Phytochemicals from Allium tuberosum Rottler ex Spreng Show Potent Inhibitory Activity against B-Raf, EGFR, K-Ras, and PI3K of Non-Small Cell Lung Cancer Targets
Abstract
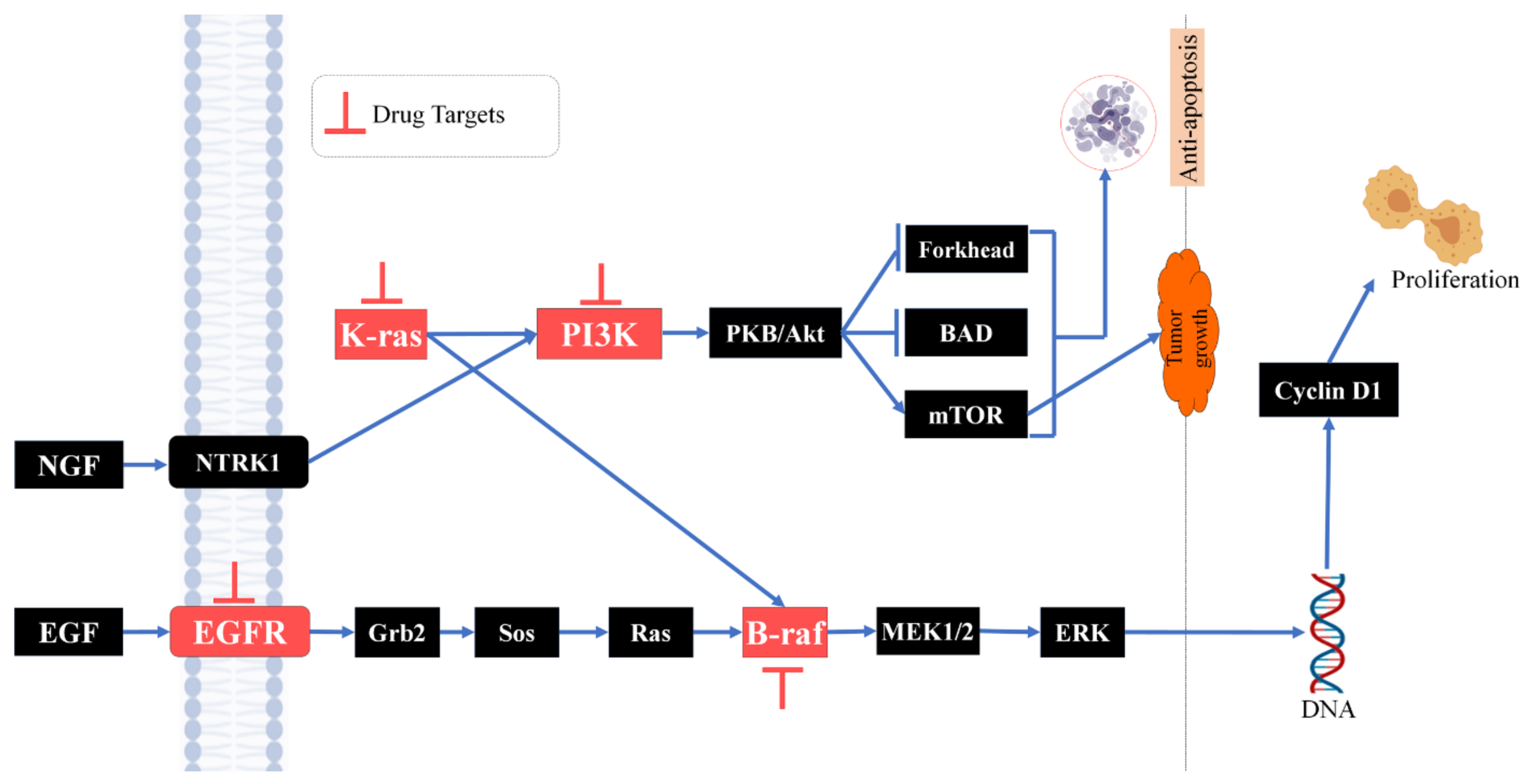
:1. Introduction
2. Methodology
2.1. Preparation of Plant Extracts
2.2. Quantitative Phytochemical Screening
2.2.1. Determination of Total Phenolic Content (TPC)
2.2.2. Determination of Total Flavonoid Content (TFC)
2.3. Antioxidant Activity by DPPH
2.4. Metabolites Profiling
2.5. Selection and Preparation of Receptor
2.6. Preparation of Ligands
2.7. Molecular Docking
2.8. Prediction of ADME Profile and Drug-likeness
3. Result
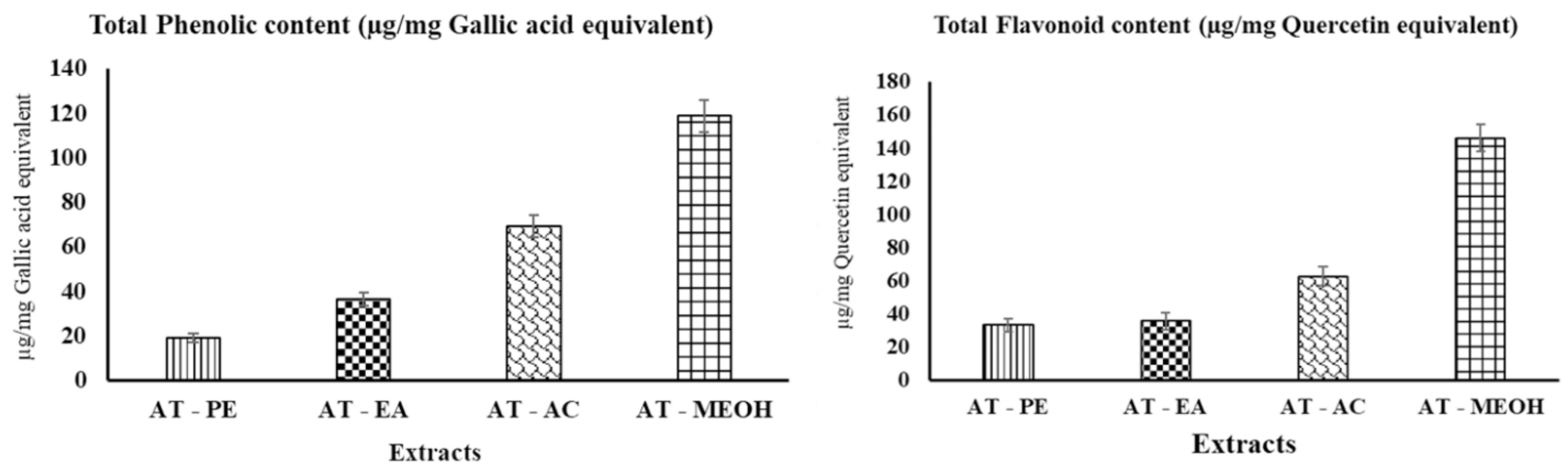
3.1. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.2. DPPH Free Radical Scavenging Assay
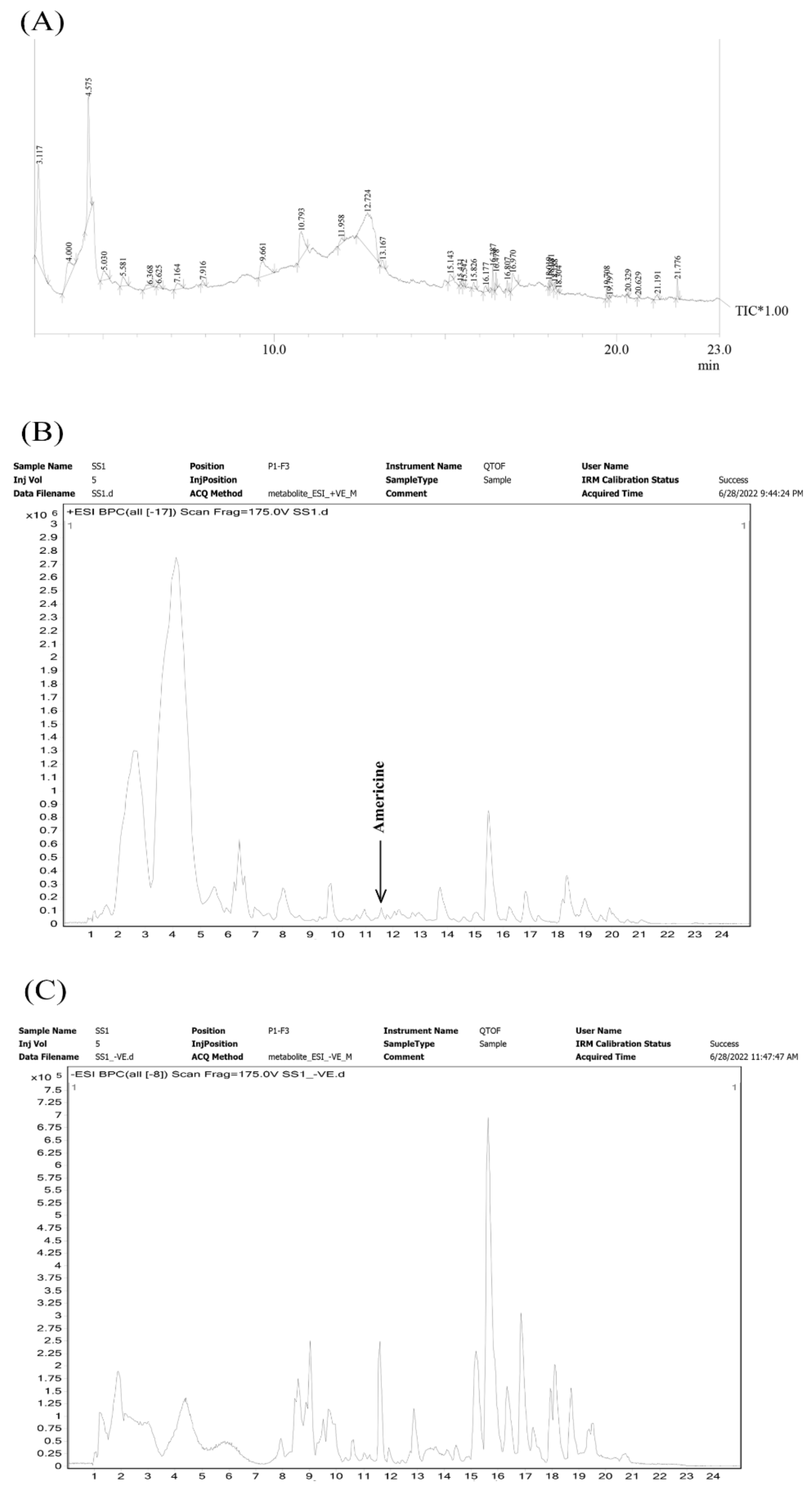
3.3. Metabolites Profiling
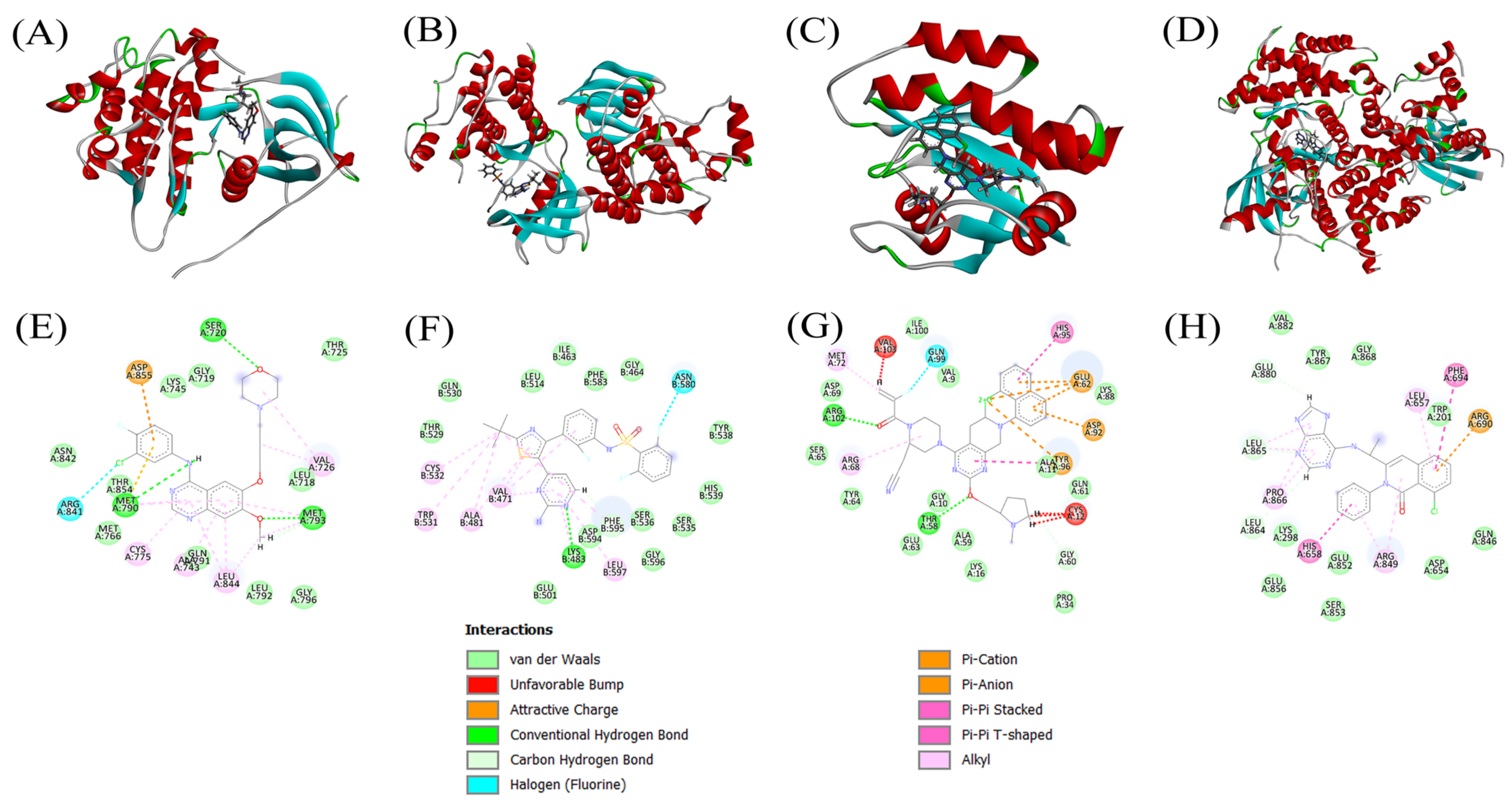
3.4. Docking Scores and Inhibition of Receptors
3.5. ADMET Profile Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bailar, J.C.; Gornik, H.L. Cancer undefeated. N. Engl. J. Med. 1997, 336, 1569–1574. [Google Scholar] [CrossRef] [PubMed]
- Alberg, A.J.; Samet, J.M. Epidemiology of lung cancer. Chest 2003, 123, 21S–49S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldstraw, P.; Ball, D.; Jett, J.R.; Le Chevalier, T.; Lim, E.; Nicholson, A.G.; Shepherd, F.A. Non-small-cell lung cancer. Lancet 2011, 378, 1727–1740. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Akerley, W.; Borghaei, H.; Chang, A.C.; Cheney, R.T.; Chirieac, L.R.; D’Amico, T.A.; Demmy, T.L.; Ganti, A.K.P.; Govindan, R. Non-small cell lung cancer: Clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2012, 10, 1236–1271. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Heigener, D.F.; Mok, T.; Soria, J.-C.; Rabe, K.F. Management of non-small-cell lung cancer: Recent developments. Lancet 2013, 382, 709–719. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Zappa, C.; Mousa, S.A. Non-small cell lung cancer: Current treatment and future advances. Transl. Lung Cancer Res. 2016, 5, 288. [Google Scholar] [CrossRef] [Green Version]
- Gridelli, C.; Rossi, A.; Carbone, D.P.; Guarize, J.; Karachaliou, N.; Mok, T.; Petrella, F.; Spaggiari, L.; Rosell, R. Non-small-cell lung cancer. Nat. Rev. Dis. Primers 2015, 1, 15009. [Google Scholar] [CrossRef]
- Iyer, S.; Roughley, A.; Rider, A.; Taylor-Stokes, G. The symptom burden of non-small cell lung cancer in the USA: A real-world cross-sectional study. Supportive Care Cancer 2014, 22, 181–187. [Google Scholar] [CrossRef]
- Park, K.M.; Kim, D.W.; Lee, S.H. Extract of Allium tuberosum Rottler ex Spreng promoted the hair growth through regulating the expression of IGF-1. Evid. Based Complementary Altern. Med. 2015, 2015, 413538. [Google Scholar] [CrossRef]
- Jannat, K.; Rahman, T.; Rahmatullah, M. Traditional uses, phytochemicals and pharmacological properties of Allium tuberosum Rottler ex spreng. J. Med. Plants Stud. 2019, 7, 214–220. [Google Scholar]
- Havey, M. Onion and other cultivated alliums. Evol. Crop Plants 1995, 344–350. [Google Scholar]
- Fattorusso, E.; Lanzotti, V.; Taglialatela-Scafati, O.; Cicala, C. The flavonoids of leek, Allium porrum. Phytochemistry 2001, 57, 565–569. [Google Scholar] [CrossRef]
- Athokpam, R.; Bawari, M.; Choudhury, M. A review on medicinal plants of Manipur with special reference to hepatoprotection. Int. J. Adv. Pharm. Res. 2014, 5, 182–191. [Google Scholar]
- Tang, X.; Olatunji, O.J.; Zhou, Y.; Hou, X. Allium tuberosum: Antidiabetic and hepatoprotective activities. Food Res. Int. 2017, 102, 681–689. [Google Scholar] [CrossRef]
- Park, K.-W.; Kim, S.-Y.; Jeong, I.-Y.; Byun, M.-W.; Park, K.-H.; Yamada, K.; Seo, K.-I. Cytotoxic and antitumor activities of thiosulfinates from Allium tuberosum L. J. Agric. Food Chem. 2007, 55, 7957–7961. [Google Scholar] [CrossRef]
- Roviello, G.; D’Angelo, A.; Sirico, M.; Pittacolo, M.; Conter, F.U.; Sobhani, N. Advances in anti-BRAF therapies for lung cancer. Investig. New Drugs 2021, 39, 879–890. [Google Scholar] [CrossRef]
- Brose, M.S.; Volpe, P.; Feldman, M.; Kumar, M.; Rishi, I.; Gerrero, R.; Einhorn, E.; Herlyn, M.; Minna, J.; Nicholson, A. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res. 2002, 62, 6997–7000. [Google Scholar]
- Brustugun, O.T.; Khattak, A.M.; Trømborg, A.K.; Beigi, M.; Beiske, K.; Lund-Iversen, M.; Helland, Å. BRAF-mutations in non-small cell lung cancer. Lung Cancer 2014, 84, 36–38. [Google Scholar] [CrossRef]
- Paez, J.G.; Janne, P.A.; Lee, J.C.; Tracy, S.; Greulich, H.; Gabriel, S.; Herman, P.; Kaye, F.J.; Lindeman, N.; Boggon, T.J. EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004, 304, 1497–1500. [Google Scholar] [CrossRef] [Green Version]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48. [Google Scholar] [PubMed]
- Riely, G.J.; Marks, J.; Pao, W. KRAS mutations in non–small cell lung cancer. Proc. Am. Thorac. Soc. 2009, 6, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Ghimessy, A.; Radeczky, P.; Laszlo, V.; Hegedus, B.; Renyi-Vamos, F.; Fillinger, J.; Klepetko, W.; Lang, C.; Dome, B.; Megyesfalvi, Z. Current therapy of KRAS-mutant lung cancer. Cancer Metastasis Rev. 2020, 39, 1159–1177. [Google Scholar] [CrossRef] [PubMed]
- Skoulidis, F.; Li, B.T.; Dy, G.K.; Price, T.J.; Falchook, G.S.; Wolf, J.; Italiano, A.; Schuler, M.; Borghaei, H.; Barlesi, F. Sotorasib for lung cancers with KRAS p. G12C mutation. N. Engl. J. Med. 2021, 384, 2371–2381. [Google Scholar] [CrossRef]
- Downward, J. Targeting RAS and PI3K in lung cancer. Nat. Med. 2008, 14, 1315–1316. [Google Scholar] [CrossRef]
- Pérez-Ramírez, C.; Cañadas-Garre, M.; Molina, M.Á.; Faus-Dáder, M.J.; Calleja-Hernández, M.Á. PTEN and PI3K/AKT in non-small-cell lung cancer. Pharmacogenomics 2015, 16, 1843–1862. [Google Scholar] [CrossRef]
- Berg, M.; Soreide, K. EGFR and downstream genetic alterations in KRAS/BRAF and PI3K/AKT pathways in colorectal cancer—implications for targeted therapy. Discov. Med. 2012, 14, 207–214. [Google Scholar]
- Phua, L.C.; Ng, H.W.; Yeo, A.H.L.; Chen, E.; Lo, M.S.M.; Cheah, P.Y.; Chan, E.C.Y.; Koh, P.K.; Ho, H.K. Prevalence of KRAS, BRAF, PI3K and EGFR mutations among Asian patients with metastatic colorectal cancer. Oncol. Lett. 2015, 10, 2519–2526. [Google Scholar] [CrossRef] [Green Version]
- Trusheva, B.; Trunkova, D.; Bankova, V. Different extraction methods of biologically active components from propolis: A preliminary study. Chem. Cent. J. 2007, 1, 13. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Arvouet-Grand, A.; Vennat, B.; Pourrat, A.; Legret, P. Standardization of propolis extract and identification of principal constituents. J. De Pharm. De Belg. 1994, 49, 462–468. [Google Scholar]
- Kumarasamy, Y.; Byres, M.; Cox, P.J.; Jaspars, M.; Nahar, L.; Sarker, S.D. Screening seeds of some Scottish plants for free radical scavenging activity. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Suda, K.; Onozato, R.; Yatabe, Y.; Mitsudomi, T. EGFR T790M mutation: A double role in lung cancer cell survival? J. Thorac. Oncol. 2009, 4, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, M.-F.; Chang, T.-H.; Wu, S.-G.; Yang, H.-Y.; Hsu, Y.-C.; Yang, P.-C.; Shih, J.-Y. EGFR-L858R mutant enhances lung adenocarcinoma cell invasive ability and promotes malignant pleural effusion formation through activation of the CXCL12-CXCR4 pathway. Sci. Rep. 2015, 5, 13574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggirala, K.B.; Lee, Y.; Lee, K. Chronicles of EGFR Tyrosine Kinase Inhibitors: Targeting EGFR C797S Containing Triple Mutations. Biomol. Ther. 2022, 30, 19. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, J.G.B.; Otterson, G.A. Agents to treat BRAF-mutant lung cancer. Drugs Context 2019, 8, 212566. [Google Scholar] [CrossRef]
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716. [Google Scholar] [CrossRef]
- Scheffler, M.; Bos, M.; Gardizi, M.; König, K.; Michels, S.; Fassunke, J.; Heydt, C.; Künstlinger, H.; Ihle, M.; Ueckeroth, F.; et al. PIK3CA mutations in non-small cell lung cancer (NSCLC): Genetic heterogeneity, prognostic impact and incidence of prior malignancies. Oncotarget 2015, 6, 1315–1326. [Google Scholar] [CrossRef] [Green Version]
- Begum, S.F.M.; Priya, S.; Sundararajan, R.; Hemalatha, S. Novel anticancerous compounds from Sargassum wightii: In silico and in vitro approaches to test the antiproliferative efficacy. J. Adv. Pharm. Educ. Res. 2017, 7, 1–6. [Google Scholar]
- Bitencourt-Ferreira, G.; Azevedo, W.F.d. Molegro virtual docker for docking. In Docking Screens for Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2019; pp. 149–167. [Google Scholar]
- Wei, J.; Yan, Y.; Chen, X.; Qian, L.; Zeng, S.; Li, Z.; Dai, S.; Gong, Z.; Xu, Z. The roles of plant-derived triptolide on non-small cell lung cancer. Oncol. Res. 2019, 27, 849. [Google Scholar] [CrossRef]
- Rajasekar, N.; Sivanantham, A.; Ravikumar, V.; Rajasekaran, S. An overview on the role of plant-derived tannins for the treatment of lung cancer. Phytochemistry 2021, 188, 112799. [Google Scholar] [CrossRef] [PubMed]
- Kummalue, T. Molecular mechanism of herbs in human lung cancer cells. J. Med. Assoc. Thail. 2005, 88, 1725. [Google Scholar]
- Ouyang, L.; Luo, Y.; Tian, M.; Zhang, S.Y.; Lu, R.; Wang, J.H.; Kasimu, R.; Li, X. Plant natural products: From traditional compounds to new emerging drugs in cancer therapy. Cell Prolif. 2014, 47, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Ahmed, I.; Latif, M.S.Z.; Rafique, T.; Fawad, S.A. Comparison of antimicrobial activity, phytochemical profile and minerals composition of garlic Allium sativum and Allium tuberosum. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 311–317. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, Y.; Yang, J.; Pu, X.; Du, J.; Yang, X.; Yang, T.; Yang, S. Therapeutic role of functional components in alliums for preventive chronic disease in human being. Evid. Based Complementary Altern. Med. 2017, 2017, 9402849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsim, S.; O’dowd, C.; Milroy, R.; Davidson, S. Staging of non-small cell lung cancer (NSCLC): A review. Respir. Med. 2010, 104, 1767–1774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Li, X.-B.; Sun, J.; Xia, E.-D.; Tang, F.; Cao, H.-Q.; Xun, H. Isolation and identification of new chemical constituents from Chinese chive (Allium tuberosum) and toxicological evaluation of raw and cooked Chinese chive. Food Chem. Toxicol. 2018, 112, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, T.-T.; Hu, P.; Yang, J.; Wang, S.-Y. Purification and characterization of an antioxidant peptide (GSQ) from Chinese leek (Allium tuberosum Rottler) seeds. J. Funct. Foods 2014, 10, 144–153. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, J.-Y.; Lee, J.-H.; Young, H.-S.; Lee, T.-W. Isolation of adenosine and free amino acid composition from the leaves of Allium tuberosum. J. Korean Soc. Food Sci. Nutr. 1992, 21, 286–290. [Google Scholar]
- Park, Y.-J.; Kim, M.-H.; Bae, S.-J. Anticarcinogenic effects of Allium tuberosum on human cancer cells. Korean J. Food Sci. Technol. 2002, 34, 688–693. [Google Scholar]
- Yilmaz-Ozden, T.; Hasbal-Celikok, G.; Aksoy-Sagirli, P.; Altiparmak-Ulbegi, G.; Kocyigit, M.; Can, A.; Akev, N. Phenolic profile, antioxidant, antidiabetic, DNA protection, and cytotoxic activities of Allium kurtzianum. Int. Food Res. J. 2020, 27, 1001–1009. [Google Scholar]
- Oh, M.; Kim, S.-Y.; Park, S.; Kim, K.-N.; Kim, S.H. Phytochemicals in Chinese Chive (Allium tuberosum) Induce the Skeletal Muscle Cell Proliferation via PI3K/Akt/mTOR and Smad Pathways in C2C12 Cells. Int. J. Mol. Sci. 2021, 22, 2296. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tsumagari, H.; Okawa, M.; Nohara, T. Pregnane-and furostane-type oligoglycosides from the seeds of Allium tuberosum. Chem. Pharm. Bull. 2004, 52, 142–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K., II; Moon, Y.H.; Choi, S.U.; Park, K.H. Antibacterial activity of S-methyl methanethiosulfinate and S-methyl 2-propene-1-thiosulfinate from Chinese chive toward Escherichia coli O157: H7. Biosci. Biotechnol. Biochem. 2001, 65, 966–968. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, K.W.; Kim, J.Y.; Jeong, I.Y.; Byun, M.W.; Park, J.E.; Yee, S.T.; Kim, K.H.; Rhim, J.S.; Yamada, K.; et al. Thiosulfinates from Allium tuberosum L. induce apoptosis via caspase-dependent and -independent pathways in PC-3 human prostate cancer cells. Bioorg. Med. Chem. Lett. 2008, 18, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yang, H.S.; Park, K.W.; Kim, J.Y.; Lee, M.K.; Jeong, I.Y.; Shim, K.H.; Kim, Y.S.; Yamada, K.; Seo, K.I. Mechanisms of thiosulfinates from Allium tuberosum L.-induced apoptosis in HT-29 human colon cancer cells. Toxicol. Lett. 2009, 188, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tsumagari, H.; Nohara, T. Steroidal oligoglycosides from the seeds of Allium tuberosum. Chem. Pharm. Bull. 2000, 48, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.-C.; Liu, Z.-R.; Li, Y.-L.; Wang, Y.-A.; Kong, J.-W.; Ge, D.-Y.; Peng, G.-Y. Allium tuberosum alleviates pulmonary inflammation by inhibiting activation of innate lymphoid cells and modulating intestinal microbiota in asthmatic mice. J. Integr. Med. 2021, 19, 158–166. [Google Scholar] [CrossRef]
- Sutejo, I.R.; Ariesaka, K.M.; Prasetyo, F.A.; Taufiqurrqhman, M.; Insani, A.Y.; Ariansari, B.G. Immunostimulant Effect of Garlic Chives Leaf Ethanolic Extract (Allium tuberosum) by Increasing Level of Antioxidant at Rats Doxorubicin-Induced Rats. Indones. J. Cancer Chemoprev. 2016, 7, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Huang, L.-L.; Chen, J.-H.; Wu, J.; Xu, Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal Transduct. Target. Ther. 2019, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhou, Y.; Li, Y.; Zhou, J.; Wu, Y.; Cui, Y.; Yang, G.; Hong, Y. Mutations of p53 and KRAS activate NF-κB to promote chemoresistance and tumorigenesis via dysregulation of cell cycle and suppression of apoptosis in lung cancer cells. Cancer Lett. 2015, 357, 520–526. [Google Scholar] [CrossRef]
- Park, S.J.; More, S.; Murtuza, A.; Woodward, B.D.; Husain, H. New targets in non–small cell lung cancer. Hematol. Oncol. Clin. 2017, 31, 113–129. [Google Scholar] [CrossRef]
- Trabalzini, L.; Ercoli, J.; Trezza, A.; Schiavo, I.; Macrì, G.; Moglia, A.; Spiga, O.; Finetti, F. Pharmacological and In Silico Analysis of Oat Avenanthramides as EGFR Inhibitors: Effects on EGF-Induced Lung Cancer Cell Growth and Migration. Int. J. Mol. Sci. 2022, 23, 8534. [Google Scholar] [CrossRef]
- Kiriwan, D.; Seetaha, S.; Jiwacharoenchai, N.; Tabtimmai, L.; Sousa, S.F.; Songtawee, N.; Choowongkomon, K. Identification of tripeptides against tyrosine kinase domain of EGFR for lung cancer cell inhibition by in silico and in vitro studies. Chem. Biol. Drug Des. 2022, 99, 456–469. [Google Scholar] [CrossRef]
- Seto, K.; Shimizu, J.; Masago, K.; Araki, M.; Katayama, R.; Sagae, Y.; Fujita, S.; Horio, Y.; Sasaki, E.; Kuroda, H. Sensitivity to dabrafenib and trametinib treatments in patients with non-small-cell cancer harboring BRAF compound mutations: A pooled analysis of BRAF p. V600E-positive advanced non-small-cell lung cancer. Cancer Genet. 2022, 266–267, 1–6. [Google Scholar] [CrossRef]
- Fatima, A.; Yee, H.F. In silico screening of mutated K-ras inhibitors from Malaysian Typhonium flagelliforme for non-small cell lung cancer. Adv. Bioinform. 2014, 2014, 431696. [Google Scholar] [CrossRef]
- Wang, H.; Lv, Q.; Xu, Y.; Cai, Z.; Zheng, J.; Cheng, X.; Dai, Y.; Jänne, P.A.; Ambrogio, C.; Köhler, J. An integrative pharmacogenomics analysis identifies therapeutic targets in KRAS-mutant lung cancer. EBioMedicine 2019, 49, 106–117. [Google Scholar] [CrossRef]
- Shukuya, T.; Yamada, T.; Koenig, M.J.; Xu, J.; Okimoto, T.; Li, F.; Amann, J.M.; Carbone, D.P. The effect of LKB1 activity on the sensitivity to PI3K/mTOR inhibition in non–small cell lung cancer. J. Thorac. Oncol. 2019, 14, 1061–1076. [Google Scholar] [CrossRef]
- Paul, S.B.; Choudhury, S. Computational analysis of the activity of pongachalcone I against highly resistant bacteria Pseudomonas putida. Bioinformation 2010, 4, 473. [Google Scholar] [CrossRef] [Green Version]
- Laskar, Y.B.; Mazumder, P.B.; Talukdar, A.D. Hibiscus sabdariffa anthocyanins are potential modulators of estrogen receptor alpha activity with favourable toxicology: A computational analysis using molecular docking, ADME/Tox prediction, 2D/3D QSAR and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2021, 1–23. [Google Scholar] [CrossRef]
- Mahaffey, C.M.; Davies, A.M.; Lara, J.P.N.; Pryde, B.; Holland, W.; Mack, P.C.; Gumerlock, P.H.; Gandara, D.R. Schedule-dependent apoptosis in K-ras mutant non–small-cell lung cancer cell lines treated with docetaxel and erlotinib: Rationale for pharmacodynamic separation. Clin. Lung Cancer 2007, 8, 548–553. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Q.; Peng, X.; Zhou, C.; Zhong, Y.; Chen, X.; Qiu, Y.; Jin, M.; Gong, M.; Kong, D. Stellettin B induces G1 arrest, apoptosis and autophagy in human non-small cell lung cancer A549 cells via blocking PI3K/Akt/mTOR pathway. Sci. Rep. 2016, 6, 27071. [Google Scholar] [CrossRef] [Green Version]
- Pal, H.C.; Sharma, S.; Strickland, L.R.; Agarwal, J.; Athar, M.; Elmets, C.A.; Afaq, F. Delphinidin reduces cell proliferation and induces apoptosis of non-small-cell lung cancer cells by targeting EGFR/VEGFR2 signaling pathways. PLoS ONE 2013, 8, e77270. [Google Scholar] [CrossRef] [Green Version]
- Luk, P.P.; Yu, B.; Ng, C.C.; Mercorella, B.; Selinger, C.; Lum, T.; Kao, S.; O’Toole, S.A.; Cooper, W.A. BRAF mutations in non-small cell lung cancer. Transl. Lung Cancer Res. 2015, 4, 142. [Google Scholar]
- Singh, J.; Meena, A. Chemopreventive potential of linalool in targeting lung cancer biomarkers: An in silico & in vitro investigation. Endocr. Metab. Immune Disord. Drug Targets 2021, 22, 1416–1424. [Google Scholar] [CrossRef]
- Balaji, S.; Chempakam, B. Toxicity prediction of compounds from turmeric (Curcuma longa L). Food Chem. Toxicol. 2010, 48, 2951–2959. [Google Scholar] [CrossRef]
- Tan, W.; Mei, H.; Chao, L.; Liu, T.; Pan, X.; Shu, M.; Yang, L. Combined QSAR and molecule docking studies on predicting P-glycoprotein inhibitors. J. Comput.-Aided Mol. Des. 2013, 27, 1067–1073. [Google Scholar] [CrossRef]
- Dahlgren, D.; Lennernäs, H. Intestinal permeability and drug absorption: Predictive experimental, computational and in vivo approaches. Pharmaceutics 2019, 11, 411. [Google Scholar] [CrossRef]




| Time | Solvent A | Solvent B | Flow Rate | Pressure |
|---|---|---|---|---|
| 1 Min | 95% | 5% | 0.3 mL/min | 1200 bar |
| 20 Min | 0% | 100% | 0.3 mL/min | 1200 bar |
| 25 Min | 0% | 100% | 0.3 mL/min | 1200 bar |
| 26 Min | 95% | 5% | 0.3 mL/min | 1200 bar |
| 30 Min | 95% | 5% | 0.3 mL/min | 1200 bar |
| Sl No. | Receptor | PDB Id | Mutation | Resolution | R-Free | Experimental Methods |
|---|---|---|---|---|---|---|
| 1 | B-Raf | 4R5Y | V600E | 3.50 Å | 0.306 | XRD |
| 2 | EGFR | 6LUB | L858R/T790M/C797S | 2.31 Å | 0.226 | XRD |
| 3 | K-Ras | 8DNI | G12C | 1.50 Å | 0.254 | XRD |
| 4 | PI3K | 3APC | - | 2.54 Å | 0.290 | XRD |
| Compound Name | Docking Score (MolDock Score) | |||
|---|---|---|---|---|
| EGFR | K-Ras | PI3K | B-Raf | |
| Gefitinib (Positive Control of EGFR) | −103.14 | - | - | - |
| Adagrasib (Positive Control of K-Ras) | - | −136.77 | - | - |
| Duvelisib (Positive Control of PI3K) | - | - | −113.52 | - |
| Dabrafenib (Positive Control of B-Raf) | - | - | - | −138.89 |
| Americine | −112.82 | −139.02 | −115.76 | −144.45 * |
| 9-Hydroxy-9,11,15-octadecatrienoic acid (9-HOTE) | −121.96 | −144.61 | −123.48 | −140.27 |
| Di-n-octyl phthalate | −110.60 | −154.00 | −133.50 * | |
| 8-hydroxyoctadeca-9,12-dienoic acid (8S-HODE) | −111.91 | −140.70 * | −127.40 | |
| 9-hydroperoxyoctadeca-10,12,15-trienoic acid [9(S)-HpOTrE] | −123.38 | −142.13 | −126.70 | |
| ɑ-Linolenic Acid | −118.29 | −142.05 | −122.03 | |
| Fumaricaciddi(2-decyl)ester | −117.66 | −167.84 | −127.15 | |
| 3-ketostearic acid | −115.01 | −143.54 | −123.17 | |
| 1,2-Benzenedicarboxylicacidbutyloctylester | −103.81 | Low Score | −114.19 | |
| N,N′-Pentamethylenebis[s-3-aminopropyl thiosulfuric acid | −110.92 | −150.51 | ||
| Ethyl2E,4Z-hexadecadienoate | −124.85 * | −144.21 | ||
| 9,12-Octadecadien-1-Ol | −110.65 | −142.31 | ||
| Eicosanoicacid, Methyl ester | −106.81 | −141.91 | ||
| Petroselinicacid | −115.01 | −138.96 | ||
| 10,16-dihydroxy-palmitic acid | −120.06 | −137.31 | ||
| Nonadecane,9-methyl | −114.58 | |||
| Methaphenilene | −109.04 | |||
| 10,11-Epoxy-3,7,11-trimethyl-2E,6E-tridecadienoic acid | −105.83 | |||
| Glycerol1,2-diacetate | −105.46 | |||
| Leucyl-glutamate | −104.83 | |||
| 1,2-Benzenedicarboxylicacid,butyloctylester | 3-Ketostearic Acid | 9-Hydroperoxy-10,12,15-octadecatrienoic Acid | 9-Hydroxy-9,11,15-octadecatrienoic Acid | (9Z,12Z)-(8S)-Hydroxyoctadeca-9,12-dienoic Acid | ɑ-Linolenic Acid | Americine | Di-n-octyl phthalate | Fumaricacid,di(2-decyl)ester | |
|---|---|---|---|---|---|---|---|---|---|
| Pharmacokinetics | |||||||||
| GI absorption | High | High | High | High | High | High | High | High | Low |
| BBB permeant | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| P-GP substrate | No | No | No | No | No | No | Yes | No | Yes |
| Drug-likeness | |||||||||
| Lipinski (Pfizer) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Ghose (Amgen) | Yes | Yes | Yes | Yes | Yes | Yes | No | No | No |
| Veber (GSK) | No | No | No | No | No | No | Yes | No | No |
| Egan (Pharmacia) | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Muegge (Bayer) | No | No | Yes | No | No | No | Yes | No | No |
| Bioavailability Score | 0.55 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.55 | 0.55 | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, R.; Singha, S.; Nath, D.; Das, G.; Patra, J.K.; Talukdar, A.D. Phytochemicals from Allium tuberosum Rottler ex Spreng Show Potent Inhibitory Activity against B-Raf, EGFR, K-Ras, and PI3K of Non-Small Cell Lung Cancer Targets. Appl. Sci. 2022, 12, 11749. https://doi.org/10.3390/app122211749
Nath R, Singha S, Nath D, Das G, Patra JK, Talukdar AD. Phytochemicals from Allium tuberosum Rottler ex Spreng Show Potent Inhibitory Activity against B-Raf, EGFR, K-Ras, and PI3K of Non-Small Cell Lung Cancer Targets. Applied Sciences. 2022; 12(22):11749. https://doi.org/10.3390/app122211749
Chicago/Turabian StyleNath, Rajat, Shreeta Singha, Deepa Nath, Gitishree Das, Jayanta Kumar Patra, and Anupam Das Talukdar. 2022. "Phytochemicals from Allium tuberosum Rottler ex Spreng Show Potent Inhibitory Activity against B-Raf, EGFR, K-Ras, and PI3K of Non-Small Cell Lung Cancer Targets" Applied Sciences 12, no. 22: 11749. https://doi.org/10.3390/app122211749






