Roshan, U.; Amarasinghe, R.; Jayathilaka, D.; Wang, P. Vision-Based Performance Analysis of an Active Microfluidic Droplet Generation System Using Droplet Images. Sensors 2022, 22, 6900. [Google Scholar] [CrossRef]
- Erkoc, P.; Yasa, I.C.; Ceylan, H.; Yasa, O.; Alapan, Y.; Sitti, M. Mobile Microrobots for Active Therapeutic Delivery. Adv. Therap. 2019, 2, 1800064. [Google Scholar] [CrossRef] [Green Version]
- Wijegunawardana, I.D.; Amarasinghe, Y.W.R. The Role of MEMS in In-Vitro-Fertilization. Adv. Technol. 2021, 1, 235–255. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.; Piel, B.; Rai, P. Cancer Nanomedicine: A Review of Recent Success in Drug Delivery. Clin. Transl. Med. 2017, 6, 44. [Google Scholar] [CrossRef] [Green Version]
- Ceylan, H.; Giltinan, J.; Kozielski, K.; Sitti, M. Mobile Microrobots for Bioengineering Applications. Lab Chip 2017, 17, 1705–1724. [Google Scholar] [CrossRef] [Green Version]
- Yesin, K.B.; Exner, P.; Vollmers, K.; Nelson, B.J. Design and Control of In-Vivo Magnetic Microrobots. In Medical Image Computing and Computer-Assisted Intervention–MICCAI 2005; Duncan, J.S., Gerig, G., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2005; Volume 3749, pp. 819–826. ISBN 978-3-540-29327-9. [Google Scholar]
- Alapan, Y.; Yasa, O.; Yigit, B.; Yasa, I.C.; Erkoc, P.; Sitti, M. Microrobotics and Microorganisms: Biohybrid Autonomous Cellular Robots. Annu. Rev. Control Robot. Auton. Syst. 2019, 2, 205–230. [Google Scholar] [CrossRef]
- Soto, F.; Karshalev, E.; Zhang, F.; Esteban Fernandez de Avila, B.; Nourhani, A.; Wang, J. Smart Materials for Microrobots. Chem. Rev. 2022, 122, 5365–5403. [Google Scholar] [CrossRef]
- Schmidt, C.K.; Medina-Sánchez, M.; Edmondson, R.J.; Schmidt, O.G. Engineering Microrobots for Targeted Cancer Therapies from a Medical Perspective. Nat. Commun. 2020, 11, 5618. [Google Scholar] [CrossRef]
- Elgeti, J.; Winkler, R.G.; Gompper, G. Physics of Microswimmers—Single Particle Motion and Collective Behavior: A Review. Rep. Prog. Phys. 2015, 78, 056601. [Google Scholar] [CrossRef] [Green Version]
- Vyskočil, J.; Mayorga-Martinez, C.C.; Jablonská, E.; Novotný, F.; Ruml, T.; Pumera, M. Cancer Cells Microsurgery via Asymmetric Bent Surface Au/Ag/Ni Microrobotic Scalpels Through a Transversal Rotating Magnetic Field. ACS Nano 2020, 14, 8247–8256. [Google Scholar] [CrossRef]
- Aghakhani, A.; Yasa, O.; Wrede, P.; Sitti, M. Acoustically Powered Surface-Slipping Mobile Microrobots. Proc. Natl. Acad. Sci. USA 2020, 117, 3469–3477. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.M.; Nam, J.K.; Choi, K.; Jang, G.H. A Self-Positioning and Rolling Magnetic Microrobot on Arbitrary Thin Surfaces. J. Appl. Phys. 2014, 115, 17E303. [Google Scholar] [CrossRef]
- Behkam, B.; Sitti, M. Bacteria Integrated Swimming Microrobots. In 50 Years of Artificial Intelligence; Lungarella, M., Iida, F., Bongard, J., Pfeifer, R., Eds.; Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2007; Volume 4850, pp. 154–163. ISBN 978-3-540-77295-8. [Google Scholar]
- Wu, Z.; Troll, J.; Jeong, H.-H.; Wei, Q.; Stang, M.; Ziemssen, F.; Wang, Z.; Dong, M.; Schnichels, S.; Qiu, T.; et al. A Swarm of Slippery Micropropellers Penetrates the Vitreous Body of the Eye. Sci. Adv. 2018, 4, eaat4388. [Google Scholar] [CrossRef] [Green Version]
- Palima, D.; Glückstad, J. Gearing up for Optical Microrobotics: Micromanipulation and Actuation of Synthetic Microstructures by Optical Forces. Laser Photonics Rev. 2013, 7, 478–494. [Google Scholar] [CrossRef] [Green Version]
- Solovev, A.A.; Mei, Y.; Bermúdez Ureña, E.; Huang, G.; Schmidt, O.G. Catalytic Microtubular Jet Engines Self-Propelled by Accumulated Gas Bubbles. Small 2009, 5, 1688–1692. [Google Scholar] [CrossRef]
- Karpelson, M.; Wei, G.-Y.; Wood, R.J. Driving High Voltage Piezoelectric Actuators in Microrobotic Applications. Sens. Actuators A Phys. 2012, 176, 78–89. [Google Scholar] [CrossRef]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-Inspired Magnetic Swimming Microrobots for Biomedical Applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yu, J.; Yan, X.; Choi, H.; Zhang, L. Magnetic Actuation Based Motion Control for Microrobots: An Overview. Micromachines 2015, 6, 1346–1364. [Google Scholar] [CrossRef]
- Schuerle, S.; Pané, S.; Pellicer, E.; Sort, J.; Baró, M.D.; Nelson, B.J. Helical and Tubular Lipid Microstructures That Are Electroless-Coated with CoNiReP for Wireless Magnetic Manipulation. Small 2012, 8, 1498–1502. [Google Scholar] [CrossRef]
- Kim, E.; Jeon, S.; An, H.-K.; Kianpour, M.; Yu, S.-W.; Kim, J.; Rah, J.-C.; Choi, H. A Magnetically Actuated Microrobot for Targeted Neural Cell Delivery and Selective Connection of Neural Networks. Sci. Adv. 2020, 6, eabb5696. [Google Scholar] [CrossRef]
- Sitti, M. Microscale and Nanoscale Robotics Systems [Grand Challenges of Robotics]. IEEE Robot. Automat. Mag. 2007, 14, 53–60. [Google Scholar] [CrossRef]
- Dodampegama, S.K.; Konara, K.M.T.M.B.; Munasinghe, M.A.A.; Amarasinghe, Y.W.R. Design and Analysis of Hybrid Robotic Mechanisms Using SCARA and RCM Mechanisms. In Proceedings of the 2020 From Innovation to Impact (FITI), Colombo, Sri Lanka, 15 December 2020. [Google Scholar]
- Perera, K.N.M.; Amarasinghe, Y.W.R.; Dao, D.V. An Artificial Appendage for Swimming Microrobots in Non-Newtonian Fluids. In Proceedings of the 2021 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 27 July 2021; pp. 723–727. [Google Scholar]
- Naser, F.A.; Rashid, M.T. Effect of Reynold Number and Angle of Attack on the Hydrodynamic Forces Generated from A Bionic Concave Pectoral Fins. Proceedings of the Fourth Scientific Conference for Engineering and Postgraduate Research Volume 745, 012026. [CrossRef]
- Purcell, E.M. Life at Low Reynolds Number. Am. J. Phys. 1977, 45, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Palagi, S.; Fischer, P. Bioinspired Microrobots. Nat. Rev. Mater. 2018, 3, 113–124. [Google Scholar] [CrossRef]
- Yuan, J.; Cho, S.K. Bio-Inspired Micro/Mini Propulsion at Air-Water Interface: A Review. J. Mech. Sci. Technol. 2012, 26, 3761–3768. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Zhang, H.; Chang, X.; Song, W.; Hu, Y.; Shao, G.; Sandraz, E.; Zhang, G.; Li, L.; et al. Magnetically Propelled Fish-Like Nanoswimmers. Small 2016, 12, 6098–6105. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Dijkslag, H.C.; Abelmann, L.; Misra, S. MagnetoSperm: A Microrobot That Navigates Using Weak Magnetic Fields. Appl. Phys. Lett. 2014, 104, 223701. [Google Scholar] [CrossRef]
- Salazar, R.; Fuentes, V.; Abdelkefi, A. Classification of Biological and Bioinspired Aquatic Systems: A Review. Ocean Eng. 2018, 148, 75–114. [Google Scholar] [CrossRef]
- Konara, M.; Munasinghe, M.A.A.; Dodampegama, S.K.; Amarasinghe, R. Design and Analysis of an Autonomously Guided Vehicle to Minimize the Impact of COVID-19. In Proceedings of the 2020 From Innovation to Impact (FITI), Colombo, Sri Lanka, 15 December 2020. [Google Scholar]
- Adam, G.A.; Chowdhury, S.; Guix, M.; Johnson, B.V.; Bi, C.; Cappelleri, D. Towards Functional Mobile Microrobotic Systems. Robotics 2019, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Perera, K.; Premachandra, H.; Amarasinghe, Y. Design of a Magnetostrictive Bimorph for Micromanipulation; KDU: Yokosuka, Japan, 2021; p. 4. [Google Scholar]
- Ha, N.; Goo, N.; Yoon, H. Development of a Propulsion System for a Biomimetic Thruster. Chin. Sci. Bull. 2011, 56, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Peyer, K.E.; Nelson, B.J. Artificial Bacterial Flagella for Micromanipulation. Lab Chip 2010, 10, 2203. [Google Scholar] [CrossRef]
- Schuhmacher, J.S.; Thormann, K.M.; Bange, G. How Bacteria Maintain Location and Number of Flagella? FEMS Microbiol. Rev. 2015, 39, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Hwang, G.; Andreff, N.; Regnier, S. Modeling and Swimming Property Characterizations of Scaled-Up Helical Microswimmers. IEEE/ASME Trans. Mechatron. 2014, 19, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Abbott, J.J.; Dong, L.; Kratochvil, B.E.; Bell, D.; Nelson, B.J. Artificial Bacterial Flagella: Fabrication and Magnetic Control. Appl. Phys. Lett. 2009, 94, 064107. [Google Scholar] [CrossRef] [Green Version]
- Bell, D.J.; Leutenegger, S.; Hammar, K.M.; Dong, L.X.; Nelson, B.J. Flagella-like Propulsion for Microrobots Using a Nanocoil and a Rotating Electromagnetic Field. In Proceedings of the 2007 IEEE International Conference on Robotics and Automation, Rome, Italy, 10–14 April 2007; pp. 1128–1133. [Google Scholar]
- Ghosh, A.; Paria, D.; Singh, H.J.; Venugopalan, P.L.; Ghosh, A. Dynamical Configurations and Bistability of Helical Nanostructures under External Torque. Phys. Rev. E 2012, 86, 031401. [Google Scholar] [CrossRef] [Green Version]
- Hwang, G.; Braive, R.; Couraud, L.; Cavanna, A.; Abdelkarim, O.; Robert-Philip, I.; Beveratos, A.; Sagnes, I.; Haliyo, S.; Régnier, S. Electro-Osmotic Propulsion of Helical Nanobelt Swimmers. Int. J. Robot. Res. 2011, 30, 806–819. [Google Scholar] [CrossRef]
- Tottori, S.; Zhang, L.; Qiu, F.; Krawczyk, K.K.; Franco-Obregón, A.; Nelson, B.J. Magnetic Helical Micromachines: Fabrication, Controlled Swimming, and Cargo Transport. Adv. Mater. 2012, 24, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Abbott, J.J.; Dong, L.; Peyer, K.E.; Kratochvil, B.E.; Zhang, H.; Bergeles, C.; Nelson, B.J. Characterizing the Swimming Properties of Artificial Bacterial Flagella. Nano Lett. 2009, 9, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Localized Non-Contact Manipulation Using Artificial Bacterial Flagella. Appl. Phys. Lett. 2011, 99, 174101. [Google Scholar] [CrossRef]
- Peyer, K.E.; Tottori, S.; Qiu, F.; Zhang, L.; Nelson, B.J. Magnetic Helical Micromachines. Chem. Eur. J. 2013, 19, 28–38. [Google Scholar] [CrossRef]
- Qiu, F.; Fujita, S.; Mhanna, R.; Zhang, L.; Simona, B.R.; Nelson, B.J. Magnetic Helical Microswimmers Functionalized with Lipoplexes for Targeted Gene Delivery. Adv. Funct. Mater. 2015, 25, 1666–1671. [Google Scholar] [CrossRef]
- Ullrich, F.; Qiu, F.; Pokki, J.; Huang, T.; Pane, S.; Nelson, B.J. Swimming Characteristics of Helical Microrobots in Fibrous Environments. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 470–475. [Google Scholar]
- Mhanna, R.; Qiu, F.; Zhang, L.; Ding, Y.; Sugihara, K.; Zenobi-Wong, M.; Nelson, B.J. Artificial Bacterial Flagella for Remote-Controlled Targeted Single-Cell Drug Delivery. Small 2014, 10, 1953–1957. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, S.; Ha, S.; Lee, S.; Kim, E.; Kim, S.Y.; Park, S.H.; Jeon, J.H.; Kim, S.W.; Moon, C.; et al. Magnetically Actuated Microrobots as a Platform for Stem Cell Transplantation. Sci. Robot. 2019, 4, eaav4317. [Google Scholar] [CrossRef]
- Xu, T.; Hwang, G.; Andreff, N.; Regnier, S. Scaled-up Helical Nanobelt Modeling and Simulation at Low Reynolds Numbers. In Proceedings of the 2012 IEEE International Conference on Robotics and Automation, St. Paul, MN, USA, 14–18 May 2012; pp. 4045–4051. [Google Scholar]
- Gong, D.; Cai, J.; Celi, N.; Feng, L.; Jiang, Y.; Zhang, D. Bio-Inspired Magnetic Helical Microswimmers Made of Nickel-Plated Spirulina with Enhanced Propulsion Velocity. J. Magn. Magn. Mater. 2018, 468, 148–154. [Google Scholar] [CrossRef]
- Gao, W.; Feng, X.; Pei, A.; Kane, C.R.; Tam, R.; Hennessy, C.; Wang, J. Bioinspired Helical Microswimmers Based on Vascular Plants. Nano Lett. 2014, 14, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Zhou, Q.; Yu, J.; Xu, T.; Deng, Y.; Tang, T.; Feng, Q.; Bian, L.; Zhang, Y.; Ferreira, A.; et al. Magnetite Nanostructured Porous Hollow Helical Microswimmers for Targeted Delivery. Adv. Funct. Mater. 2015, 25, 5333–5342. [Google Scholar] [CrossRef]
- Servant, A.; Qiu, F.; Mazza, M.; Kostarelos, K.; Nelson, B.J. Controlled In Vivo Swimming of a Swarm of Bacteria-Like Microrobotic Flagella. Adv. Mater. 2015, 27, 2981–2988. [Google Scholar] [CrossRef]
- Tottori, S.; Nelson, B.J. Artificial Helical Microswimmers with Mastigoneme-Inspired Appendages. Biomicrofluidics 2013, 7, 061101. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Kagan, D.; Pak, O.S.; Clawson, C.; Campuzano, S.; Chuluun-Erdene, E.; Shipton, E.; Fullerton, E.E.; Zhang, L.; Lauga, E.; et al. Cargo-Towing Fuel-Free Magnetic Nanoswimmers for Targeted Drug Delivery. Small 2012, 8, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hu, C.; Pane, S.; Nelson, B.J. Dynamic Modeling of Magnetic Helical Microrobots. IEEE Robot. Autom. Lett. 2022, 7, 1682–1688. [Google Scholar] [CrossRef]
- Quispe, J.E.; Bolopion, A.; Renaud, P.; Regnier, S. Enhancing Swimming and Pumping Performance of Helical Swimmers at Low Reynolds Numbers. IEEE Robot. Autom. Lett. 2021, 6, 6860–6867. [Google Scholar] [CrossRef]
- Konara, K.M.T.M.B.; Amarasinghe, Y.W.R. Design and Simulation of a 4-DoF Vibratory Gyroscope. In Proceedings of the 2021 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 27–29 July 2021; pp. 728–733. [Google Scholar]
- Schamel, D.; Pfeifer, M.; Gibbs, J.G.; Miksch, B.; Mark, A.G.; Fischer, P. Chiral Colloidal Molecules And Observation of The Propeller Effect. J. Am. Chem. Soc. 2013, 135, 12353–12359. [Google Scholar] [CrossRef]
- Xu, T.; Vong, C.-I.; Wang, B.; Liu, L.; Wu, X.; Zhang, L. Rotating Soft-Tail Millimeter-Scaled Swimmers with Superhydrophilic or Superhydrophobic Surfaces. In Proceedings of the 2016 6th IEEE International Conference on Biomedical Robotics and Biomechatronics (BioRob), Singapore, 26–29 June 2016; pp. 502–507. [Google Scholar]
- Wang, X.; Qin, X.-H.; Hu, C.; Terzopoulou, A.; Chen, X.-Z.; Huang, T.-Y.; Maniura-Weber, K.; Pané, S.; Nelson, B.J. 3D Printed Enzymatically Biodegradable Soft Helical Microswimmers. Adv. Funct. Mater. 2018, 28, 1804107. [Google Scholar] [CrossRef]
- Lee, H.; Kim, D.; Kwon, S.; Park, S. Magnetically Actuated Drug Delivery Helical Microrobot with Magnetic Nanoparticle Retrieval Ability. ACS Appl. Mater. Interfaces 2021, 13, 19633–19647. [Google Scholar] [CrossRef]
- Dong, M.; Wang, X.; Chen, X.; Mushtaq, F.; Deng, S.; Zhu, C.; Torlakcik, H.; Terzopoulou, A.; Qin, X.; Xiao, X.; et al. 3D-Printed Soft Magnetoelectric Microswimmers for Delivery and Differentiation of Neuron-Like Cells. Adv. Funct. Mater. 2020, 30, 1910323. [Google Scholar] [CrossRef]
- Oulmas, A.; Andreff, N.; Regnier, S. Closed-Loop 3D Path Following of Scaled-up Helical Microswimmers. In Proceedings of the 2016 IEEE International Conference on Robotics and Automation (ICRA), Stockholm, Sweden, 16–21 May 2016; pp. 1725–1730. [Google Scholar]
- Mahoney, A.W.; Sarrazin, J.C.; Bamberg, E.; Abbott, J.J. Velocity Control with Gravity Compensation for Magnetic Helical Microswimmers. Adv. Robot. 2011, 25, 1007–1028. [Google Scholar] [CrossRef]
- Xu, T.; Hwang, G.; Andreff, N.; Regnier, S. Planar Path Following of 3-D Steering Scaled-Up Helical Microswimmers. IEEE Trans. Robot. 2015, 31, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Chaumette, F.; Hutchinson, S. Visual Servo Control. I. Basic Approaches. IEEE Robot. Automat. Mag. 2006, 13, 82–90. [Google Scholar] [CrossRef]
- Guan, Y.; Xu, T.; Liu, J.; Wu, X. Image-Based Visual Servoing of Helical Microswimmers for Arbitrary Planar Path Following at Low Reynolds Numbers. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 1883–1888. [Google Scholar]
- Tottori, S.; Sugita, N.; Kometani, R.; Ishihara, S.; Mitsuishi, M. Selective Control Method for Multiple Magnetic Helical Microrobots. J. Micro-Nano Mech. 2011, 6, 89–95. [Google Scholar] [CrossRef]
- Wang, X.; Hu, C.; Schurz, L.; De Marco, C.; Chen, X.; Pané, S.; Nelson, B.J. Surface-Chemistry-Mediated Control of Individual Magnetic Helical Microswimmers in a Swarm. ACS Nano 2018, 12, 6210–6217. [Google Scholar] [CrossRef]
- Ishimoto, K.; Gadêlha, H.; Gaffney, E.A.; Smith, D.J.; Kirkman-Brown, J. Human Sperm Swimming in a High Viscosity Mucus Analogue. J. Theor. Biol. 2018, 446, 1–10. [Google Scholar] [CrossRef]
- Magdanz, V.; Medina-Sánchez, M.; Schwarz, L.; Xu, H.; Elgeti, J.; Schmidt, O.G. Spermatozoa as Functional Components of Robotic Microswimmers. Adv. Mater. 2017, 29, 1606301. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Bi, C.; Cappelleri, D.J.; Ciuti, G.; Conn, A.T.; Faivre, D.; Habibi, N.; Hošovský, A.; Iacovacci, V.; Khalil, I.S.M.; et al. Magnetic Actuation Methods in Bio/Soft Robotics. Adv. Funct. Mater. 2021, 31, 2005137. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Fatih Tabak, A.; Klingner, A.; Sitti, M. Magnetic Propulsion of Robotic Sperms at Low-Reynolds Number. Appl. Phys. Lett. 2016, 109, 033701. [Google Scholar] [CrossRef]
- Lagomarsino, M.C.; Capuani, F.; Lowe, C.P. A Simulation Study of the Dynamics of a Driven Filament in an Aristotelian Fluid. J. Theor. Biol. 2003, 224, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Oulmas, A.; Andreff, N.; Regnier, S. 3D Closed-Loop Motion Control of Swimmer with Flexible Flagella at Low Reynolds Numbers. In Proceedings of the 2017 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Vancouver, BC, Canada, 24–28 September 2017; pp. 1877–1882. [Google Scholar]
- Lowe, C.P. Dynamics of Filaments: Modelling the Dynamics of Driven Microfilaments. Phil. Trans. R. Soc. Lond. B 2003, 358, 1543–1550. [Google Scholar] [CrossRef]
- Khalil, I.S.M.; Tabak, A.F.; Abou Seif, M.; Klingner, A.; Sitti, M. Controllable Switching between Planar and Helical Flagellar Swimming of a Soft Robotic Sperm. PLoS ONE 2018, 13, e0206456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pak, O.S.; Gao, W.; Wang, J.; Lauga, E. High-Speed Propulsion of Flexible Nanowire Motors: Theory and Experiments. Soft Matter 2011, 7, 8169. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Sattayasamitsathit, S.; Manesh, K.M.; Weihs, D.; Wang, J. Magnetically Powered Flexible Metal Nanowire Motors. J. Am. Chem. Soc. 2010, 132, 14403–14405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, I.S.M.; Youakim, K.; Sanchez, A.; Misra, S. Magnetic-Based Motion Control of Sperm-Shaped Microrobots Using Weak Oscillating Magnetic Fields. In Proceedings of the 2014 IEEE/RSJ International Conference on Intelligent Robots and Systems, Chicago, IL, USA, 4–18 September2014; pp. 4686–4691. [Google Scholar]
- Khalil, I.S.M.; Klingner, A.; Hamed, Y.; Magdanz, V.; Toubar, M.; Misra, S. Characterization of Flagellar Propulsion of Soft Microrobotic Sperm in a Viscous Heterogeneous Medium. Front. Robot. AI 2019, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Magdanz, V.; Vivaldi, J.; Mohanty, S.; Klingner, A.; Vendittelli, M.; Simmchen, J.; Misra, S.; Khalil, I.S.M. Impact of Segmented Magnetization on the Flagellar Propulsion of Sperm-Templated Microrobots. Adv. Sci. 2021, 8, 2004037. [Google Scholar] [CrossRef]
- Magdanz, V.; Khalil, I.S.M.; Simmchen, J.; Furtado, G.P.; Mohanty, S.; Gebauer, J.; Xu, H.; Klingner, A.; Aziz, A.; Medina-Sánchez, M.; et al. IRONSperm: Sperm-Templated Soft Magnetic Microrobots. Sci. Adv. 2020, 6, eaba5855. [Google Scholar] [CrossRef]
- Bray, D. Cell Movements: From Molecules to Motility, 2nd ed.; Garland Science: New York, NY, USA, 2000; ISBN 978-0-203-83358-2. [Google Scholar]
- Sanchez, T.; Welch, D.; Nicastro, D.; Dogic, Z. Cilia-Like Beating of Active Microtubule Bundles. Science 2011, 333, 456–459. [Google Scholar] [CrossRef] [Green Version]
- Brokaw, C.J. Thinking about Flagellar Oscillation. Cell Motil. Cytoskeleton 2009, 66, 425–436. [Google Scholar] [CrossRef]
- Mitchison, T.J.; Mitchison, H.M. How Cilia Beat. Nature 2010, 463, 308–309. [Google Scholar] [CrossRef]
- Lauga, E.; Powers, T.R. The Hydrodynamics of Swimming Microorganisms. Rep. Prog. Phys. 2009, 72, 096601. [Google Scholar] [CrossRef]
- Khaderi, S.N.; den Toonder, J.M.J.; Onck, P.R. Magnetic Artificial Cilia for Microfluidic Propulsion. In Advances in Applied Mechanics; Elsevier: Amsterdam, The Netherlands, 2015; Volume 48, pp. 1–78. ISBN 978-0-12-802128-6. [Google Scholar]
- Hanasoge, S.; Hesketh, P.J.; Alexeev, A. Metachronal Motion of Artificial Magnetic Cilia. Soft Matter 2018, 14, 3689–3693. [Google Scholar] [CrossRef] [Green Version]
- Khaderi, S.N.; Craus, C.B.; Hussong, J.; Schorr, N.; Belardi, J.; Westerweel, J.; Prucker, O.; Rühe, J.; den Toonder, J.M.J.; Onck, P.R. Magnetically-Actuated Artificial Cilia for Microfluidic Propulsion. Lab Chip 2011, 11, 2002. [Google Scholar] [CrossRef] [Green Version]
- Khaderi, S.N.; Baltussen, M.G.H.M.; Anderson, P.D.; Ioan, D.; den Toonder, J.M.J.; Onck, P.R. Nature-Inspired Microfluidic Propulsion Using Magnetic Actuation. Phys. Rev. E 2009, 79, 046304. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, E.; Pellicciotta, N.; Feriani, L.; Cicuta, P. Supplementary Material from “Motile Cilia Hydrodynamics: Entrainment versus Synchronization When Coupling through Flow. ” Philos. Trans. R. Soc. B 2019, 375, 20190152. [Google Scholar] [CrossRef]
- Khaderi, S.N.; den Toonder, J.M.J.; Onck, P.R. Microfluidic Propulsion by the Metachronal Beating of Magnetic Artificial Cilia: A Numerical Analysis. J. Fluid Mech. 2011, 688, 44–65. [Google Scholar] [CrossRef] [Green Version]
- Wan, K.Y. Coordination of Eukaryotic Cilia and Flagella. Essays Biochem. 2018, 62, 829–838. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, A.; Bahrami, M. A Novel Swimming Microrobot Based on Artificial Cilia for Biomedical Applications. J. Intell. Robot. Syst. 2011, 63, 399–416. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lee, J.; Nelson, B.J.; Zhang, L.; Choi, H. Fabrication and Manipulation of Ciliary Microrobots with Non-Reciprocal Magnetic Actuation. Sci. Rep. 2016, 6, 30713. [Google Scholar] [CrossRef] [Green Version]
- Sfakiotakis, M.; Lane, D.M.; Davies, J.B.C. Review of Fish Swimming Modes for Aquatic Locomotion. IEEE J. Ocean. Eng. 1999, 24, 237–252. [Google Scholar] [CrossRef] [Green Version]
- Scaradozzi, D.; Palmieri, G.; Costa, D.; Pinelli, A. BCF Swimming Locomotion for Autonomous Underwater Robots: A Review and a Novel Solution to Improve Control and Efficiency. Ocean Eng. 2017, 130, 437–453. [Google Scholar] [CrossRef]
- Wolfgang, M.J.; Anderson, J.M.; Grosenbaugh, M.A.; Yue, D.K.; Triantafyllou, M.S. Near-Body Flow Dynamics in Swimming Fish. J. Exp. Biol. 1999, 202, 2303–2327. [Google Scholar] [CrossRef] [PubMed]
- Wardle, C.; Videler, J.; Altringham, J. Tuning in to Fish Swimming Waves: Body Form, Swimming Mode and Muscle Function. J. Exp. Biol. 1995, 198, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Gutman, E.; Stucki, N.; Seitz, B.F.; Wendel-García, P.D.; Newton, T.; Pokki, J.; Ergeneman, O.; Pané, S.; Or, Y.; et al. Undulatory Locomotion of Magnetic Multilink Nanoswimmers. Nano Lett. 2015, 15, 4829–4833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.C.M.; Liao, P.; Wei, T.; Zhang, L.; Sun, D. Magnetically Powered Biodegradable Microswimmers. Micromachines 2020, 11, 404. [Google Scholar] [CrossRef]
- Choi, J.; Hwang, J.; Kim, J.; Choi, H. Recent Progress in Magnetically Actuated Microrobots for Targeted Delivery of Therapeutic Agents. Adv. Healthcare Mater. 2021, 10, 2001596. [Google Scholar] [CrossRef]
- Giltinan, J.; Sridhar, V.; Bozuyuk, U.; Sheehan, D.; Sitti, M. 3D Microprinting of Iron Platinum Nanoparticle-Based Magnetic Mobile Microrobots. Adv. Intell. Syst. 2021, 3, 2000204. [Google Scholar] [CrossRef]
- Ghosh, A.; Fischer, P. Controlled Propulsion of Artificial Magnetic Nanostructured Propellers. Nano Lett. 2009, 9, 2243–2245. [Google Scholar] [CrossRef]
- Beyrand, N.; Couraud, L.; Barbot, A.; Decanini, D.; Hwang, G. Multi-Flagella Helical Microswimmers for Multiscale Cargo Transport and Reversible Targeted Binding. In Proceedings of the 2015 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Hamburg, Germany, 28 September–2 October 2015; pp. 1403–1408. [Google Scholar]
- Qiu, F.; Zhang, L.; Peyer, K.E.; Casarosa, M.; Franco-Obregón, A.; Choi, H.; Nelson, B.J. Noncytotoxic Artificial Bacterial Flagella Fabricated from Biocompatible ORMOCOMP and Iron Coating. J. Mater. Chem. B 2014, 2, 357–362. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Sun, L.; Zhang, S.; Gong, D.; Li, X.; Yue, S.; Feng, L.; Zhang, D. Facile Fabrication of Magnetic Microrobots Based on Spirulina Templates for Targeted Delivery and Synergistic Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2019, 11, 4745–4756. [Google Scholar] [CrossRef]
- Milana, E.; Gorissen, B.; Peerlinck, S.; Volder, M.; Reynaerts, D. Artificial Soft Cilia with Asymmetric Beating Patterns for Biomimetic Low-Reynolds-Number Fluid Propulsion. Adv. Funct. Mater. 2019, 29, 1900462. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Fu, X.; Zhang, D.; Zhao, Y. Tailoring Flexible Arrays for Artificial Cilia Actuators. Adv. Intell. Syst. 2021, 3, 2000225. [Google Scholar] [CrossRef]
- Fusco, S.; Ullrich, F.; Pokki, J.; Chatzipirpiridis, G.; Özkale, B.; Sivaraman, K.M.; Ergeneman, O.; Pané, S.; Nelson, B.J. Microrobots: A New Era in Ocular Drug Delivery. Expert Opin. Drug Deliv. 2014, 11, 1815–1826. [Google Scholar] [CrossRef]
- Lim, H.; Jafry, A.T.; Lee, J. Fabrication, Flow Control, and Applications of Microfluidic Paper-Based Analytical Devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [Green Version]
- Sampath, W.H.P.; Hettiarachchi, S.P.; Melroy, N.H.R.G.; Amarasinghe, Y.W.R. Design and Development of a Droplet-Based Microfluidics System Using Laser Fabrication Machining Techniques for a Lab on a Chip Device. In Innovation in Medicine and Healthcare; Chen, Y.-W., Tanaka, S., Howlett, R.J., Jain, L.C., Eds.; Smart Innovation, Systems and Technologies; Springer: Singapore, 2020; Volume 192, pp. 201–210. ISBN 9789811558511. [Google Scholar]
- Hettiarachchi, S.; Melroy, G.; Mudugamuwa, A.; Perera, N.; Sampath, P.; Amarasinghe, R. 3D Printed Multi-Channel Peristaltic Pump with Active Droplet Generator for Lab-on-a-Chip Devices. In Sustainable Design and Manufacturing; Scholz, S.G., Howlett, R.J., Setchi, R., Eds.; Smart Innovation, Systems and Technologies; Springer: Singapore, 2022; Volume 262, pp. 235–244. ISBN 9789811661273. [Google Scholar]
- Rajabasadi, F.; Schwarz, L.; Medina-Sánchez, M.; Schmidt, O.G. 3D and 4D Lithography of Untethered Microrobots. Prog. Mater. Sci. 2021, 120, 100808. [Google Scholar] [CrossRef]
- Li, J.; Sattayasamitsathit, S.; Dong, R.; Gao, W.; Tam, R.; Feng, X.; Ai, S.; Wang, J. Template Electrosynthesis of Tailored-Made Helical Nanoswimmers. Nanoscale 2014, 6, 9415–9420. [Google Scholar] [CrossRef] [Green Version]
- Sitti, M.; Wiersma, D.S. Pros and Cons: Magnetic versus Optical Microrobots. Adv. Mater. 2020, 32, 1906766. [Google Scholar] [CrossRef]
- Ahmad, B.; Gauthier, M.; Laurent, G.J.; Bolopion, A. Mobile Microrobots for In Vitro Biomedical Applications: A Survey. IEEE Trans. Robot. 2022, 38, 646–663. [Google Scholar] [CrossRef]
- Yang, L.; Yu, J.; Yang, S.; Wang, B.; Nelson, B.J.; Zhang, L. A Survey on Swarm Microrobotics. IEEE Trans. Robot. 2022, 38, 1531–1551. [Google Scholar] [CrossRef]
- Rahmer, J.; Stehning, C.; Gleich, B. Remote Magnetic Actuation Using a Clinical Scale System. PLoS ONE 2018, 13, e0193546. [Google Scholar] [CrossRef] [Green Version]
- Abbott, J.J.; Peyer, K.E.; Lagomarsino, M.C.; Zhang, L.; Dong, L.; Kaliakatsos, I.K.; Nelson, B.J. How Should Microrobots Swim? Int. J. Robot. Res. 2009, 28, 1434–1447. [Google Scholar] [CrossRef]
- Hwang, G.; Paula, A.J.; Hunter, E.E.; Liu, Y.; Babeer, A.; Karabucak, B.; Stebe, K.; Kumar, V.; Steager, E.; Koo, H. Catalytic Antimicrobial Robots for Biofilm Eradication. Sci. Robot. 2019, 4, eaaw2388. [Google Scholar] [CrossRef]
- Dong, X.; Sitti, M. Controlling Two-Dimensional Collective Formation and Cooperative Behavior of Magnetic Microrobot Swarms. Int. J. Robot. Res. 2020, 39, 617–638. [Google Scholar] [CrossRef]


| Type | No. of Flagella | Placement of the Flagella | Layout | Examples |
|---|---|---|---|---|
| Monotrichous | One | One pole |  | Pseudomonas |
| Amphitrichous | Two | Two poles |  | Campylobacter |
| Lophotrichous | More than two | One pole |  | Helicobacter |
| Peritrichous | More than two | Lateral |  | Escherichia coli |
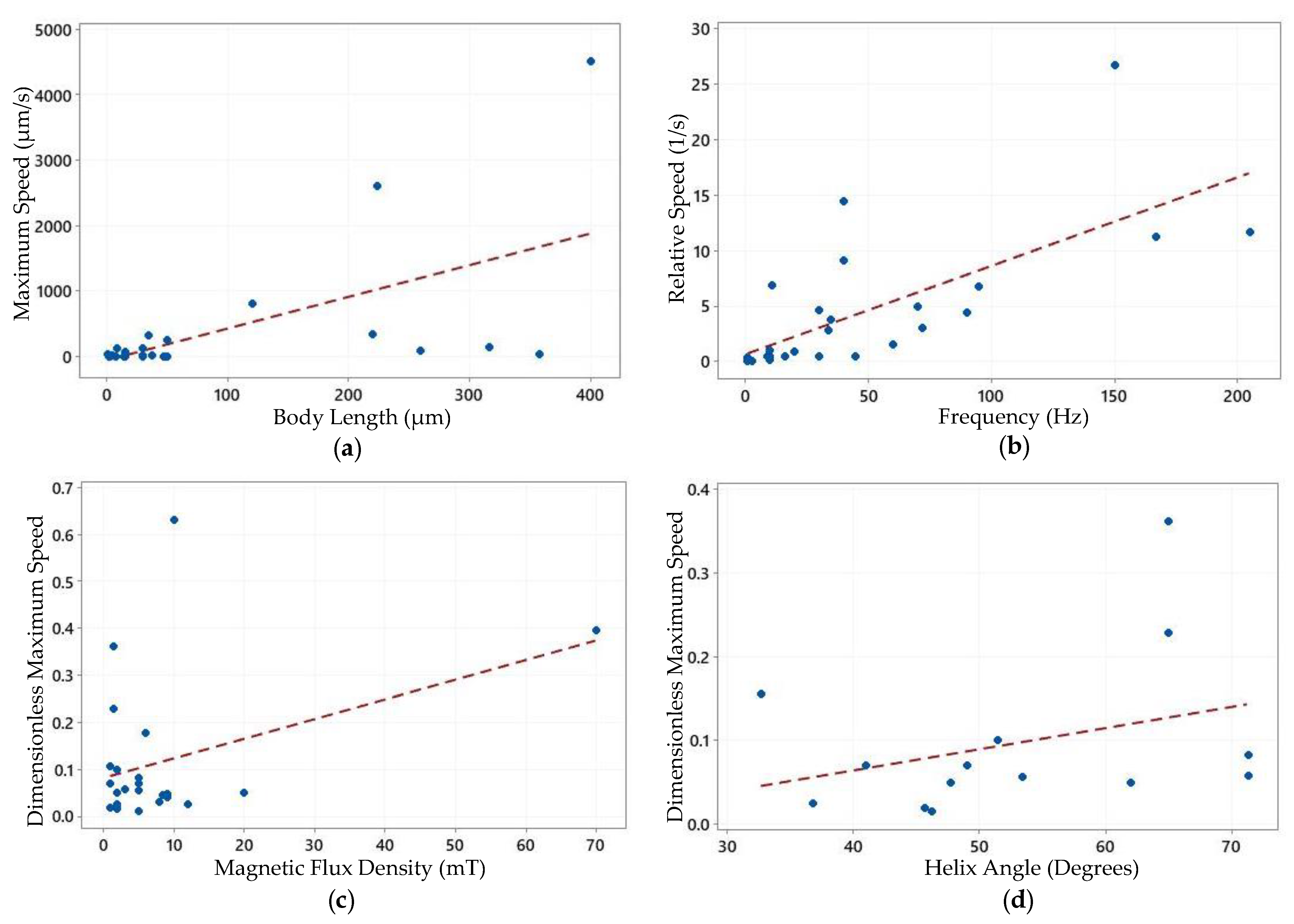
| Inspired from | Body Length L (µm) | Maximum Speed U (µm/s) | Relative Speed U/L (1/s) | Frequency f (Hz) | Dimensionless Maximum Speed U/Lf | Helix Angle (Degrees) | Magnetic Flux Density | Ref. |
|---|---|---|---|---|---|---|---|---|
| B | 8.8 | 127.0 | 14.43 | 40 | 0.3608 | 65.0 | (R) 1.5 mT | [51] |
| B | 35.0 | 320.0 | 9.14 | 40 | 0.2286 | 65.0 | (R) 1.5 mT | [51] |
| B | 1.5 | 40.0 | 26.67 | 150 | 0.1780 | - | (R) 6.0 mT | [117] |
| B | 30.0 | 140.0 | 4.67 | 30 | 0.1560 | 32.7 | (R) - | [118] |
| B | 2.0 | 2.0 | 1.00 | 10 | 0.1000 | 51.5 | (R) 2.0 mT | [44] |
| B | 16.0 | 45.0 | 2.81 | 34 | 0.0827 | 71.3 | (R) 5.0 mT | [55] |
| B | 50.0 | 250.0 | 5.00 | 70 | 0.0710 | 41.0 | (R) 1.0 mT | [61] |
| B | 121.0 | 821.2 | 6.79 | 95 | 0.0710 | 49.0 | (R) 5.0 mT | [60] |
| B | 16.0 | 8.4 | 0.53 | 9 | 0.0580 | 71.3 | (R) 3.0 mT | [57] |
| B | 224.0 | 2613.8 | 11.67 | 205 | 0.0570 | 53.4 | (R) 5.0 mT | [60] |
| B | 8.0 | 4.0 | 0.50 | 10 | 0.0500 | 47.7 | (R) 2.0 mT | [44] |
| B | 30.0 | 3.9 | 0.13 | 2.6 | 0.0500 | 62.0 | (R) 20.0 mT | [48] |
| B | 16.0 | 70.4 | 4.40 | 90 | 0.0490 | - | (R) 9.0 mT | [63] |
| B | 16.0 | 48.9 | 3.06 | 72 | 0.0420 | - | (R) 9.0 mT | [119] |
| B | 30.0 | 15.0 | 0.50 | 16 | 0.0313 | - | (R) 8.0 mT | [71] |
| B | 47.0 | 1.2 | 0.03 | 1 | 0.0255 | 36.8 | (R) 2.0 mT | [47] |
| B | 30.0 | 6.0 | 0.20 | 10 | 0.0200 | 45.7 | (R) 2.0 mT | [44] |
| B | 50.0 | 10.0 | 0.20 | 10 | 0.0200 | - | (R) 1.0 mT | [65] |
| B | 38.0 | 18.0 | 0.47 | 30 | 0.0158 | 46.2 | (R) 2.0 mT | [52] |
| B | 15.0 | 3.7 | 0.25 | - | - | - | (R) 80.0 T, (G) 40.0 T/m | [28] |
| S | 260.0 | 103.0 | 0.40 | 1 | 0.3960 | - | (R) 70 mT | [92] |
| S | 358.0 | 39.0 | 0.11 | 1 | 0.1080 | - | (R), (O) 70.0 mT | [88] |
| S | 5.5 | 20.8 | 3.78 | 35 | 0.1080 | - | (R) 0.95 mT, (G) 1.0 mT | [89] |
| S | 316.0 | 158.0 | 0.50 | 45 | 0.0110 | - | (O) 5.0 mT | [91] |
| C | 400.0 | 4500.0 | 11.25 | 167 | 0.0675 | - | - | [107] |
| C | 220.0 | 340.0 | 1.55 | 60 | 0.0258 | - | (G) 12.0 mT | [108] |
| F | 4.8 | 30.9 | 6.90 | 11 | 0.6300 | - | (O) 10.0 mH | [37] |
| F | 15.5 | 14.4 | 0.93 | 20 | 0.0465 | - | (O) 8.4 mT | [113] |
| Material/s | Key Features |
|---|---|
| Pure iron, ferric oxide, ferro-ferric oxide, nickel, cobalt | Used as the magnetic particles or magnetic material to generate a magnetic moment [38]. |
| Polydimethylsiloxane (PDMS) | Used as the matrix material [121]. Flexible and transparent material used for biomedical applications [122]. |
| Polyurethane (PU), polystyrene (PS), polymethyl methacrylate (PMMA), epoxy | Used as the matrix material [122]. |
| Titanium | Improves biocompatibility [63]. Prevents oxidation of nickel [119]. Facilitates the surface functionalization of the structures with other medical and biological substances such as liposomes [57]. |
| Silver | Used to fabricate hinges by partially dissolving in hydrogen peroxide [37]. |
| Polypyrrole (PPy) | Flexible material and used to fabricate tails of nanowires [113]. Biocompatible [123]. |
| Gold | Used to fabricate solid linkages in nanowires [84]. |
| Hydrogels | These are used to fabricate biodegradable microrobots [114]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dodampegama, S.; Mudugamuwa, A.; Konara, M.; Perera, N.; De Silva, D.; Roshan, U.; Amarasinghe, R.; Jayaweera, N.; Tamura, H. A Review on the Motion of Magnetically Actuated Bio-Inspired Microrobots. Appl. Sci. 2022, 12, 11542. https://doi.org/10.3390/app122211542
Dodampegama S, Mudugamuwa A, Konara M, Perera N, De Silva D, Roshan U, Amarasinghe R, Jayaweera N, Tamura H. A Review on the Motion of Magnetically Actuated Bio-Inspired Microrobots. Applied Sciences. 2022; 12(22):11542. https://doi.org/10.3390/app122211542
Chicago/Turabian StyleDodampegama, Shanuka, Amith Mudugamuwa, Menaka Konara, Nisal Perera, Dinindu De Silva, Uditha Roshan, Ranjith Amarasinghe, Nirosh Jayaweera, and Hiroki Tamura. 2022. "A Review on the Motion of Magnetically Actuated Bio-Inspired Microrobots" Applied Sciences 12, no. 22: 11542. https://doi.org/10.3390/app122211542
APA StyleDodampegama, S., Mudugamuwa, A., Konara, M., Perera, N., De Silva, D., Roshan, U., Amarasinghe, R., Jayaweera, N., & Tamura, H. (2022). A Review on the Motion of Magnetically Actuated Bio-Inspired Microrobots. Applied Sciences, 12(22), 11542. https://doi.org/10.3390/app122211542










