Cluster Analysis of Soluble Organic Fractions in Two Low-Rank Coals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Reagents
2.2. Experimental Raw Materials
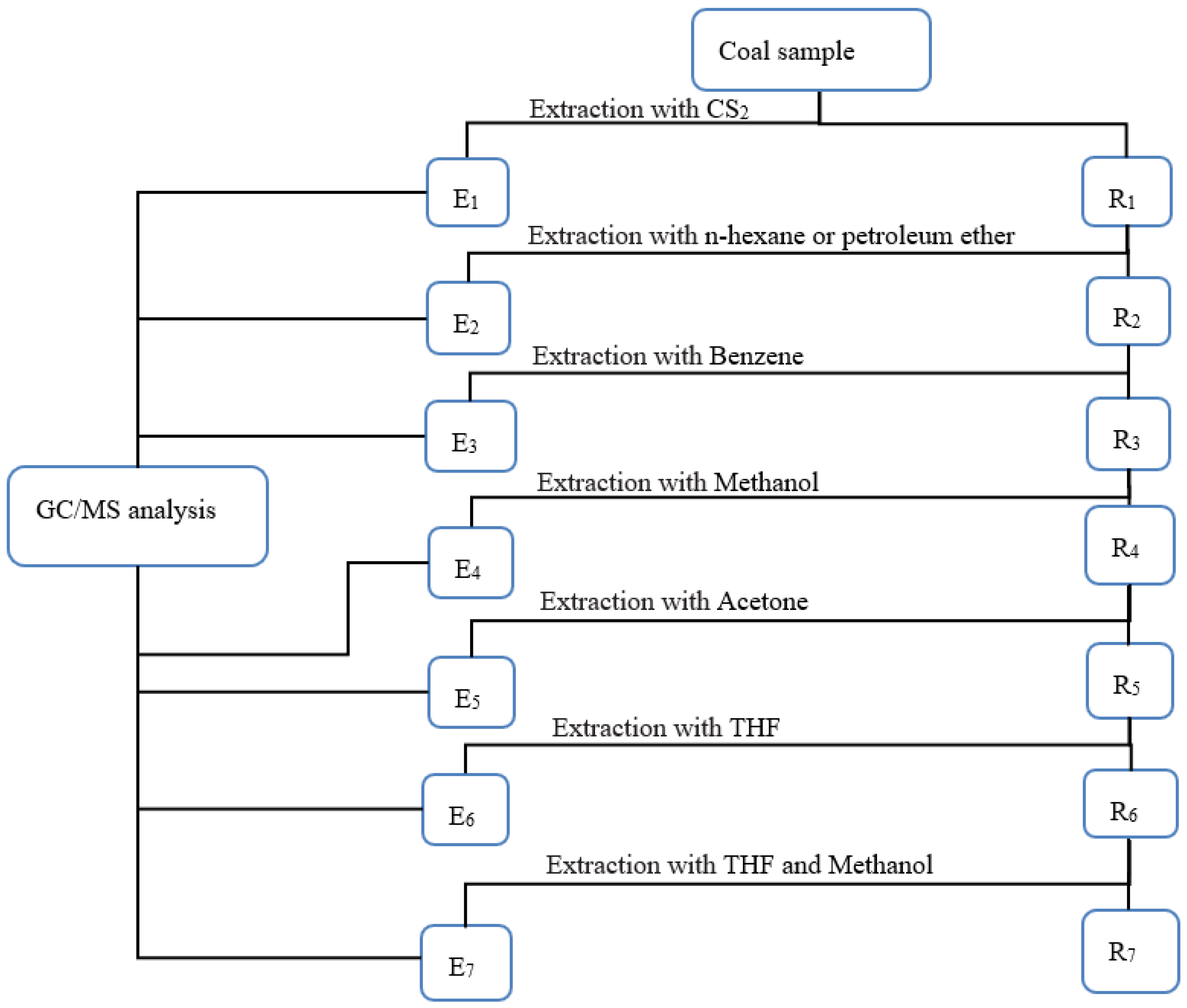
2.3. Graded Extraction of Coal Samples
2.4. Analytical Methods
3. Results and Discussion
3.1. Coal Extraction Rate
3.2. Analysis of the Family Composition of GC/MS Results of Coal Extracts
3.3. Principal Component Analysis of GC/MS Results of Coal Extracts
3.4. Clustering Analysis of GC/MS Results of Coal Extracts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.M.; Cao, J.P.; Wan, T. Extraction Effect of Different Solvents on Different Coal-rank Coal in Ningxia. Chem. Eng. Des. Commun. 2017, 43, 22–23. [Google Scholar]
- Bai, S.; Xiu, A.R.; Liu, X.C.; Tang, Y.; Xie, R.L.; Zhao, Z.G.; Cui, P. Effects of Freezing Pretreatment on Extraction Ratio of Shenfu Long-flame Coal and Its Mechanism. Coal Convers. 2022, 45, 8. [Google Scholar]
- Peng, Y.J. Effect of Small Molecule Organics on Enhancing Gas Occurrence in Coal; China University of Mining and Technology: Beijing, China, 2015. [Google Scholar]
- Peng, Y.J.; Li, Z.H.; Ji, H.J.; Tang, Y.B. Effect of Soluble Organic Matter in Coal on Gas Sorption and Desorption Characteristics. J. China Coal Soc. 2012, 37, 1472–1476. [Google Scholar]
- Li, S. Shaanxi Coal’s Coal Grading and Separation Utilization Case. Energy 2017, 12, 74–75. [Google Scholar]
- Liu, M. Study on the Morphological Evolution of Sulfur-containing Compounds in Coal THF Extracts. Coal Chem. Ind. 2020, 48, 56–61. [Google Scholar]
- Yu, K.K.; Zhang, X.D.; Zhang, S.; Du, Z.G. The FTIR Characteristics of Extracted Coking Coal in Different Macrolithotype. Spectrosc. Spectr. Anal. 2018, 38, 7. [Google Scholar]
- Zubkova, V.; Witkiewicz, Z. Chromatographic analysis of chemical compositions of coals and changes in them during technological processing. Crit. Rev. Environ. Sci. Technol. 2016, 2016, 701–755. [Google Scholar]
- Herod, A.A.; Bartle, K.D.; Kandiyoti, R. Characterization of Heavy Hydrocarbons by Chromatographic and Mass Spectrometric Methods: An Overview. Energy Fuels 2007, 21, 2176–2203. [Google Scholar] [CrossRef]
- Wang, X.H.; Wei, X.Y.; Zong, Z.M. GC/MS Analysis of Soluble Fraction with CS2 of Pingshuo Coal. Coal Convers. 2004, 2004, 23–25. [Google Scholar]
- Tang, J.W.; Feng, L.; Zhao, G.Y.; Tang, H.Y. Structure and Composition Analysis of Mildly Oxidized Lingnite Products by TG-GC-MS. J. China Univ. Min. Technol. 2016, 45, 821–827. [Google Scholar]
- Ju, C.X.; Li, F.G.; Li, X.; Yang, J.; Neng, C.L.; Zong, Z.M.; Zhang, H.; Wei, X.Y. GC/MS Analysis Extract of Yanzhou Coal in Tetrahydrofuran on Microwave. Appl. Chem. Ind. 2013, 42, 917–920. [Google Scholar]
- Zhang, L.P.; Hu, S.; Xiang, J.; Sun, L.S.; Su, S. Molecular Structure of ZhunDong Coal by Microwave-Assisted Extraction. J. Combust. Sci. Technol. 2015, 21, 260–266. [Google Scholar]
- Ping, X.D.; Zhang, X.D.; Zhang, S.; Ren, D.L.; Miao, H.Y.; Chen, F.J. GC/MS and FTIR Analysis of Coking Coal Extracted with Different Volume Fractions of THF. Coal Convers. 2021, 44, 45–55. [Google Scholar]
- Yu, Y.R. Analysis of Molecular Features of Eight Lignites with Chemometrics; China University of Mining and Technology: Beijing, China, 2018. [Google Scholar]
- Xia, D.P.; Liu, C.L.; Chen, Z.H.; Huang, S. The interaction between pore structure of different rank coals and coal-to biomethan. Coal Convers. 2022, 45, 1–17. [Google Scholar]
- Zhang, X.Y.; Wang, F.; Li, G.S.; Fan, X.; Yu, Y.R.; Zhao, Y.P.; Wei, X.Y.; Ma, F.Y.; Yu, G. Cluster Analysis of Molecular Characteristics for Soluble Organic Matter in Coals. Chin. J. Anal. Chem. 2019, 47, 99–105. [Google Scholar]
- Zhang, X.Y.; Wang, R.Y.; Ma, F.Y. Structural characteristics of soluble organic matter in four low-rank coals. Fuel 2020, 267, 117230. [Google Scholar] [CrossRef]
- Hansen, C.M. 50 Years with solubility parameters—Past and future. Prog. Org. Coat. 2004, 51, 77–84. [Google Scholar] [CrossRef]
- Liu, Z.X.; Wei, X.Y.; Zong, Z.M. GC/MS Analysis of Step-by-Step Extracts Using Organic Solvents from DongSheng Coal. Coal Convers. 2003, 2003, 37–40. [Google Scholar]
- Huang, J.B. Study on the Effect of Extractant on the Extraction Behavior for Coal Liquefaction Residue and the Separation of Extraction Products. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2021. [Google Scholar]
- Ouyang, X.D.; Ding, M.J.; Zong, Y.; Zong, Z.M.; Wei, X.Y. Analysis of Methanol-Extracts from Shenfu Coal by GC/MS and FTI R. Coal Convers. 2007, 2007, 6–9 + 27. [Google Scholar]






| Extractant | δ/(cal·cm−3)1/2 | Polarity Parameters | Extraction Rate/% | Aliphatic Hydrocarbon Content/10−4 g | Aromatic Content/10−4 g | Heterocyclic Substance Content/10−4 g |
|---|---|---|---|---|---|---|
| CS2 | 10 | 0.15 | 0.7160 | 32.12 | 105.51 | 13.63 |
| n-hexane | 7.3 | 0.06 | 0.2830 | 2.4141 | 0.1318 | 0.076 |
| Benzene | 9.2 | 3 | 0.3067 | 1.795 | 0.91 | 0.889 |
| Methanol | 14.5 | 6.6 | 0.6247 | 0.259 | 0.054 | 4.838 |
| Acetone | 9.9 | 5.4 | 2.7197 | 7.275 | 8.166 | 14.176 |
| THF | 9.5 | 4.2 | 7.3968 | 1.485 | 0.583 | 0.22 |
| THF and methanol | 12 | 5.4 | 0.5142 | 0.7409 | 0.5815 | 0.1452 |
| Extractant | δ/(cal·cm−3)1/2 | Polarity Parameters | Extraction Rate/% | Aliphatic Hydrocarbon Content/10−4 g | Aromatic Content/10−4 g | Heterocyclic Substance Content/10−4 g |
|---|---|---|---|---|---|---|
| CS2 | 10 | 0.15 | 1.0988 | 23.78 | 53.889 | 13.63 |
| Petroleum ether | 16.1 | 0.01 | 0.0253 | 0.0466 | 0 | 0.014 |
| Benzene | 9.2 | 3 | 0.0440 | 0.016 | 0.101 | 1.187 |
| Methanol | 14.5 | 6.6 | 2.1682 | 0.126 | 0 | 1.348 |
| Acetone | 9.9 | 5.4 | 0.5201 | 0 | 0 | 144.952 |
| THF | 9.5 | 4.2 | 0.9214 | 0 | 0 | 32.571 |
| THF and methanol | 12 | 5.4 | 1.6829 | 0 | 0 | 6.5115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; He, X. Cluster Analysis of Soluble Organic Fractions in Two Low-Rank Coals. Appl. Sci. 2022, 12, 11562. https://doi.org/10.3390/app122211562
Wang X, He X. Cluster Analysis of Soluble Organic Fractions in Two Low-Rank Coals. Applied Sciences. 2022; 12(22):11562. https://doi.org/10.3390/app122211562
Chicago/Turabian StyleWang, Xiaohua, and Xin He. 2022. "Cluster Analysis of Soluble Organic Fractions in Two Low-Rank Coals" Applied Sciences 12, no. 22: 11562. https://doi.org/10.3390/app122211562





