Health Parameters of Potato Tubers under the Influence of Soil Applied Bio-Preparations and Bio-Stimulants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Experiment Design
2.3. Characteristics of the Preparations Used
- Biological control agents (BCAs):
- -
- Polyversum WP—106 oospor Pythium oligandrum in 1 g of the agent (Biopreparaty Ltd., Uherce, Czech Republic);
- -
- Serenade ASO—Bacillus subtillis str. QST 713—13.96 g L−1 (minimal concentration 1.042·1012 CFU·L-1) (Bayer AG, Leverkusen, Germany).
- Plant growth promoter (PGP):
- -
- Kelpak SL—auxins—11.0 mg·L−1 and cytokinins—0.031 mg∙ L−1 obtained from Ecklonia maxima algae (Kelp Products International (Pty) Ltd., Simon’s Town, South Africa).
- Microbial soil additives (MSADs):
- -
- Em Farma™—microorganism strains or consortia thereof produced by natural fermentation processes based on species commonly occurring in nature, involving microorganisms, not genetically modified, with the highest standards of hygiene and quality (a useful blend of bacteria that supports the natural growth of useful microorganisms found in agricultural environments and is safe for humans and the environment), organic cane molasses, revitalized, unchlorinated water, salt, mineral complex (Probiotics, Poland);
- -
- UG Max soil conditioner—a liquid concentrate containing microorganisms (acid bacteria, photosynthetic bacteria, Azotobacter, Pseudomonas, actinomycetes, and yeasts) as well as macronutrients (g·L−1: K—3.5; N—1.2; S—1.0; P—0.5; Na—0.2; Mg—0.1) and micronutrients (g·L−1: Zn—20.0; Mn—0.3), (Bogdan Sp. zoo, Bolesławowo, Poland);
- -
- Biogen Rewital—contains 108 CFU·g−1, a microbiological composite of non-pathogenic bacteria (cellulolytic, nitrifying, sanitary, lipolytic) and a starter medium (Bio-Gen Ltd., Łódź, Poland).
- -
- A biotechnical agent for controlling Leptinotarsa decemlineata—SpinTor 240 SC—spinosad: Spinosyn A, Spinosyn D (a substance from the group of macrocyclonic lactones)—240 g·L−1, (22.72%) (Dow AgroSciences Polska Sp. z o.o., Poland);
- -
- Miedzian 50 WP—copper in the form of copper oxychloride—50% (500 g Cu·kg−1) (Synthos Agro Sp. z o.o., Oświęcim, Poland).
2.4. Soil Conditions
2.5. Assessment of the Occurrence of Infectious Diseases of the Skins of Potato Tubers and Yields
2.6. Statistical Calculations
3. Results
3.1. The Effect of Hydrothermal Conditions and Protection Measures on Potato Tubers Health
3.2. Total Potato Yield Depending on Hydrothermal Conditions and the Preparations Used
3.3. Assessment of the Effect of Potato Tuber Skin Diseases on Total Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismail, S.; Jiang, B.; Nasimi, Z.; Inam-ul-Haq, M.; Yamamoto, N.; Danso Ofori, A.; Khan, N.; Arshad, M.; Abbas, K.; Zheng, A. Investigation of Streptomyces scabies Causing Potato Scab by Various Detection Techniques, Its Pathogenicity and Determination of Host-Disease Resistance in Potato Germplasm. Pathogens 2020, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, R.; Singh, N.; Sharma, S.; Kaur, A. Beneficial phytochemicals in potato—A review. Food Res. Int. 2013, 50, 487–496. [Google Scholar] [CrossRef]
- Cwalina-Ambroziak, B.; Damszel, M.M.; Głosek-Sobieraj, M. The effect of biological and chemical control agents on the health status of the very early potato cultivar Rosara. J. Plant Prot. Res. 2015, 50, 389–395. [Google Scholar] [CrossRef]
- Tolessa, E.S. Importance, Nutrient Content and Factors Affecting Nutrient Content of Potato. Am. J. Food Nutr. Health 2018, 3, 37–41. [Google Scholar]
- Gustavsen, G.W. Sustainability and Potato Consumption. Potato Res. 2021, 64, 571–586. [Google Scholar] [CrossRef]
- Im, H.W.; Suhnh, B.; Lee, S.U.; Kozukue, N.; Ohnisi-Kameyama, M.; Levin, C.E.; Friedman, M. Analysis of phenolic compounds by high-performance liquid chromatography/mass spectrometry in potato plant flowers, leaves, stems, and tubers and in home-processed potatoes. J. Agric. Food Chem. 2008, 56, 3341–3349. [Google Scholar] [CrossRef]
- Hu, C.; Tsao, R.; Liu, R.; Sullivan, J.A.; McDonald, M.R. Influence of cultivar and year on phytochemical and antioxidant activity of potato (Solanum tuberosum L.) in Ontario. Can. J. Plant Sci. 2012, 92, 485–493. [Google Scholar] [CrossRef]
- Burgos, G.; Felde, T.Z.; Andre, C.; Kubow, S. The Potato and Its Contribution to the Human Diet and Health. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Burlingame, B.; Mouille’, B.; Charrondie´re, R. Nutrients, bioactive non-nutrients and anti-nutrients in potatoes. J. Food Compos. Anal. 2009, 22, 494–502. [Google Scholar] [CrossRef]
- Simakov, E.A.; Anisimov, B.V.; Zhevora, S.V.; Mitiushkin, A.V.; Zhuravlev, A.A.; Mitiushkin, A.V.; Yu Kordabovsky, V. Prospects of potato selection for the improvement of nutritional value of tubers. IOP Conf. Ser. Earth Environ. Sci. 2021, 659, 012096. [Google Scholar] [CrossRef]
- FAO, Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture. Alternative Pathways to 2050. 2018. Available online: http://www.fao.org/global-perspectives-studies/resources/detail/en/c/1157074/ (accessed on 11 November 2022).
- Naumann, M.; Koch, M.; Thiel, H.; Gransee, A.; Pawelzik, E. The Importance of Nutrient Management for Potato Production Part II: Plant Nutrition and Tuber Quality. Potato Res. 2020, 63, 121–137. [Google Scholar] [CrossRef]
- Osowski, J.; Urbanowicz, J. Silver scab (Helminthosporium solani)—Symptoms and control. Biuletyn Instytutu Hodowli I Aklimatyzacji Roślin 2021, 294, 35–50. [Google Scholar] [CrossRef]
- Wale, S.; Platt, H.W.; Cattlin, N. Fungal and fungal like diseases. In Diseases, Pests and Disorders of Potatoes; Manson Publishing Ltd.: London, UK, 2008; pp. 28–70. [Google Scholar]
- Avis, T.J.; Martinez, C.; Tweddel, R.J. Integrated management of potato silver scurf (Helminthosporium solani). Can. J. Plant Pathol. 2010, 32, 287–297. [Google Scholar] [CrossRef]
- Massana-Codina, J.; Schnee, S.; Lecoultre, N.; Droz, E.; Dupuis, B.; Keiser, A.; de Werra, P.; Wolfender, J.-L.; Gindro, K.; Schürch, S. Influence of abiotic factors, inoculum source and cultivar susceptibility on the potato tuber blemish diseases black dot (Colletotrichum coccodes) and silver scurf (Helminthosporium solani). Plant Pathol. 2021, 70, 885–897. [Google Scholar] [CrossRef]
- Loria, R.; Kers, J.; Joshi, M. Evolution of plant patogenicity in streptomyces. Annu. Rev. Phytopathol. 2006, 44, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, M.; Tamaki, H.; Manome, A.; Koyama, O.; Kamagata, Y. Development of a genotyping method for potato scab pathogens based on multiplex PCR. Biosci. Biotechnol. Biochem. 2008, 72, 2324–2334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, L.A.; Kirk, W.W. Streptomyces—From basic microbiology to role as a plant pathogen. Am. J. Potato Res. 2015, 92, 236–242. [Google Scholar] [CrossRef]
- Leiminger, J.; Frank, M.; Wenk, C.; Poschenrieder, G.; Kellermann, A.; Schwarzfischer, A. Distribution and characterization of Streptomyces species causing potato common scab in Germany. Plant Pathol. 2013, 62, 611–623. [Google Scholar] [CrossRef]
- Loria, R.; Bukhalid, R.A.; Fry, B.A.; King, R.R. Plant pathogenicity in the genus Streptomyces. Plant Dis. 1997, 81, 836–846. [Google Scholar] [CrossRef] [Green Version]
- Ogoshi, A. Introduction—The Genus Rhizoctonia. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Sneh, B., Jabaji-Hare, S., Neate, S., Dijst, G., Eds.; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Dhingra, O.D.; Costa, M.L.N.; Silva, G.J.; Mizubuti, E.S.G. Essential oil of mustard to control Rhizoctona solani seedling damping off and seedling blight in nursery. Fitopatol. Bras. 2004, 29, 683–686. [Google Scholar] [CrossRef]
- El-Tarabily, K.A. Suppression of Rhizoctonia solani diseases of sugar beet by antagonistic and plant growth-promoting yeasts. J. Appl. Microbiol. 2004, 96, 69–75. [Google Scholar] [CrossRef]
- Lemańczyk, G. Occurrence of sharp eyespot in spring cereals grown in some regions of Poland. J. Plant Prot. Res. 2010, 50, 505–512. [Google Scholar] [CrossRef]
- Nagrodzka, K.; Moliszewska, M.; Grata, K.; Nabrdalik, M. Biological control of Rhizoctonia solani AG 2-2IIIB by Bacillus subtilis metabolites. In Proceedings of the ECOpole’16 Conference, Zakopane, Poland, 5–8 October 2016; Volume 10, pp. 741–748. [Google Scholar] [CrossRef]
- Ogoshi, A. Ecology and pathogenicity of anastomosis and intraspecific groups of Rhizoctonia solani. Annu. Rev. Phytopathol. 1987, 25, 125–143. [Google Scholar] [CrossRef]
- Ithurrart, M.E.F.; Büttner, G.; Peterson, J. Rhizoctonia root rot in sugar beet (Beta vulgaris ssp. altissima)—Epidemiological aspects in relation to maize (Zea mays) as a host plant. J. Plant Dis. Prot. 2004, 111, 302–312. [Google Scholar]
- Harveson, R.M. Identifying and distinguishing seedling and root rot diseases of sugar beets. Online. Plant Health Prog. 2006. [Google Scholar] [CrossRef] [Green Version]
- Gawińska-Urbanowicz, H. Evaluation of the incidence of fungal and bacterial diseases in potatoes under field conditions. Biul. IHAR 2007, 243, 191–197. (In Polish) [Google Scholar]
- Atkinson, D.; Thornton, M.K.; Miller, J.S. Development of Rhizoctonia solani on Stems, Stolons and Tubers of Potatoes I. Effect of Inoculum Source. Am. J. Potato Res. 2010, 87, 374–381. [Google Scholar] [CrossRef]
- Baranowska, A.; Zarzecka, K.; Mystkowska, I.; Gugała, M. The presence of Rhizoctonia solani on potato tubers of ware potatoes depending on the method of application of the soil fertilizer UGmax. Prog. Plant Prot. 2017, 57, 115–120. (In Polish) [Google Scholar] [CrossRef]
- Tjimune, R.; Mangwende, E.; Lekota, M.; Muzhinji, N. First Report of Rhizoctonia solani AG 3-PT causing black scurf on potato tubers in Namibia. New Dis. Rep. 2022, 45, e12066. [Google Scholar] [CrossRef]
- Lehtonen, M.; Somervuo, P.; Valkonen, J. Infection with Rhizoctonia solani induces defence genes and systemic resistance in potato sprouts grown without light: Implications of dynamic plant-pathogen interplay underground. Phytopathology 2008, 98, 1190–1198. [Google Scholar] [CrossRef] [Green Version]
- Wales, S.; Platt, H.W.; Cattlin, N. Diseases, Pests and Disorders of Potatoes. In A Colour Handbook; Manson Publishing Ltd.: London, UK, 2008; pp. 75–76. [Google Scholar]
- Errampalli, D.; Saunders, J.M.; Holley, J.D. Emergence of silver scurf (Helminthosporium solani) as an economically important disease of potato. Plant Pathol. 2001, 50, 141–153. [Google Scholar] [CrossRef]
- Martinez, C.; Rioux, D.; Tweddell, R.J. Ultrastructure of the infection process of potato tuber by Helminthosporium solani, causal agent of potato silver scurf. Mycol. Res. 2004, 108, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Hide, G.A.; Hall, S.M.; Boorer, K.J. Resistance to thiabendazole in isolates of Helminthosporium solani, the cause of silver scurf disease of potatoes. Plant Pathol. 1988, 37, 377–380. [Google Scholar] [CrossRef]
- Rodriguez, D.A.; Secor, G.A.; Gudmestad, N.C.; Franci, L.J. Sporulation of Helminthosporium solani and infection of potato tubers in seed and commercial storages. Plant Dis. 1996, 80, 1063–1070. [Google Scholar] [CrossRef]
- Lec, L. Rhizoctonia solani and Streptomyces scabies on sprouts and tubers of potato grown in organic and integrated systems, and fungal communities in the soil habitat. Phytopathol. Pol. 2006, 42, 13–28. [Google Scholar]
- Khatri, B.B.; Tegg, R.S.; Brown, P.H.; Wilson, C.R. Infection of Potato Tubers with the Common Scab Pathogen Streptomyces scabiei in a Soil-less System. J. Phytopathol. 2010, 158, 453–455. [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Antonkiewicz, J. Organic fertilization shapes the biodiversity of fungal communities associated with potato dry rot. Appl. Soil Ecol. 2018, 129, 43–51. [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Filipiak-Florkiewicz, A.; Florkiewicz, A.; Luty, L. The Effect of Biopreparations and Biostimulants on the Chemical Composition and Microorganisms Associated with Verticillium Wilt of Horseradish Roots (Armoracia rusticana Gaertn.). Appl. Sci. 2021, 11, 680. [Google Scholar] [CrossRef]
- Gleń-Karolczyk, K.; Boligłowa, E.; Gospodarek, J.; Antonkiewicz, J.; Luty, L. Effect of Seed Dressing and Soil Chemical Properties on Communities of Microorganisms Associated with Pre-Emergence Damping-Off of Broad Bean Seedlings. Agronomy 2021, 11, 1889. [Google Scholar] [CrossRef]
- Enciso-Rodriguez, F.; Douches, D.; Lopez-Cruz, M.; Coombs, J.; de Los Campos, G. Genomic Selection for Late Blight and Common Scab Resistance in Tetraploid Potato (Solanum tuberosum). G3 Genes Genomes Genet. 2018, 8, 2471–2481. [Google Scholar] [CrossRef] [Green Version]
- Nowicki, M.; Foolad, M.R.; Nowakowska, M.; Kozik, E.U. Potato and Tomato Late Blight Caused by Phytophthora infestans: An Overview of Pathology and Resistance Breeding. Plant Dis. 2011, 96, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Pomerantz, A.; Cohen, Y.; Shufan, E.; Ben-Naim, Y.; Mordechai, S. Characterization of Phytophthora infestans resistance to mefenoxam using FTIR spectroscopy. J. Photochem. Photobiol. 2014, 141, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Gleń-Karolczk, K. Protective treatments shaping the yielding, healthiness and diversity of microorganisms associated with Verticillium wilt of horseradish roots (Armoracia rusticana Gaertn.). Zesz Nauk UR Krakowie 2019, 544, 136, (In Polish, English Abstract). [Google Scholar]
- Mierzwa-Hersztek, M.; Gleń-Karolczyk, K.; Gondek, K. Fungistatic activity of composts with the addition of polymers obtained from thermoplastic corn starch and polyethylene—An innovative cleaner production alternative. Sci. Total Environ. 2018, 635, 1063–1075. [Google Scholar] [CrossRef]
- Paśmionka, I.; Bulski, K.; Boligłowa, E. Microbiota in the Transformation of Nitrogen Compounds in the Soil—A Review. Agronomy 2021, 11, 977. [Google Scholar] [CrossRef]
- Gałązka, A.; Kocoń, A. Ocena efektywności działania preparatów z mikroorganizmami pożytecznymi na aktywność enzymatyczną gleby. Studia i Raporty IUNG-PIB 2015, 45, 143–154. [Google Scholar] [CrossRef]
- Wichrowska, D. Antioxidant Capacity and Nutritional Value of Potato Tubers (Solanum tuberosum L.) as a Dependence of Growing Conditions and Long-Term Storage. Agriculture 2022, 12, 21. [Google Scholar] [CrossRef]
- Witkowicz, R.; Biel, W.; Chłopicka, J.; Galanty, A.; Gleń-Karolczyk, K.; Skrzypek, E.; Krupa, M. Biostimulants and Microorganisms Boost the Nutritional Composition of Buckwheat (Fagopyrum esculentum Moench) Sprouts. Agronomy 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Witkowicz, R.; Biel, W.; Skrzypek, E.; Chłopicka, J.; Gleń-Karolczyk, K.; Krupa, M.; Prochownik, E.; Galanty, A. Microorganisms and Biostimulants Impact on the Antioxidant Activity of Buckwheat (Fagopyrum esculentum Moench) Sprouts. Antioxidants 2020, 9, 584. [Google Scholar] [CrossRef]
- Witkowicz, R.; Skrzypek, E.; Gleń-Karolczyk, K.; Krupa, M.; Biel, W.; Chłopicka, J.; Galanty, A. Effects of application of plant growth promoters, biological control agents and microbial soil additives on photosynthetic efficiency, canopy vegetation indices and yield of common buckwheat (Fagopyrum esculentum Moench). Biol. Agric. Hortic. 2021, 37, 234–251. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Krochmal-Marczak, B.; Sawicka, B.; Pszczółkowski, M. The Impact of Effective Microorganisms on Flesh Color and Chemical Composition of Raw Potato Tubers. Appl. Sci. 2021, 11, 8959. [Google Scholar] [CrossRef]
- Sawicka, B.; Pszczółkowski, P.; Kiełtyka-Dadasiewicz, A.; Barbaś, P.; Ćwintal, M.; Krochmal-Marczak, B. The Effect of Effective Microorganisms on the Quality of Potato Chips and French Fries. Appl. Sci. 2021, 11, 1415. [Google Scholar] [CrossRef]
- Boligłowa, E.; Gleń, K. 2008. Assessment of effective microorganism activity (EM) in winter wheat production against fungal diseases. Ecol. Chem. Eng. A 2008, 15, 23–27. [Google Scholar]
- PN ISO 10390-1997; Soil Quality—Determination of Ph. Polish Committee for Standardization (NP): Warsaw, Poland, 1997.
- PN-R-04029:1997; Chemical and Agricultural Analysis of Soil. Methods for Soil Collection and Determination of Nitrate Ions in Organic Soils. Polish Committee for Standardization (NP): Warsaw, Poland, 1997.
- PN-R-04023:1996; Agrochemical Soil Analyses—Determination of Assimilated Phosphorus Contents. Polish Committee for Standardization (NP): Warsaw, Poland, 1996.
- PN-R-04022:1996/Az1:2002; Chemical and Agricultural Soil Analysis. Determination of the Amount of Assimilable Potassium. Polish Committee for Standardization (PN): Warsaw, Poland, 2002.
- PN-R-04020:1994/Az1:2004; Chemical and Agricultural Soil Analysis. Determination of the Amount of Assimilable Magnesium. Polish Committee for Standardization (PN): Warsaw, Poland, 2004.
- PN-R-04016:1992; Chemical and Agricultural Soil Analysis. Determination of the Amount of Assimilable Zinc. Polish Committee for Standardization (PN): Warsaw, Poland, 1992.
- PN-R-04017:1992; Chemical and Agricultural Soil Analysis. Determination of the Amount of Assimilable Copper. Polish Committee for Standardization (PN): Warsaw, Poland, 1992.
- PN-R-04018:1993; Chemical and Agricultural Soil Analysis. Determination of the Amount of Assimilable Boron. Polish Committee for Standardization (PN): Warsaw, Poland, 1993.
- PN-R-04019:1993; Chemical and Agricultural Soil Analysis. Determination of the Amount of Assimilable Manganese. Polish Committee for Standardization (PN): Warsaw, Poland, 1993.
- PN-R-04021:1994; Chemical and Agricultural Soil Analysis. Determination of the Amount of Assimilable Iron. Polish Committee for Standardization (PN): Warsaw, Poland, 1994.
- Polish Soil Classification. Soil Sci. Ann. 2011, 62, 1–193. Available online: http://www.ptg.sggw.pl (accessed on 11 November 2022).
- Świtoniak, M.; Kabała, C.; Charzyński, P. Proposal of English equivalents for the soil taxa names in the Polish Soils Classification. Soil. Sci. Ann. 2016, 67, 103–116. [Google Scholar] [CrossRef]
- Skowera, B. Zmiany warunków hydrotermicznych na obszarze Polski (1971–2010). Fragm. Agron. 2014, 31, 74–87. [Google Scholar]
- OEPP/EPPO. Bulletin OEPP/EPPO PP 1/32 (3). Rhizoctonia Solani Potato 2013, 43, 380–382. [Google Scholar]
- Driscoll, J.; Coombs, J.; Hammerschmidt, R.; Kirk, W.; Wanner, L.; Douches, D. Greenhouse and Field Nursery Evaluation for Potato Common Scab Tolerance in a Tetraploid Population. Am. J. Potato Res. 2009, 86, 96–101. [Google Scholar] [CrossRef]
- Wenzel, H. Zur erfassung des schadenausmasses in pflanzenschutz versuchen. Pflanzenschutz-Ber 1948, 15, 81–84. [Google Scholar]
- Cwalina-Ambroziak, B. The efficiency of biological and chemical protection of potato plants against late blight (Phytophthora infestans [Mont.] de Bary) and early blight (Alternaria spp.). Pol. J. Agron. 2012, 11, 3–9. [Google Scholar]
- Donatelli, M.; Magarey, R.D.; Bregaglio, S.; Willocquet, L.; Whish, J.P.M.; Savary, S. Modelling the impacts of pests and diseases on agricultural systems. Agric. Syst. 2017, 55, 213–224. [Google Scholar] [CrossRef]
- Shuping, D.S.S.; Eloff, J.N. The use of plants to protect plants and food against fungal pathogens: A review. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Kurzawińska, H.; Mazur, S. The effect of bio-preparations on the infestation of tubers by Streptomyces spp. Folia Hort. 2008, 20, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Osowski, J.; Bernat, E. The silver scab problem on selected potato cultivars registered in Poland. Prog. Plant Prot. 2005, 1, 336–341. (In Polish) [Google Scholar]
- Sadowski, C. Health status of the potato in poland and the threats of its production. Zesz. Probl. Postep. Nauk. Rol. 2006, 511, 37–51. (In Polish) [Google Scholar]
- Tsror, L. Biology, Epidemiology and Management of Rhizoctonia solani on Potato. J. Phytopathol. 2010, 158, 649–658. [Google Scholar] [CrossRef]
- Porto, J.S.; Rebouças, T.N.H.; José, A.R.S.; José, A.R.S.; Tebaldi, N.D.; Luz, J.M.Q. Biocontrol of Potato Common Scab Cultivated on Different Soil Mulch. Agronomy 2022, 12, 904. [Google Scholar] [CrossRef]
- Rehman, A.; Sandhu, J.; Alam, M.W.; Mehboob, S. A perspective on common scab (Streptomyces scabiei) disease management strategies in potato crop. Int. J. Phytopathol. 2021, 10, 195–201. [Google Scholar] [CrossRef]
- Reznikov, S.; Vellicce, G.R.; González, V.; de Lisi, V.; Castagnaro, A.P.; Ploper, L.D. Evaluation of chemical and biological seed treatments to control charcoal rot of soybean. J. Gen. Plant Pathol. 2016, 82, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.P.; Sperschneider, J.; Win, J.; Kidd, B.; Yoshida, K.; Hane, J.; Singh, K.B. Comparative secretome analysis of Rhizoctonia solani isolates with different host ranges reveals unique secretomes and cell death inducing effectors. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Joshi, H.; Duttand, S.; Choudhary, P.; Mundra, S.L. Role of Effective Microorganisms (EM) in Sustainable Agriculture. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 172–181. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.M.D.; Babar, A. Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS ONE 2020, 15, e0231426. [Google Scholar] [CrossRef] [Green Version]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahillon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Kong, Z.-Q.; Zhang, D.-D.; Chen, J.-Y.; Dai, X.-F.; Li, R. Rhizosphere Microbiomes of Potato Cultivated under Bacillus subtilis Treatment Influence the Quality of Potato Tubers. Int. J. Mol. Sci. 2021, 22, 12065. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Y.; Zhuang, L.; Yu, Y.; Liu, J.; Zhang, L.; Gao, Z.; Wu, Y.; Gao, W.; Ding, G.; et al. A Rhizosphere-Derived Consortium of Bacillus subtilis and Trichoderma harzianum Suppresses Common Scab of Potato and Increases Yield. Comput. Struct. Biotechnol. J. 2019, 17, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tsai, C.H.; Chen, P.Y.; Wu, C.Y.; Chang, Y.L.; Yang, Y.L.; Chen, Y.L. Biological Control of Potato Common Scab by Bacillus Amyloliquefaciens Ba01. PLoS ONE 2018, 13, e0196520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Wang, B.; Pan, P.; Li, P.; Qi, Z.; Zhang, Q.; Shi, C.; Hao, W.; Zhou, B.; Lin, R. Bacillus Altitudinis Strain AMCC 101304: A Novel Potential Biocontrol Agent for Potato Common Scab. Biocontrol. Sci. Technol. 2019, 29, 1009–1022. [Google Scholar] [CrossRef]
- Schmiedeknecht, G.; Bochow, H.; Junge, H. Use of Bacillus subtilis as biocontrol agent. II. Biological control of potato diseases/Anwendung von Bacillus subtilis als Mittel für den biologischen Pflanzenschutz. II. Biologische Bekämpfung von Kartoffelkrankheiten. Zeitschrift Für Pflanzenkrankheiten Und Pflanzenschutz/J. Plant Dis. Prot. 1998, 105, 376–386. [Google Scholar]
- Khedher, S.B.; Kilani-Feki, O.; Dammak, M.; Jabnoun-Khiareddine, H.; Daami-Remadi, M.; Tounsi, S. Efficacy of Bacillus subtilis V26 as a biological control agent against Rhizoctonia solani on potato. Comptes Rendus Biol. 2015, 338, 784–792. [Google Scholar] [CrossRef]
- Gleń, K.; Boligłowa, E.; Gospodarek, J. Fungi colonising broad bean seeds depending on protection method. Pol. J. Agron. 2013, 12, 9–16. (In Polish) [Google Scholar] [CrossRef]
- Wachowska, U.; Duba, A.; Goriewa, K.; Wiwart, M. The effectiveness of Aureobasidium pullulans, Debaryomyces hansenii and Rhodotorula glutinis yeasts in inhibiting the development of septoria leaf blotch (Zymoseptoria tritici) in wheat. Zesz. Probl. Post. Nauk. Rol. 2018, 592, 97–106. [Google Scholar] [CrossRef]
- Brožová, J. Exploitation of the mycoparasitic fungus Pythium oligandrum in plant protection. Plant Prot. Sci. 2002, 38, 29–35. [Google Scholar]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
- Berg, G.; Köberl, M.; Rybakova, D.; Müller, H.; Grosch, R.; Smalla, K. Plant microbial diversity is suggested as the key to future biocontrol and health trends. FEMS Microbiol. Ecol. 2017, 93, fix050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurzawińska, H.; Mazur, S. 2007. Usefulness of Pythium oligandrum in the protection of potato against some diseases. Prog. Plant Prot. 2007, 47, 185–188. [Google Scholar]
- Berendsen, R.L.; Pieterse, C.M.; Bakker, P.A. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. 2018. The effect of application of biopreparations and fungicides on the yield and selected parameters of seed value of seed potatoes. Acta Agrophysica 2018, 25, 239–255. [Google Scholar] [CrossRef] [Green Version]
- Zarzecka, K.; Gugała, M.; Sikorska, A.; Mystkowska, I. The Impact Of The Soil Conditioner Ugmax On Selected Qualitative Characteristics Of Potato Tubers. Appl. Ecol. Environ. Res. 2018, 16, 39–50. [Google Scholar] [CrossRef]
- Mystkowska, I.T. Biostimulators as a factor affecting the yield of edible potato. Acta Agrophysica 2018, 25, 307–315. [Google Scholar] [CrossRef]
- Gugała, M.; Zarzecka, K.; Sikorska, A.; Mystkowska, I.; Dołęga, H. Effect of herbicides and growth biostimulants on weed reduction and yield of edible potato. Fragm. Agron. 2017, 34, 59–66. [Google Scholar]
- Stankeviciene, A.; Snieskiene, V.; Varkuleviciene, V. The diversity of pathogenic fungi in the rhizosphere of pot-plants of different phytopathologic state. Agron. Res. 2009, 7, 505–510. [Google Scholar]
- Anisimov, M.M.; Chaikina, E.L.; Klykov, A.G.; Rasskazov, V.A. Effect of seaweeds extracts on the growth on Seedling roots of buckwheat (Fagopyrum esculentum Moench) is depended on the season of algae collection. Agric. Sci. Dev. 2013, 2, 67–75. [Google Scholar]
- Kocira, A.; Kornas, R.; Kocira, S. Effect assessment of Kelpak SL on the bean yield Phaseolus vulgaris L. J. Cent. Eur. Agric. 2013, 14, 67–76. [Google Scholar] [CrossRef]
- Sosnowski, J.; Jankowski, K.; Wiśniewska-Kadźajan, B. Effect of growth regulator Kelpak SL on the formation of aboveground biomass of Festulolium braunii (K. Richt.) A. Camus. Acta Agrobotanica 2013, 66, 149–154. [Google Scholar] [CrossRef]
- Gleń-Karolczk, K.; Boligłowa, E. The effect of Kelpak SL bioregulator on fungi isolated from the roots of horseradish (Armoracia rusticana Gaertn.). J. Res. Appl. Agric. Eng. 2015, 60, 63–67. [Google Scholar]





| No. | Highlighted Characteristics | Description |
|---|---|---|
| 1. | Maturity time | Medium early |
| 2. | Flesh color | Pale yellow |
| 3. | Skin color | Yellow |
| 4. | Tuber shape | Oval |
| Shape regularity 1–9 | Regular 7.0 | |
| 5. | The size of the tubers 1–9 | Very big 9.0 |
| 6. | Shallowness of eyes 1–9 | Shallow 7.1 |
| 7. | Fertility | Very big |
| 8. | Cooking type | B-BC (B—general purpose, C—mealy) |
| 9. | Resistance to late blight on a 9° | Susceptible (5°) |
| 10. | Resistance to PVY on a 9° | Field resistant (7°) |
| 11. | Resistance to PLRV on a 9° | Resistant (8°) |
| 12. | Resistance to nematode | Quite resistant to the potato cyst nematode |
| Designation of Protection Treatments | Preparate | Preparations and Their Doses | |||
|---|---|---|---|---|---|
| Before Planting | During Vegetation | ||||
| Application to Soil | Dressing Seed Potatoes | The Foliar Application | |||
| BCAs | BCAPo * | Polyversum WP | 200 g·ha−1 in 300 L of water | 10 g·kg−1 of seed potatoes (soaking for 30 min) | 3 × SpinTor 240 SC 0.1 l ha−1 + 3 × Miedzian 50 WP 2 kg ha−1 |
| BCABs | Serenade ASO | 10 L ha−1 in 500 L of water | 400 mL·kg−1 of seed potatoes (soaking for 30 min) | ||
| PGP | PGPEm | Kelpak SL | 4 L ha−1 in 800 L of water | 0.4% (soaking for 5 min) | |
| MSADs | EM | Em Farma™ | 20 L ha−1 in 300 L of water | 1% (soaking for 5 min) | |
| UG | UG Max soil conditioner | 1.2 L∙ha−1 in 300 L of water | 0.5% (soaking for 30 min) | ||
| BR | Biogen Rewital | 1 L∙ha−1 in 500 L of water | 0.2% (soaking for 4 h) | ||
| Control | C | no protection | 300 L of water | In distilled water for 30 min | |
| Specification | 2015 | 2016 | 2017 | Mean |
|---|---|---|---|---|
| pH H2O | 5.69 | 6.08 | 5.83 | 5.87 |
| Salinity (g NaCl·L−1) | 0.21 | 0.14 | 0.24 | 0.20 |
| Humus content [%] | 1.73 | 1.89 | 1.45 | 1.69 |
| The content available in the soil layer of 0–30 cm (mg·L−1) | ||||
| N-NO3 | 16.00 | <14.00 | <14.00 | 14.67 |
| P | 29.00 | 36.00 | 28.00 | 31.00 |
| K | 108.00 | 118.00 | 199.00 | 141.67 |
| Ca | 182.00 | 270.00 | 258.00 | 236.67 |
| Mg | 10.00 | 44.00 | 38.00 | 30.67 |
| Cl | <27.00 | <27.00 | 39.00 | 31.00 |
| Cu | 0.89 | 2.02 | 1.02 | 1.31 |
| Zn | 2.59 | 12.54 | 5.02 | 6.72 |
| Mn | 60.12 | 76.00 | 78.00 | 71.37 |
| Fe | 234.60 | 268.64 | 173.8 | 225.68 |
| B | 0.10 | 0.20 | 1.10 | 0.47 |
| S-SO4 (mg∙100 g−1 soil) | 0.35 | 0.32 | 1.72 | 0.80 |
| Pb (mg·kg−1) | 12.26 | 16.81 | 12.63 | 13.90 |
| Cd (mg·kg−1) | <0.33 | 0.33 | 0.37 | 0.34 |
| Ni (mg·kg−1) | 1.50 | 4.04 | 3.02 | 2.85 |
| Infection of Potato Tubers by: | Parameters of Potato Tuber Infection | Efficiency of Protection [E] | ||
|---|---|---|---|---|
| Disease Severity Index [DSI] | Average Degree of Infection [ADI] | Percentage of Infested Tubers [PIT] | ||
| Rhizoctonia solani | ||||
| Preparates | *** | *** | *** | *** |
| Years | *** | *** | *** | *** |
| Preparates × years | *** | *** | *** | *** |
| Streptomyces scabies | ||||
| Preparates | *** | *** | *** | *** |
| Years | *** | *** | *** | *** |
| Preparates × years | *** | *** | *** | *** |
| Helminthosporium solani | ||||
| Preparates | *** | *** | *** | *** |
| Years | *** | *** | *** | *** |
| Preparates × years | *** | *** | *** | *** |
| Specification | C ** | BCAPo | BCABs | PGPEm | EM | UG | BR | |
|---|---|---|---|---|---|---|---|---|
| Rhizoctonia solani | ||||||||
| Disease Severity Index | 2015 | 55.4 b * ± 1.2 | 47.4 d ± 3.1 | 33.3 fgh ± 4.9 | 26.9 ijk ± 7.0 | 29.6 hi j ± 1.9 | 38.6 e ± 1.2 | 28.5 hij ± 3.0 |
| 2016 | 50.3 cd ± 3.8 | 15.5 m ± 7.2 | 20.0 lm ± 7.9 | 31.5 ghi ± 5.7 | 25.8 jk ± 5.7 | 35.5 efg ± 5.1 | 22.8 kl ± 12.2 | |
| 2017 | 68.0 a ± 4.8 | 37.8 ef ± 5.1 | 38.8 e ± 5.9 | 54.8 bc ± 1.5 | 54.8 bc ± 2.0 | 50.0 cd ± 2.8 | 45.5 d ± 2.5 | |
| Average degree of infection | 2015 | 2.2 b ± 3.8 | 1.9 c ± 3.4 | 1.3 efg ± 5.2 | 1.1 hij ± 7.8 | 1.2 fghi ± 5.4 | 1.5 de ± 2.2 | 1.1 ghij ± 5.1 |
| 2016 | 2.0 bc ± 3.8 | 0.6 l ± 7.2 | 0.8 kl ± 7.9 | 1.3 fgh ± 5.7 | 1.0 ij ± 5.7 | 1.4 def ± 5.1 | 0.9 jk ± 12.2 | |
| 2017 | 2.7 a ± 4.8 | 1.5 de ± 6.2 | 1.6 d ± 5.9 | 2.2 b ± 1.5 | 2.2 b ± 2.0 | 2.0 b ± 2.8 | 1.8 c ± 2.5 | |
| Percentage of infested tubers | 2015 | 74 abc ± 2.7 | 72 abcd ± 5.6 | 63 cdef ± 5.3 | 32 jk ± 15.3 | 45 ghij ± 3.8 | 56 dfgh ± 16.8 | 40 hijk ± 10.0 |
| 2016 | 83 ab ± 9.3 | 27 k ± 12.3 | 36 ijk ± 7.9 | 53 efghi ± 6.3 | 47 fghij ± 15.2 | 47 fghij ± 7.1 | 38 ijk ± 17.5 | |
| 2017 | 88 a ± 10.2 | 63 cdfe ± 5.3 | 60 cdfg ± 4.7 | 86 ab ± 11.6 | 82 ab ± 12.7 | 69 bcdf ± 2.5 | 62 cdfg ± 7.2 | |
| Effectiveness of protection | 2015 | 14.3 l ± 18.7 | 40.0 fgh ± 7.4 | 51.5 cd ± 6.6 | 46.6 cdef ± 2.2 | 30.4 ij ± 2.6 | 48.5 cde ±3.2 | |
| 2016 | 69.2 a ± 3.2 | 60.2 b ± 5.2 | 37.3 ghi ± 9.6 | 48.8 cde ± 6.0 | 29.3 ij ± 12.2 | 54.7 bc ± 10.1 | ||
| 2017 | 44.5 defg ± 6.4 | 43.0 efg ± 7.8 | 19.5 kl ± 6.3 | 19.5 kl ± 8.2 | 26.5 jk ± 7.9 | 33.1 hij ± 5.0 | ||
| Streptomyces scabies | ||||||||
| Disease Severity Index | 2015 | 73.2 a ± 2.0 | 66.7 b ± 3.0 | 62.8 bc ± 4.1 | 45.4 hij ± 3.8 | 52.4 efg ± 4.5 | 57.2 cde ± 4.9 | 67.3 ab ± 2.8 |
| 2016 | 59.0 cd ± 3.9 | 37.8 klm ± 6.6 | 32.4 mno ± 3.7 | 41.4 ijkl ± 2.9 | 47.0 ghi ± 4.1 | 50.5 fgh ± 4.3 | 50.6 fgh ± 6.3 | |
| 2017 | 53.8 def ± 3.5 | 30.0 no ± 4.4 | 26.6 o ± 5.4 | 39.8 jkl ± 2.2 | 35.6 lmn ± 5.1 | 41.0 ijkl ± 6.1 | 43.2 ijk ± 2.3 | |
| Average degree of infection | 2015 | 3.7 a ± 2.0 | 3.3 ab ± 3.2 | 3.1 bc ± 4.1 | 2.3 gh ± 3.8 | 2.6 def ± 4.5 | 2.9 cde ± 4.9 | 3.4 ab ± 3.0 |
| 2016 | 3.0 cd ± 3.9 | 1.9 ij ± 6.6 | 1.6 jkl ± 3.7 | 2.1 hi ± 2.9 | 2.4 fgh ± 4.1 | 2.5 efg ± 4.2 | 2.5 efg ± 6.3 | |
| 2017 | 2.7 de ± 3.5 | 1.5 kl ± 5.2 | 1.3 l ± 5.4 | 1.9 ij ± 11.3 | 1.8 ijk ± 5.1 | 2.1 hi ± 6.1 | 2.1 hi ± 3.3 | |
| Percentage of infested tubers | 2015 | 97 a ± 3.4 | 91 abc ± 5.7 | 86 bcd ± 2.3 | 77 de ± 4.3 | 80 cde ± 3.5 | 94 ab ± 2.1 | 93 ab ± 3.6 |
| 2016 | 92 ab ± 3.1 | 73 efg ± 6.0 | 56 hi ± 5.1 | 75 ef ± 4.4 | 73 efg ± 8.1 | 80 cde ± 3.5 | 88 abc ± 3.2 | |
| 2017 | 86 bcd ± 2.3 | 63 gh ± 5.3 | 52 i ± 5.4 | 71 efg ± 6.1 | 65 fgh ± 2.7 | 71 efg ± 6.1 | 80 cde ± 6.1 | |
| Efficiency of protection | 2015 | 8.9 h ± 30.9 | 14.1 fgh ± 24.5 | 38.0 bc ± 6.3 | 28.4 cdef ± 11.4 | 21.9 efg ± 17.5 | 8.0 h ± 31.5 | |
| 2016 | 35.9 bc ± 11.7 | 45.1 ab ± 4.5 | 29.7 cde ± 6.8 | 20.3 efg ± 15.9 | 14.4 fgh ± 25.4 | 14.2 fgh ± 37.7 | ||
| 2017 | 44.2 ab ± 5.6 | 50.8 a ± 5.7 | 26.0 def ± 6.2 | 33.8 cd ± 10.1 | 23.8 efg ± 19.5 | 19.7 fg ± 9.2 | ||
| Helminthosporium solani | ||||||||
| Disease Severity Index | 2015 | 63.6 a ± 2.9 | 54.3 b ± 2.8 | 53.6 b ± 1.1 | 39.2 ef ± 4.6 | 50.4 bc ± 2.2 | 43.2 de ± 2.3 | 46.0 cd ± 2.9 |
| 2016 | 52.6 b ± 5.0 | 28.8 ij ± 3.9 | 27.2 jk ± 7.5 | 34.3 gh ± 3.9 | 45.8 cd ± 2.3 | 42.0 de ± 2.1 | 39.0 ef ± 1.7 | |
| 2017 | 46.5 cd ± 3.3 | 23.6 kl ± 6.1 | 20.2 l ± 5.9 | 31.0 hij ± 7.4 | 33.0 hi ± 4.7 | 38.6 efg ± 5.9 | 34.8 fgh ± 4.1 | |
| Average degree of infection | 2015 | 3.2 a ± 2.9 | 2.7 b ± 2.8 | 2.5 bc ± 11.1 | 2.0 ef ± 4.6 | 2.5 bc ± 2.2 | 2.2 de ± 2.3 | 2.3 cd ± 2.9 |
| 2016 | 2.6 b ± 5.0 | 1.4 ijk ± 3.9 | 1.4 jk ± 7.5 | 1.7 fghi ± 3.3 | 2.3 cd ± 2.3 | 2.1 de ± 2.1 | 2.0 ef ± 1.7 | |
| 2017 | 2.3 cd ± 3.1 | 1.2 kl ± 6.1 | 1.0 l ± 5.9 | 1.5 hij ± 7.0 | 1.7 fghij ± 4.7 | 1.9 efg ± 5.9 | 1.7 fgh ± 4.1 | |
| Percentage of infested tubers | 2015 | 78 a ± 2.6 | 70 abcd ± 2.9 | 66 abcde ± 3.0 | 56 efg ± 5.1 | 66 abcde ± 5.2 | 59 cdefg ± 2.9 | 62 bcdef ± 3.2 |
| 2016 | 73 ab ± 7.1 | 54 efg ± 3.7 | 55 efg ± 6.0 | 66 abcde ± 3.0 | 69 abcd ± 8.6 | 76 a ± 3.7 | 70 abcd ± 2.9 | |
| 2017 | 71 abc ± 6.1 | 50 fg ± 8.9 | 48 g ± 19.5 | 55 efg ± 10.8 | 58 defg ± 7.7 | 61 bcdef ± 7.1 | 62 bcdef ± 7.2 | |
| Efficiency of protection | 2015 | 14.4 j ± 14.4 | 15.7 j ± 5.7 | 38.4 cd ± 7.3 | 20.8 ghij ± 8.6 | 32.1 def ± 4.8 | 27.7 efgh ± 7.5 | |
| 2016 | 45.2 bc ± 4.8 | 48.3 ab ± 8.0 | 34.8 de ± 7.2 | 12.9 j ± 15.3 | 20.2 hij ± 8.4 | 25.9 fgh ± 4.9 | ||
| 2017 | 49.3 ab ± 6.3 | 56.6 a ± 4.5 | 33.4 def ± 14.8 | 29.1 efg ± 11.5 | 16.9 ij ± 28.5 | 25.2 fghi ± 12.4 |
| Effects | Parameter | |
|---|---|---|
| Crop | Yield Increase | |
| Preparates | *** | *** |
| Years | *** | ** |
| Preparates × years | *** | *** |
| Specification | C | BCAPo | BCABs | PGPEm | EM | UG | BR |
|---|---|---|---|---|---|---|---|
| Crop [t] | |||||||
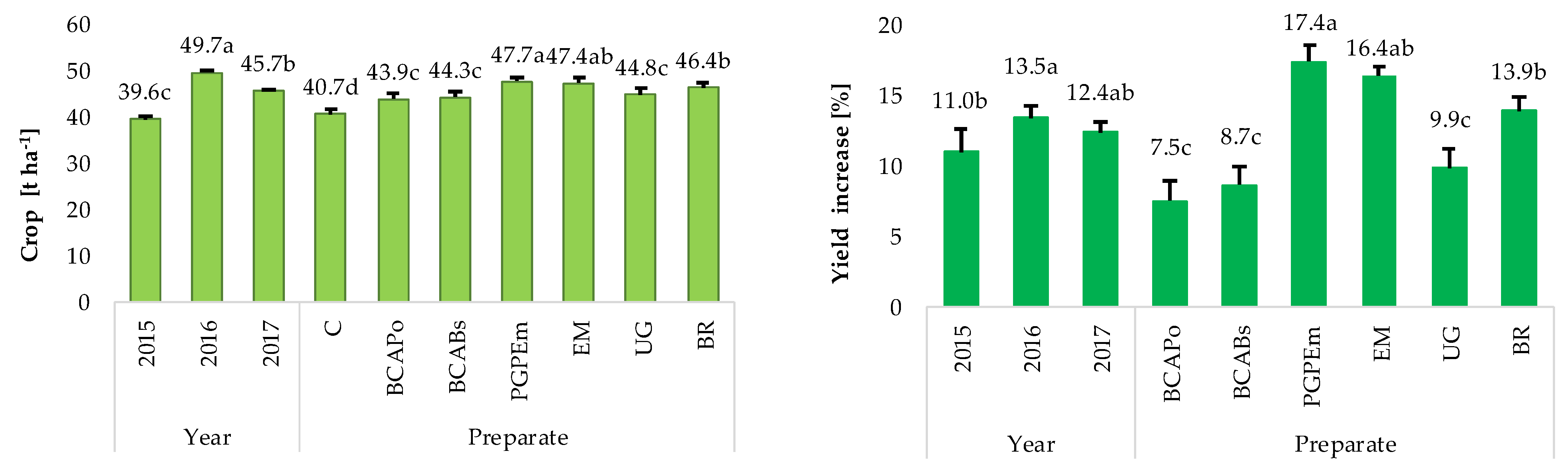
| 2015 | 36.2 j * ± 1.5 | 36.7 j ± 1.9 | 38.0 j ± 5.6 | 44.2 gh ± 1.7 | 42.1 hi ± 1.7 | 38.4 j ± 1.8 | 41.7 i ± 1.3 |
| 2016 | 44.6 fg ± 1.4 | 48.4 cd ± 0.8 | 49.0 bc ± 1.2 | 51.8 a ± 1.3 | 51.2 ab ± 0.6 | 51.2 ab ±1.7 | 52.0 a ± 0.9 |
| 2017 | 41.3 i ± 1.4 | 46.5 def ± 1.4 | 45.9 efg ± 0.7 | 47.1 cde ± 0.8 | 48.8 c ±1.3 | 44.9 efg ±1.1 | 45.4 efg ± 0.3 |
| Yield increase [%] | |||||||
| 2015 | 1.4 g ± 140.2 | 4.9 fg ± 97.1 | 22.1 a ± 13.5 | 16.3 abc ± 18.9 | 6.1 efg ± 47.4 | 15.2 abcd ± 16.9 | |
| 2016 | 8.5 defg ± 15.9 | 10 cdef ± 27.8 | 16.1 abc ± 18.8 | 14.8 bcd ± 11.0 | 14.8 bcd ± 20.9 | 16.6 abc ± 10.1 | |
| 2017 | 12.6 bcde ± 5.9 | 11.2 bcdef ± 19.0 | 14.1 bcd ± 5.7 | 18.2 ab ± 2.0 | 8.7 def ± 18.1 | 9.9 cdef ± 14.2 | |
| Specification | Disease Severity Index—Crop | Summary Disease Severity Index—Crop | ||
|---|---|---|---|---|
| Black Scurf | Common Scab | Silver Scurf | ||
| C | −0.18 | −0.78 ** | −0.68 * | −0.96 *** |
| BCAPo * | −0.82 *** | −0.92 *** | −0.94 *** | −0.99 *** |
| BCABs | −0.46 | −0.89 *** | −0.86 *** | −0.95 *** |
| PGPEm | 0.03 | −0.53 | −0.45 | −0.35 |
| EM | 0.14 | −0.53 | −0.48 | −0.90 *** |
| UG | −0.18 | −0.41 | −0.23 | −0.83 *** |
| BR | −0.39 | −0.53 | −0.47 | −0.92 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gleń-Karolczyk, K.; Bolligłowa, E.; Luty, L. Health Parameters of Potato Tubers under the Influence of Soil Applied Bio-Preparations and Bio-Stimulants. Appl. Sci. 2022, 12, 11593. https://doi.org/10.3390/app122211593
Gleń-Karolczyk K, Bolligłowa E, Luty L. Health Parameters of Potato Tubers under the Influence of Soil Applied Bio-Preparations and Bio-Stimulants. Applied Sciences. 2022; 12(22):11593. https://doi.org/10.3390/app122211593
Chicago/Turabian StyleGleń-Karolczyk, Katarzyna, Elżbieta Bolligłowa, and Lidia Luty. 2022. "Health Parameters of Potato Tubers under the Influence of Soil Applied Bio-Preparations and Bio-Stimulants" Applied Sciences 12, no. 22: 11593. https://doi.org/10.3390/app122211593





