An Optimized Methodology for Patient-Specific Therapeutic Activity Administration in Liver Radioembolization
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Phantom Studies
2.1.1. Phantom Imaging
2.1.2. Phantom Image Processing
2.1.3. Phantom Dose Calculation and Analysis
2.2. Patient Studies
2.2.1. Patient Data Set
2.2.2. Patient Imaging
- (1)
- Five multi-modality fiducial markers (Izi Medical) were fixed on the skin of the patient, one at the level of the xyphoid, one at the level of the inferior rib cage of the right, one at the level of the inferior rib cage on the left, one at the level of the spinous process of the second lumbar vertebra (L2), and finally, the other one at the level of the spinous process of the fourth lumbar vertebra (L4). These fiducial markers are small discs of CT radiopaque material that, when loaded with 99mTc, become visible during SPECT imaging [35]. Each fiducial marker was loaded with approximately 0.5 MBq of 99mTc, diluted in a physiological saline solution at a concentration of 50 MBq/0.5 mL immediately before the acquisition of the SPECT-MAA images. During the registration of the SPECT and CT images of the patients with fiducial markers attached to their skin, these were easily located on both SPECT and CT images and were used as reference points (i.e., landmarks) in the registration algorithm.
- (2)
- All these fiducial markers were used to assist in the registration of SPECT-MAA images with the corresponding CT images. The five fiducial markers were always applied on each of the last seven treatments.
2.2.3. Patient Image Processing
2.2.4. Patient Dose Calculation and Analysis
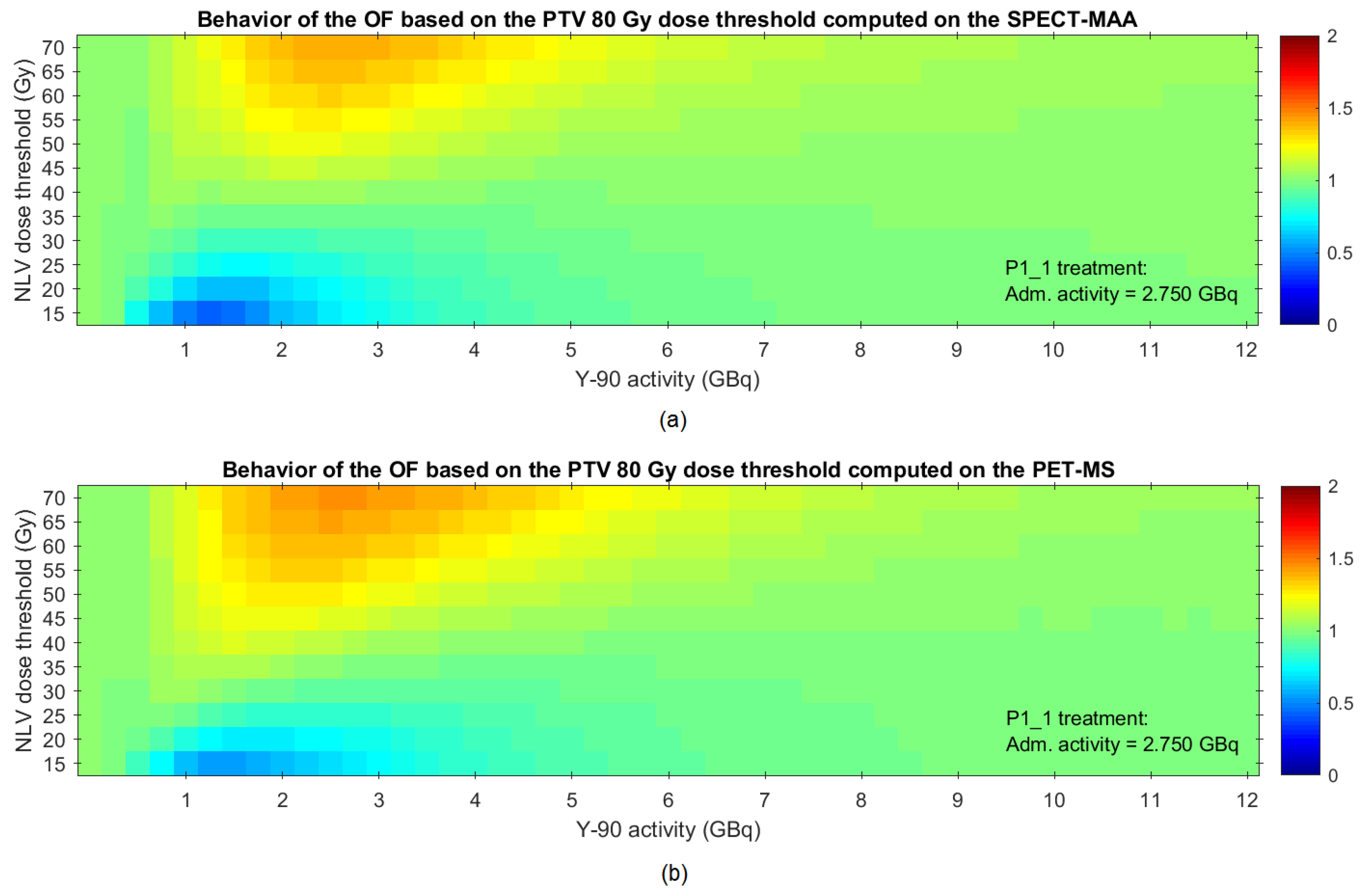
2.2.5. Proposed Methodology for Optimization of the Activity to Be Administered
3. Results
3.1. Phantom Studies
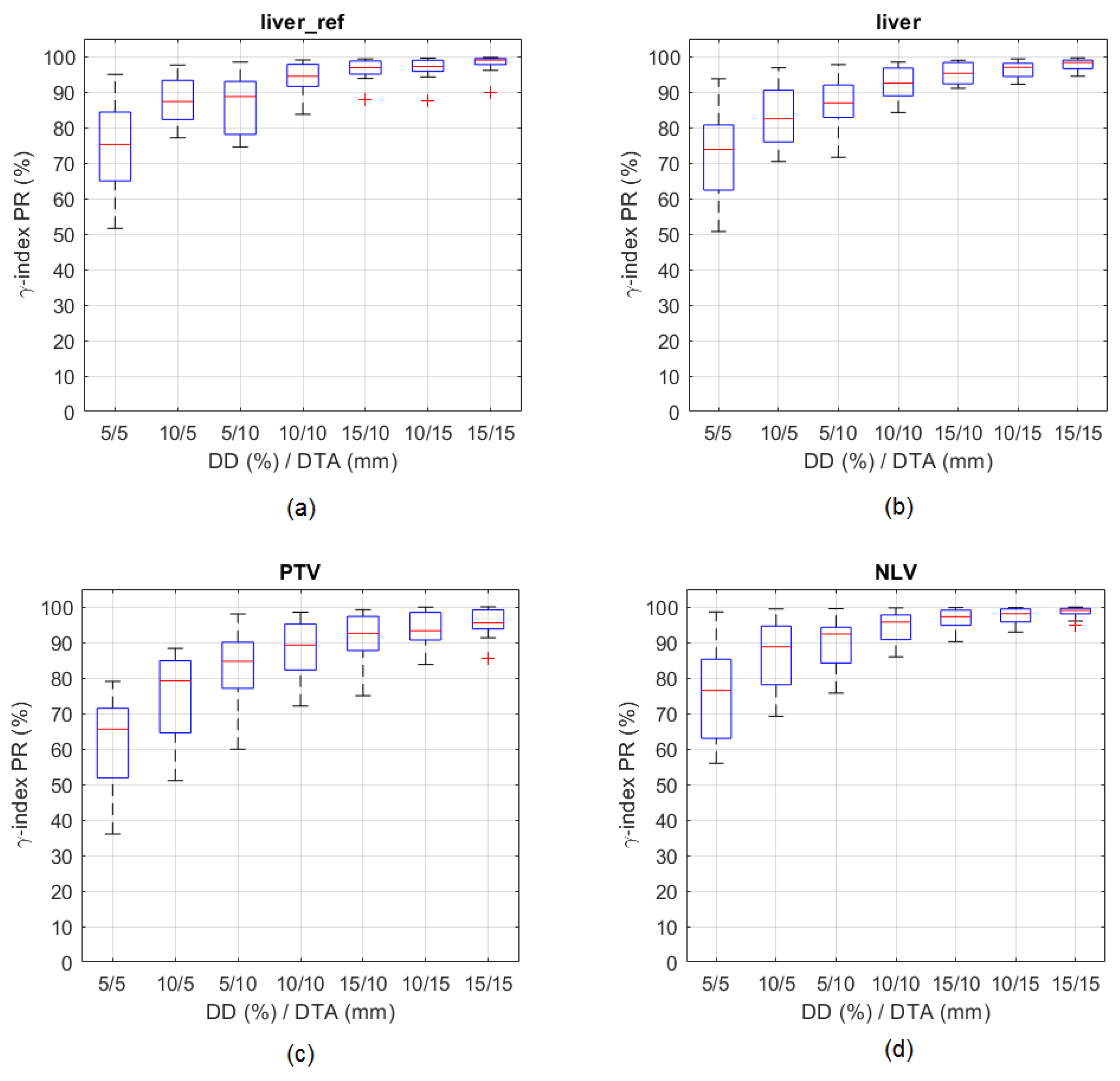
3.1.1. Accuracy of SPECT and PET Derived Absorbed Dose Distributions
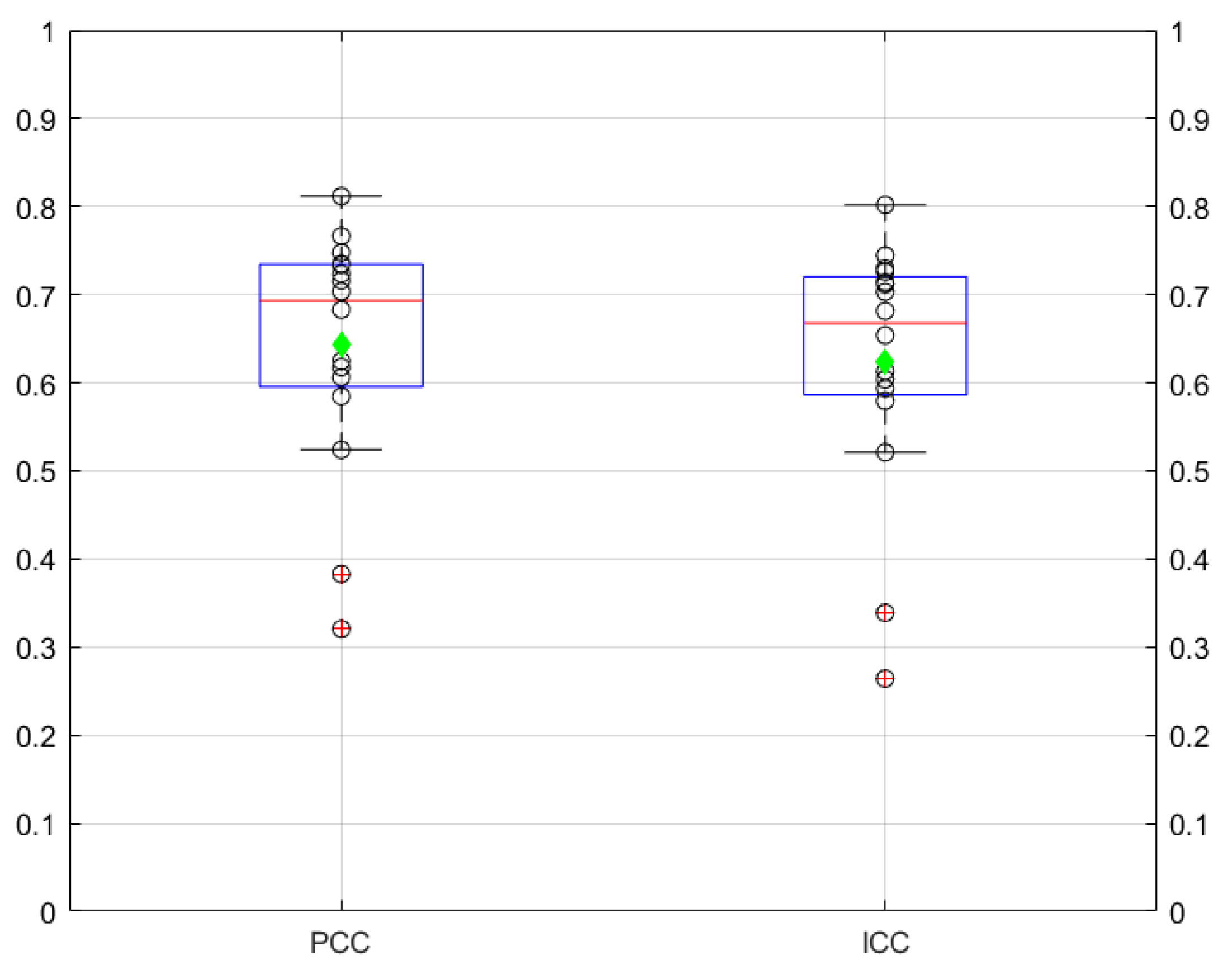
3.1.2. Correlation and Agreement between Absorbed Dose Distributions Derived from SPECT and PET Images
3.2. Patient Studies
Correlation and Agreement between Absorbed Dose Distributions Derived from Spect and Pet Images
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Trends over Time for Cancer Mortality Worldwide. 2008. Available online: http://www.cancerresearchuk.org (accessed on 27 February 2018).
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, 359–386. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Center, M.M.; DeSantis, C.; Ward, E.M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1893–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M. Global Cancer Observatory: Cancer Today. 2018. Available online: https://gco.iarc.fr/today (accessed on 28 March 2019).
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2018, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freddie, B.; Jacques, F.; Isabelle, S.; Rebecca, L.S.; Lindsey, A.T.; Ahmedin, J. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Erdoğan, E.B.; Özdemir, H.; Aydin, M. Radioembolization Treatment for Liver Cancer. Bezmialem Sci. 2016, 4, 25–32. [Google Scholar] [CrossRef]
- Murthy, R.; Kamat, P.; Nuñez, R.; Salem, R. Radioembolization of Yttrium-90 Microspheres for Hepatic Malignancy. Semin. Interv. Radiol. 2008, 25, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Gardini, A.C.; Tamburini, E.; Sangro, B. Radioembolization versus chemoembolization for unresectable hepatocellular carcinoma: A meta-analysis of randomized trials. OncoTargets Ther. 2018, 11, 7315. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.; Nag, S.; Salem, R.; Murthy, R.; McEwan, A.J.; Nutting, C.; Benson, A., 3rd; Espat, J.; Bilbao, J.I.; Sharma, R.A.; et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: A consensus panel report from the radioembolization brachytherapy oncology consortium. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 13–23. [Google Scholar] [CrossRef]
- Smits, M.; Nijsen, J.; van den Bosch, M.; Lam, M.; Vente, M.; Huijbregts, J.; Schip, A.D.V.H.; Elschot, M.; Bult, W.; de Jong, H.W.; et al. Holmium-166 radioembolization for the treatment of patients with liver metastases: Design of the phase I HEPAR trial. J. Exp. Clin. Cancer Res. 2010, 29, 70. [Google Scholar] [CrossRef] [Green Version]
- Prince, J.F. Holmium Radioembolization: Efficacy and Safety. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2016. [Google Scholar]
- Kallini, J.R.; Gabr, A.; Salem, R.; Lewandowski, R.J. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv. Ther. 2016, 33, 699–714. [Google Scholar] [CrossRef] [Green Version]
- Garin, E.; Lenoir, L.; Rolland, Y.; Edeline, J.; Mesbah, H.; Laffont, S.; Porée, P.; Clément, B.; Raoul, J.L.; Boucher, E. Dosimetry based on 99mTc-macroaggregated albumin SPECT/CT accurately predicts tumor response and survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microspheres: Preliminary results. J. Nucl. Med. 2012, 53, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Pasciak, A.; Bradley, Y.; McKinney, J. Handbook of Radioembolization; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Boston Scientific International. TheraSphere™ Yttrium-90 Glass Microspheres—Instructions for Use; Boston Scientific International: Fremont, CA, USA, 2005; pp. 1–16. [Google Scholar]
- Ferreira, P. Voxel-Based Dosimetry Using Multimodal Images for Patient-Specific Liver Radioembolization with Yttrium-90 Charged Glass Microspheres. Ph.D. Thesis, Department of Electrical and Computer Engineering, Universidade de Lisboa, Lisboa, Portugal, 2019. [Google Scholar]
- Ferreira, P.; Oliveira, F.P.M.; Parafita, R.; Girão, P.S.; Correia, P.L.; Costa, D.C. Patient-specific gamma-index analysis to evaluate 99mTc-MAA as a predictor for 90Y glass microspheres liver radioembolisation dosimetry. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2018, 7, 583–589. [Google Scholar] [CrossRef]
- E.U. Council Directive 2013/59/Euratom. Off. J. Eur. Union 2013, L13/1, 1–73. [Google Scholar]
- Greer, K. NEMA IEC Body Phantom Set—User’s Manual; Data Spectrum Corporation: Durham, NC, USA, 2008. [Google Scholar]
- Bolch, W.; Bouchet, L.; Robertson, J.; Wessels, B.; Siegel, J.; Howell, R.; Erdi, A.K.; Aydogan, B.; Costes, S.; Watson, E.E.; et al. MIRD pamphlet No. 17: The dosimetry of nonuniform activity distributions—Radionuclide S values at the voxel level. Medical Internal Radiation Dose Committee. J. Nucl. Med. 1999, 40, 11–36. [Google Scholar]
- Snyder, W.S. ‘S’ Absorbed Dose Per Unit Cumulated Activity for Selected Radionuclides and Organs, 11 ed: Society of Nuclear Medicine. MIRD Pam. 1975, 11, 1–257. [Google Scholar]
- Pacilio, M.; Amato, E.; Lanconelli, N.; Basile, C.; Torres, L.A.; Botta, F.; Ferrari, M.; Diaz, N.C.; Perez, M.C.; Fernández, M.; et al. Differences in 3D dose distributions due to calculation method of voxel S-values and the influence of image blurring in SPECT. Phys. Med. Biol. 2015, 60, 1945–1964. [Google Scholar] [CrossRef]
- Lanconelli, N.; Pacilio, M.; Meo, S.L.; Botta, F.; di Dia, A.; Aroche, A.T.; Pérez, M.A.C.; Cremonesi, M. A free database of radionuclide voxel S values for the dosimetry of nonuniform activity distributions. Phys. Med. Biol. 2012, 57, 517–533. [Google Scholar] [CrossRef]
- Lanconelli, N.; Pacilio, M.; Lo Meo, S.; Botta, F.; Di Dia, A.; Aroche, A.T.; Coca Perez, M.A.; Cremonesi, M. S Values for Voxel Dosimetry. 2017. Available online: http://www.medphys.it/down_svoxel.htm (accessed on 15 July 2017).
- Pacilio, M.; Lanconelli, N.; Lo, M.S.; Betti, M.; Montani, L.; Aroche, L.A.T.; Pérez, M.A.C. Differences among Monte Carlo codes in the calculations of voxel S values for radionuclide targeted therapy and analysis of their impact on absorbed dose evaluations. Med. Phys. 2009, 36, 1543–1552. [Google Scholar] [CrossRef] [Green Version]
- Low, D.A.; Harms, W.B.; Mutic, S.; Purdy, J.A. A technique for the quantitative evaluation of dose distributions. Med. Phys. 1998, 25, 656–661. [Google Scholar] [CrossRef]
- Hussein, M.; Rowshanfarzad, P.; Ebert, M.A.; Nisbet, A.; Clark, C.H. A comparison of the gamma index analysis in various commercial IMRT/VMAT QA systems. Radiother. Oncol. 2013, 109, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Callan, L.; Beech, B.; Wong, E. Radiation in the treatment of liver cancer. Univ. West. Ont. Med. J. 2014, 83, 17–20. [Google Scholar] [CrossRef]
- Lau, W.-Y.; Kennedy, A.S.; Kim, Y.H.; Lai, H.K.; Lee, R.-C.; Leung, T.W.T.; Liu, C.-S.; Salem, R.; Sangro, B.; Shuter, B.; et al. Patient Selection and Activity Planning Guide for Selective Internal Radiotherapy with Yttrium-90 Resin Microspheres. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Dawson, L.A.; McGinn, C.J.; Normolle, D.; Haken, R.K.T.; Walker, S.; Ensminger, W.; Lawrence, T.S. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J. Clin. Oncol. 2000, 18, 2210–2218. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef]
- Yushkevich, P.A.; Gerig, G. ITK-SNAP: An Intractive Medical Image Segmentation Tool to Meet the Need for Expert-Guided Segmentation of Complex Medical Images. IEEE Pulse 2017, 8, 54–57. [Google Scholar] [CrossRef]
- Jimmy, S. Tools for NIfTI and ANALYZE Image. 2014. Available online: https://www.mathworks.com/matlabcentral (accessed on 12 November 2015).
- Multi-Modal Markers for Radiology. 2017. Available online: https://www.medcat.nl/supplies/En/radmarker.htm (accessed on 5 April 2016).
- Yushkevich, P.A.; Piven, J.; Hazlett, H.C.; Smith, R.G.; Ho, S.; Gee, J.C.; Gerig, G. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006, 31, 1116–1128. [Google Scholar] [CrossRef] [Green Version]
- Criminisi, A. Decision Forests: A Unified Framework for Classification, Regression, Density Estimation, Manifold Learning and Semi-Supervised Learning. Found. Trends Comput. Graph. Vis. 2011, 7, 81–227. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Garin, E.; Rolland, Y.; Pracht, M.; Sourd, S.L.; Laffont, S.; Mesbah, H.; Haumont, L.-A.; Lenoir, L.; Rohou, T.; Brun, V.; et al. High impact of macroaggregated albumin-based tumour dose on response and overall survival in hepatocellular carcinoma patients treated with 90Y-loaded glass microsphere radioembolization. Liver Int. 2017, 37, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.; Parafita, R.; Canudo, A.; Oliveira, C.; Rosa, L.; Girão, P.M.; Costa, D.C. Multiple Liver Metastases from Carcinoma of the Thymus Treated with Yttrium-90 Radioembolization (Glass Microspheres): Clinical Dosimetry. Clin. Oncol. 2016, 1, 1–5. [Google Scholar]
- Goedicke, A.; Berker, Y.; Verburg, F.A.; Behrendt, F.F.; Winz, O.; Mottaghy, F.M. Study-parameter impact in quantitative 90-Yttrium PET imaging for radioembolization treatment monitoring and dosimetry. IEEE Trans. Med. Imaging 2013, 32, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Johns, H.E. The Physics of Radiology, 2nd ed.; Charles C Thomas: Springfield, IL, USA, 1961. [Google Scholar]
- Cameron, J.R.; Skofronick, J.G. Medical Physics; John Wiley & Sons: Hoboken, NJ, USA, 1978. [Google Scholar]
- Chiesa, C.; Mira, M.; Maccauro, M.; Spreafico, C.; Romito, R.; Morosi, C.; Camerini, T.; Carrara, M.; Pellizzari, S.; Negri, A.; et al. Radioembolization of hepatocarcinoma with (90)Y glass microspheres: Development of an individualized treatment planning strategy based on dosimetry and radiobiology. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1718–1738. [Google Scholar] [CrossRef] [PubMed]
- Venkatanarasimha, N.; Gogna, A.; Tong, K.T.A.; Damodharan, K.; Chow, P.K.H.; Lo, R.H.G.; Chandramohan, S. Radioembolisation of hepatocellular carcinoma: A primer. Clin. Radiol. 2017, 72, 1002–1013. [Google Scholar] [CrossRef]
- Lau, W.Y.; Leung, W.T.; Ho, S.; Leung, N.W.; Chan, M.; Lin, J.; Metreweli, C.; Johnson, P.; Li, A.K. Treatment of inoperable hepatocellular carcinoma with intrahepatic arterial yttrium-90 microspheres: A phase I and II study. Br. J. Cancer 1994, 70, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, A.M.; Bailey, I.H.; Burton, M.A. Tumour dosimetry in human liver following hepatic yttrium-90 microsphere therapy. Phys. Med. Biol. 2001, 46, 487–498. [Google Scholar] [CrossRef]
- Srinivas, S.M.; Nasr, E.C.; Kunam, V.K.; Bullen, J.A.; Purysko, A.S. Administered activity and outcomes of glass versus resin (90)Y microsphere radioembolization in patients with colorectal liver metastases. J. Gastrointest. Oncol. 2016, 7, 530–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Gucht, A.; Jreige, M.; Denys, A.; Blanc-Durand, P.; Boubaker, A.; Pomoni, A.; Mitsakis, P.; Silva-Monteiro, M.; Gnesin, S.; Lalonde, M.N.; et al. Resin Versus Glass Microspheres for (90)Y Transarterial Radioembolization: Comparing Survival in Unresectable Hepatocellular Carcinoma Using Pretreatment Partition Model Dosimetry. J. Nucl. Med. 2017, 58, 1334–1340. [Google Scholar] [CrossRef] [Green Version]
- Press, W.H.; Teukolsky, S.A.; Vetterling, W.T.; Flannery, B.P. Numerical Recipes: The Art of Scientific Computing, 3rd ed.; Cambridge University Press: New York, NY, USA, 2007. [Google Scholar]
- Garin, E.; Rolland, Y.; Laffont, S.; Edeline, J. Clinical impact of (99m)Tc-MAA SPECT/CT-based dosimetry in the radioembolization of liver malignancies with (90)Y-loaded microspheres. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, C.; Mira, M.; Maccauro, M.; Romito, R.; Spreafico, C.; Sposito, C.; Bhoori, S.; Morosi, C.; Pellizzari, S.; Negri, A.; et al. A dosimetric treatment planning strategy in radioembolization of hepatocarcinoma with 90Y glass microspheres. Q. J. Nucl. Med. Mol. Imaging 2012, 56, 503–508. [Google Scholar]
- Cremonesi, M.; Chiesa, C.; Strigari, L.; Ferrari, M.; Botta, F.; Guerriero, F.; De Cicco, C.; Bonomo, G.; Orsi, F.; Bodei, L.; et al. Radioembolization of Hepatic Lesions from a Radiobiology and Dosimetric Perspective. Front. Oncol. 2014, 4, 210. [Google Scholar] [CrossRef]




| Pn_t | Diagnosis | No. Administrations MAA MS | Administration Site | No. PTVs; Volume Range (mL); Total Volume (mL) | |
|---|---|---|---|---|---|
| P1_1 | Metastatic thymoma | 1 | 1 | proper hepatic artery | 16; 2–34; 241 |
| P2_2 | Metastatic pancreatic cancer | 3 | 3 | (1) right hepatic artery (2) segment II & III (3) segment IV | 9; 4–79; 574 |
| P3_3 | Metastatic colorectal cancer | 1 | 1 | proper hepatic artery | 3; 33–230; 314 |
| P4_4 | Metastatic colorectal cancer | 2 | 2 | (1) right hepatic artery (2) segment II & IV | 1; 462; 462 |
| P4_5 | Metastatic colorectal cancer | 1 | 1 | right hepatic artery | 5; 7–508; 1626 |
| P5_6 | Multifocal cholangiocarcinoma | 2 | 2 | (1) right hepatic artery (2) left hepatic artery | 6; 3–131; 451 |
| P6_7 | Metastatic colorectal cancer | 2 | 2 | (1) right hepatic artery (2) left hepatic artery | 3; 27–300; 389 |
| P7_8 | Metastatic colorectal cancer | 1 | 1 | proper hepatic artery | 5; 13–1016; 1154 |
| P8_9 | Metastatic colorectal cancer | 1 | 1 | left hepatic artery | 8; 2–20; 123 |
| P8_10 | Metastatic colorectal cancer | 1 | 1 | right hepatic artery | 5; 5–125; 202 |
| P9_11 | Hepatocarcinoma | 1 | 1 | left hepatic artery | 1; 772; 772 |
| P10_12 | Metastatic colorectal cancer | 1 | 1 | right hepatic artery | 14; 2–49; 93 |
| P11_13 | Cholangiocarcinoma | 1 | 1 | segment IV | 1; 256; 256 |
| P12_14 | Hepatocarcinoma | 2 | 2 | (1) right hepatic artery (2) segment IV | 1; 1047; 1047 |
| P13_15 | Metastatic pancreatic cancer | 1 | 1 | right hepatic artery | 1; 510; 510 |
| P14_16 | Cholangiocarcinoma | 2 | 2 | (1) right hepatic artery (I) (2) right hepatic artery (II) | 2; 5–89; 94 |
| ROI | Cylinder 1 | Cylinder 2 | Cylinder 3 | Cylinder 4 | Cylinder 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Actual volume (mL) | 15 | 8 | 15 | 8 | 108 | ||||||
| SPECT-based volume (mL) | 23 | 9 | 24 | 10 | 117 | ||||||
| PET-based volume (mL) | 21 | 11 | 19 | 12 | 118 | ||||||
| Activity ⇒ Mean AD | MBq ⇒ Gy | MBq ⇒ Gy | MBq ⇒ Gy | MBq ⇒ Gy | MBq ⇒ Gy | ||||||
| PET-MS ground truth (simulated) | 365 | 669 | 187 | 618 | 344 | 658 | 204 | 639 | 2084 | 775 | |
| Calculated | 185 | 365 | 81 | 309 | 175 | 378 | 92 | 294 | 1871 | 745 | |
| Deviation (%) | −49 | −45 | −57 | −50 | −49 | −43 | −55 | −54 | −10 | −4 | |
| SPECT-MAA ground truth (simulated) | 408 | 669 | 150 | 618 | 421 | 658 | 173 | 639 | 2031 | 775 | |
| Calculated | 201 | 373 | 72 | 289 | 217 | 408 | 80 | 260 | 1808 | 721 | |
| Deviation (%) | −52 | −44 | −52 | −53 | −48 | −38 | −54 | −59 | −11 | −7 | |
| ROI | DD (%)/DTA (mm) | ||||||
|---|---|---|---|---|---|---|---|
| 5/5 | 10/5 | 5/10 | 10/10 | 15/10 | 10/15 | 15/15 | |
| liver_cyl | 98.3 | 98.7 | 98.7 | 98.9 | 99.2 | 99.0 | 99.3 |
| ptv_5_cyl_backg | 88.9 | 92.1 | 90.0 | 92.8 | 95.7 | 93.2 | 96.0 |
| Pn_t | Administered Activity (MBq) | SPECT-MAA Estimated Optimal Activity (MBq) | PET-MS Estimated Optimal Activity (MBq) |
|---|---|---|---|
| P1_1 | 2750 | 2598 | 2594 |
| P2_2 | 3347 | 3748 | 4009 |
| P3_3 | 3961 | 6239 | 8518 |
| P4_5 | 4231 | 10,662 | 9423 |
| P5_6 | 4305 | 4397 | 3397 |
| P6_7 | 2814 | 2274 | 2281 |
| P7_8 | 5604 | 8094 | 8481 |
| P8_9 | 1537 | 4898 | 4505 |
| P8_10 | 4227 | 2219 | 2450 |
| P10_12 | 1992 | 1471 | 1380 |
| P12_14 | 4481 | 8675 | 8539 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, P.; Oliveira, F.P.M.; Parafita, R.; Correia, P.L.; Girão, P.S.; Costa, D.C. An Optimized Methodology for Patient-Specific Therapeutic Activity Administration in Liver Radioembolization. Appl. Sci. 2022, 12, 11669. https://doi.org/10.3390/app122211669
Ferreira P, Oliveira FPM, Parafita R, Correia PL, Girão PS, Costa DC. An Optimized Methodology for Patient-Specific Therapeutic Activity Administration in Liver Radioembolization. Applied Sciences. 2022; 12(22):11669. https://doi.org/10.3390/app122211669
Chicago/Turabian StyleFerreira, Paulo, Francisco P. M. Oliveira, Rui Parafita, Paulo L. Correia, Pedro S. Girão, and Durval C. Costa. 2022. "An Optimized Methodology for Patient-Specific Therapeutic Activity Administration in Liver Radioembolization" Applied Sciences 12, no. 22: 11669. https://doi.org/10.3390/app122211669





