Clinical Applications of Low-Intensity Pulsed Ultrasound and Its Underlying Mechanisms in Dentistry
Abstract
1. Introduction
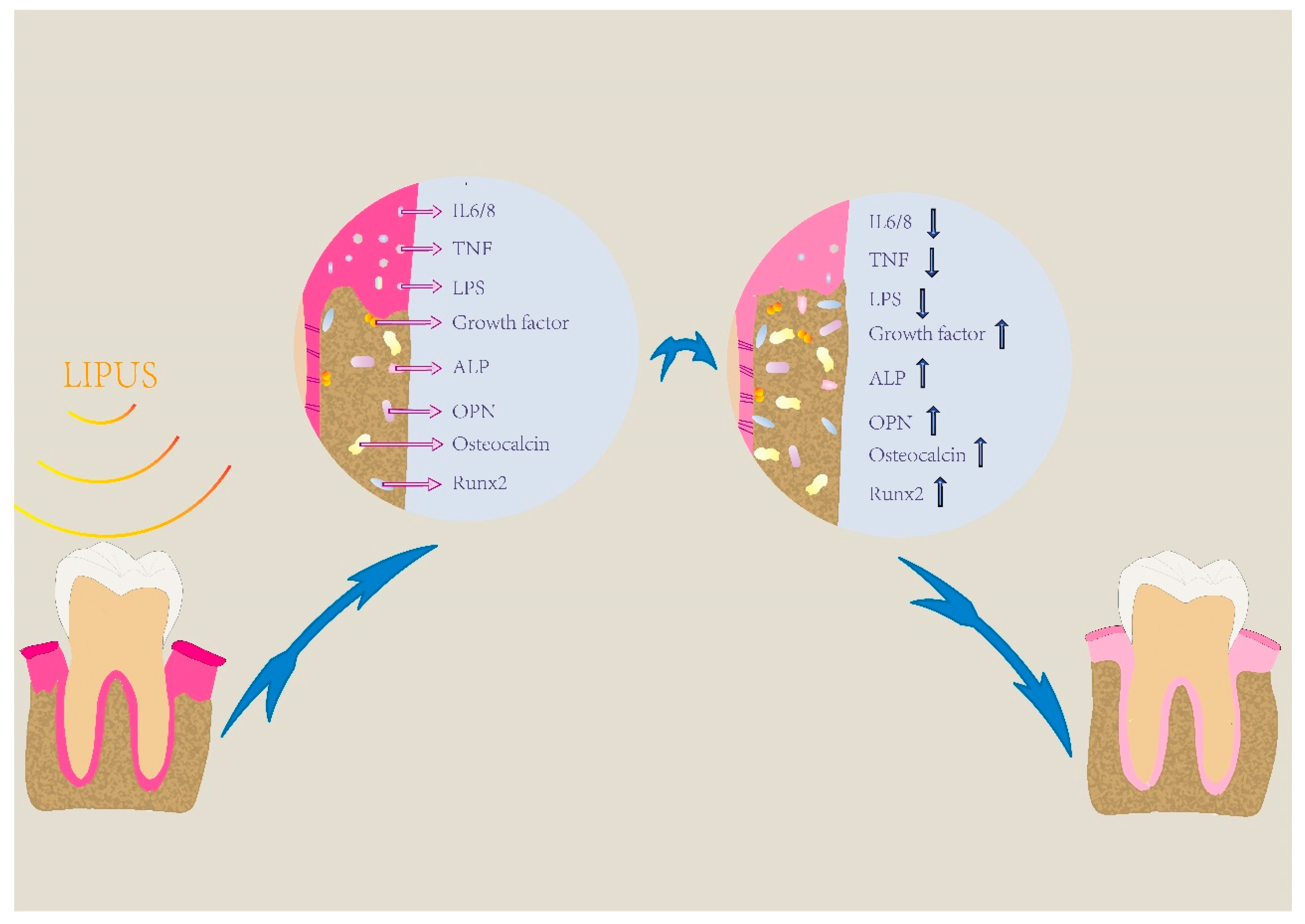
2. LIPUS Promotes Periodontal Regeneration via Increasing Osteogenic Differentiation and Inhibiting the Inflammatory Response
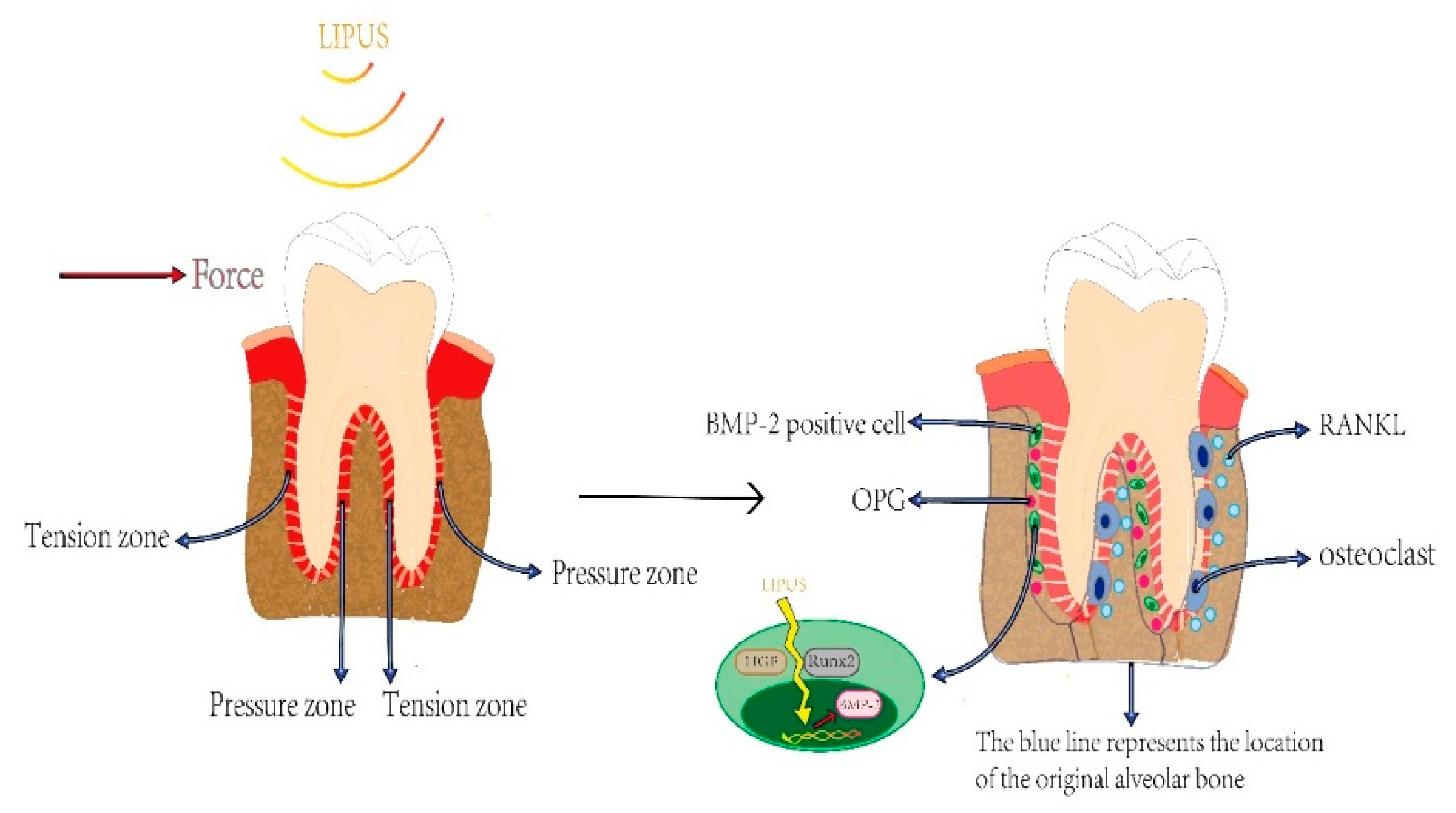
3. LIPUS Plays a Significant Role in Orthodontic Treatment via Accelerating OTM and Alleviating OITRR
4. LIPUS Contributes to the Stability of Implants via Facilitating Osseointegration
5. LIPUS Makes an Effect on TMJ-Involved Diseases via Expediting Bone Formation and Defending Chondrocytes
6. LIPUS Repairs the Dentin-Pulp Complex via Stimulating Tertiary Dentin Formation
7. Conclusions and Prospect
Author Contributions
Funding
Conflicts of Interest
References
- Berber, R.; Aziz, S.; Simkins, J.; Lin, S.S.; Mangwani, J. Low Intensity Pulsed Ultrasound Therapy (LIPUS): A review of evidence and potential applications in diabetics. J. Clin. Orthop. Trauma 2020, 11, S500–S505. [Google Scholar] [CrossRef]
- Zhang, W.; Abdelrasoul, G.N.; Savchenko, O.; Abdrabou, A.; Wang, Z.; Chen, J. Ultrasound-assisted magnetic nanoparticle-based gene delivery. PLoS ONE 2020, 15, e0239633. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.; Wu, S.; Dong, Y.; Chen, X.; Wang, S.; Li, X.; Zou, C. Review on experimental study and clinical application of low-intensity pulsed ultrasound in inflammation. Quant. Imaging Med. Surg. 2021, 11, 443–462. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Y.; Guo, S.; He, Y.; Bai, G.; Zhang, W. Low-intensity pulsed ultrasound stimulation facilitates in vitro osteogenic differentiation of human adipose-derived stem cells via up-regulation of heat shock protein (HSP)70, HSP90, and bone morphogenetic protein (BMP) signaling pathway. Biosci. Rep. 2018, 38, BSR20180087. [Google Scholar] [CrossRef]
- Harrison, A.; Lin, S.; Pounder, N.; Mikuni-Takagaki, Y. Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair. Ultrasonics 2016, 70, 45–52. [Google Scholar] [CrossRef]
- McCarthy, C.; Camci-Unal, G. Low Intensity Pulsed Ultrasound for Bone Tissue Engineering. Micromachines 2021, 12, 1488. [Google Scholar] [CrossRef]
- Rego, E.B.; Takata, T.; Tanne, K.; Tanaka, E. Current status of low intensity pulsed ultrasound for dental purposes. Open Dent. J. 2012, 6, 220–225. [Google Scholar] [CrossRef]
- Tanaka, E.; Kuroda, S.; Horiuchi, S.; Tabata, A.; El-Bialy, T. Low-intensity pulsed ultrasound in dentofacial tissue engineering. Ann. Biomed. Eng. 2015, 43, 871–886. [Google Scholar] [CrossRef]
- Atherton, P.; Lausecker, F.; Harrison, A.; Ballestrem, C. Low-intensity pulsed ultrasound promotes cell motility through vinculin-controlled Rac1 GTPase activity. J. Cell Sci. 2017, 130, 2277–2291. [Google Scholar] [CrossRef]
- Kusuyama, J.; Nakamura, T.; Ohnishi, T.; Albertson, B.G.; Ebe, Y.; Eiraku, N.; Noguchi, K.; Matsuguchi, T. Low-intensity pulsed ultrasound promotes bone morphogenic protein 9-induced osteogenesis and suppresses inhibitory effects of inflammatory cytokines on cellular responses via Rho-associated kinase 1 in human periodontal ligament fibroblasts. J. Cell. Biochem. 2019, 120, 14657–14669. [Google Scholar] [CrossRef]
- Harrison, A.; Alt, V. Low-intensity pulsed ultrasound (LIPUS) for stimulation of bone healing—A narrative review. Injury 2021, 52 (Suppl. S2), S91–S96. [Google Scholar] [CrossRef]
- Kang, P.L.; Huang, H.H.; Chen, T.; Ju, K.C.; Kuo, S.M. Angiogenesis-promoting effect of LIPUS on hADSCs and HUVECs cultured on collagen/hyaluronan scaffolds. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 102, 22–33. [Google Scholar] [CrossRef]
- Alhazmi, K.S.; El-Bialy, T.; Afify, A.R.; Merdad, L.A.; Hassan, A.H. Ultrasound Enhances Dentoalveolar Remodeling in an Ex Vivo Orthodontic, Ovariectomy-Induced Osteoporotic Model. Ultrasound Med. Biol. 2017, 43, 1963–1974. [Google Scholar] [CrossRef]
- Li, H.; Zhou, J.; Zhu, M.; Ying, S.; Li, L.; Chen, D.; Li, J.; Song, J. Low-intensity pulsed ultrasound promotes the formation of periodontal ligament stem cell sheets and ectopic periodontal tissue regeneration. J. Biomed. Mater. Res. Part A 2021, 109, 1101–1112. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Y.; Li, J.; Zhao, C.; Song, J. Low-intensity pulsed ultrasound promotes alveolar bone regeneration in a periodontal injury model. Ultrasonics 2018, 90, 166–172. [Google Scholar] [CrossRef]
- Kwon, T.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Li, H.; Deng, Y.; Tan, M.; Feng, G.; Kuang, Y.; Li, J.; Song, J. Low-intensity pulsed ultrasound upregulates osteogenesis under inflammatory conditions in periodontal ligament stem cells through unfolded protein response. Stem Cell Res. Ther. 2020, 11, 215. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, M.; Li, J.; Hu, B.; Jiang, D.; Huang, H.; Song, J. LIPUS inhibited the expression of inflammatory factors and promoted the osteogenic differentiation capacity of hPDLCs by inhibiting the NF-κB signaling pathway. J. Periodontal Res. 2020, 55, 125–140. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Zhou, J.; Qiu, Y.; Song, J. Low-intensity pulsed ultrasound enhances bone marrow-derived stem cells-based periodontal regenerative therapies. Ultrasonics 2022, 121, 106678. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015, 972313. [Google Scholar] [CrossRef]
- Basu, A.; Rothermund, K.; Ahmed, M.N.; Syed-Picard, F.N. Self-Assembly of an Organized Cementum-Periodontal Ligament-Like Complex Using Scaffold-Free Tissue Engineering. Front. Physiol. 2019, 10, 422. [Google Scholar] [CrossRef]
- Zein, N.; Harmouch, E.; Lutz, J.C.; Fernandez De Grado, G.; Kuchler-Bopp, S.; Clauss, F.; Offner, D.; Hua, G.; Benkirane-Jessel, N.; Fioretti, F. Polymer-Based Instructive Scaffolds for Endodontic Regeneration. Materials 2019, 12, 2347. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, B.C.; Tsuda, Y.; Williams, C.; Shimizu, T.; Yamato, M.; Okano, T.; Wong, J.Y. A thermoresponsive, microtextured substrate for cell sheet engineering with defined structural organization. Biomaterials 2008, 29, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.M.; Sixt, M. Mechanisms of 3D cell migration. Nat. Rev. Mol. Cell Biol. 2019, 20, 738–752. [Google Scholar] [CrossRef]
- Xu, Q.; Li, B.; Yuan, L.; Dong, Z.; Zhang, H.; Wang, H.; Sun, J.; Ge, S.; Jin, Y. Combination of platelet-rich plasma within periodontal ligament stem cell sheets enhances cell differentiation and matrix production. J. Tissue Eng. Regen. Med. 2017, 11, 627–636. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Q.; Quan, C.; Ren, Q.; He, W.; Xiao, H. Low-intensity pulsed ultrasound enhances immunomodulation and facilitates osteogenesis of human periodontal ligament stem cells by inhibiting the NF-κB pathway. Cell Tissue Bank. 2022, 1–14. [Google Scholar] [CrossRef]
- Kumagai, K.; Takeuchi, R.; Ishikawa, H.; Yamaguchi, Y.; Fujisawa, T.; Kuniya, T.; Takagawa, S.; Muschler, G.F.; Saito, T. Low-intensity pulsed ultrasound accelerates fracture healing by stimulation of recruitment of both local and circulating osteogenic progenitors. J. Orthop. Res. 2012, 30, 1516–1521. [Google Scholar] [CrossRef]
- Wei, F.Y.; Leung, K.S.; Li, G.; Qin, J.; Chow, S.K.; Huang, S.; Sun, M.H.; Qin, L.; Cheung, W.H. Low intensity pulsed ultrasound enhanced mesenchymal stem cell recruitment through stromal derived factor-1 signaling in fracture healing. PLoS ONE 2014, 9, e106722. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Qiu, Y.; Hu, B.; Chen, J.; Fu, T.; Zhou, P.; Song, J. Low-intensity pulsed ultrasound promotes periodontal ligament stem cell migration through TWIST1-mediated SDF-1 expression. Int. J. Mol. Med. 2018, 42, 322–330. [Google Scholar] [CrossRef]
- Nagata, M.; Iwasaki, K.; Akazawa, K.; Komaki, M.; Yokoyama, N.; Izumi, Y.; Morita, I. Conditioned Medium from Periodontal Ligament Stem Cells Enhances Periodontal Regeneration. Tissue Eng. Part A 2017, 23, 367–377. [Google Scholar] [CrossRef]
- Chopra, H.; Han, Y.; Zhang, C.F.; Pow, E.H.N. PDLCs and EPCs Co-Cultured on Ta Discs: A Golden Fleece for “Compromised” Osseointegration. Int. J. Mol. Sci. 2021, 22, 4486. [Google Scholar] [CrossRef]
- Liu, F.; Wang, X.; Zheng, B.; Li, D.; Chen, C.; Lee, I.S.; Zhong, J.; Li, D.; Liu, Y. USF2 enhances the osteogenic differentiation of PDLCs by promoting ATF4 transcriptional activities. J. Periodontal Res. 2020, 55, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhang, Y.; Zhou, J.; Li, J.; Deng, F.; Wang, Z.; Song, J. Low-intensity pulsed ultrasound stimulation facilitates osteogenic differentiation of human periodontal ligament cells. PLoS ONE 2014, 9, e95168. [Google Scholar] [CrossRef]
- Ren, L.; Yang, Z.; Song, J.; Wang, Z.; Deng, F.; Li, W. Involvement of p38 MAPK pathway in low intensity pulsed ultrasound induced osteogenic differentiation of human periodontal ligament cells. Ultrasonics 2013, 53, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Yang, Z.; Ren, L.; Deng, F.; Wang, Z.; Song, J. Low-intensity pulsed ultrasound induces osteogenic differentiation of human periodontal ligament cells through activation of bone morphogenetic protein-smad signaling. J. Ultrasound Med. 2014, 33, 865–873. [Google Scholar] [CrossRef]
- Pitto, L.; Ripoli, A.; Cremisi, F.; Simili, M.; Rainaldi, G. microRNA(interference) networks are embedded in the gene regulatory networks. Cell Cycle 2008, 7, 2458–2461. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Amatya, V.J.; Kushitani, K.; Kai, Y.; Kambara, T.; Takeshima, Y. miR-182 and miR-183 Promote Cell Proliferation and Invasion by Targeting FOXO1 in Mesothelioma. Front. Oncol. 2018, 8, 446. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xiang, M.; Gong, Y.; Xu, L.; Zhang, T.; He, Y.; Zhou, M.; Xin, L.; Li, J.; Song, J. LIPUS promotes FOXO1 accumulation by downregulating miR-182 to enhance osteogenic differentiation in hPDLCs. Biochimie 2019, 165, 219–228. [Google Scholar] [CrossRef]
- Schröder, A.; Meyer, A.; Spanier, G.; Damanaki, A.; Paddenberg, E.; Proff, P.; Kirschneck, C. Impact of Leptin on Periodontal Ligament Fibroblasts during Mechanical Strain. Int. J. Mol. Sci. 2021, 22, 6847. [Google Scholar] [CrossRef]
- Sokos, D.; Everts, V.; de Vries, T.J. Role of periodontal ligament fibroblasts in osteoclastogenesis: A review. J. Periodontal Res. 2015, 50, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Shirakata, Y.; Shinohara, Y.; Miron, R.J.; Hasegawa-Nakamura, K.; Fujioka-Kobayashi, M.; Noguchi, K. Comparison of the effects of recombinant human bone morphogenetic protein-2 and -9 on bone formation in rat calvarial critical-size defects. Clin. Oral Investig. 2017, 21, 2671–2679. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.Z.; Uludağ, H.; Dederich, D.N.; Doschak, M.R.; El-Bialy, T.H. Anabolic effects of low-intensity pulsed ultrasound on human gingival fibroblasts. Arch. Oral Biol. 2009, 54, 743–748. [Google Scholar] [CrossRef]
- Francis, M.; Pandya, M.; Gopinathan, G.; Lyu, H.; Ma, W.; Foyle, D.; Nares, S.; Luan, X. Histone Methylation Mechanisms Modulate the Inflammatory Response of Periodontal Ligament Progenitors. Stem Cells Dev. 2019, 28, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388. [Google Scholar] [CrossRef]
- Liu, P.; Cui, L.; Shen, L. Knockdown of TRIM52 alleviates LPS-induced inflammatory injury in human periodontal ligament cells through the TLR4/NF-κB pathway. Biosci. Rep. 2020, 40, BSR20201223. [Google Scholar] [CrossRef]
- Mederos y Schnitzler, M.; Storch, U.; Gudermann, T. AT1 receptors as mechanosensors. Curr. Opin. Pharmacol. 2011, 11, 112–116. [Google Scholar] [CrossRef]
- Nagao, M.; Tanabe, N.; Manaka, S.; Naito, M.; Sekino, J.; Takayama, T.; Kawato, T.; Torigoe, G.; Kato, S.; Tsukune, N.; et al. LIPUS suppressed LPS-induced IL-1α through the inhibition of NF-κB nuclear translocation via AT1-PLCβ pathway in MC3T3-E1 cells. J. Cell. Physiol. 2017, 232, 3337–3346. [Google Scholar] [CrossRef]
- Batista, K.B.; Thiruvenkatachari, B.; Harrison, J.E.; O’Brien, K.D. Orthodontic treatment for prominent upper front teeth (Class II malocclusion) in children and adolescents. Cochrane Database Syst. Rev. 2018, 3, CD003452. [Google Scholar] [CrossRef]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Lynch, E. Systematic Review of Orthodontic Treatment Management with Photobiomodulation Therapy. Photobiomodul. Photomed. Laser Surg. 2019, 37, 862–868. [Google Scholar] [CrossRef]
- Erbe, C.; Hartmann, L.; Schmidtmann, I.; Ohlendorf, D.; Wehrbein, H. A novel method quantifying caries following orthodontic treatment. Sci. Rep. 2021, 11, 21347. [Google Scholar] [CrossRef]
- Lin, W.; Farella, M.; Antoun, J.S.; Topless, R.K.; Merriman, T.R.; Michelotti, A. Factors associated with orthodontic pain. J. Oral Rehabil. 2021, 48, 1135–1143. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Cordasco, G.; Perillo, L.; Ramaglia, L. Mechanobiology of the tooth movement during the orthodontic treatment: A literature review. Minerva Stomatol. 2016, 65, 299–327. [Google Scholar]
- Huang, H.; Williams, R.C.; Kyrkanides, S. Accelerated orthodontic tooth movement: Molecular mechanisms. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 620–632. [Google Scholar] [CrossRef]
- Kaur, H.; El-Bialy, T. Shortening of Overall Orthodontic Treatment Duration with Low-Intensity Pulsed Ultrasound (LIPUS). J. Clin. Med. 2020, 9, 1303. [Google Scholar] [CrossRef]
- El-Bialy, T.; Farouk, K.; Carlyle, T.D.; Wiltshire, W.; Drummond, R.; Dumore, T.; Knowlton, K.; Tompson, B. Effect of Low Intensity Pulsed Ultrasound (LIPUS) on Tooth Movement and Root Resorption: A Prospective Multi-Center Randomized Controlled Trial. J. Clin. Med. 2020, 9, 804. [Google Scholar] [CrossRef]
- Alazzawi, M.M.J.; Husein, A.; Alam, M.K.; Hassan, R.; Shaari, R.; Azlina, A.; Salzihan, M.S. Effect of low level laser and low intensity pulsed ultrasound therapy on bone remodeling during orthodontic tooth movement in rats. Prog. Orthod. 2018, 19, 10. [Google Scholar] [CrossRef]
- Dahhas, F.Y.; El-Bialy, T.; Afify, A.R.; Hassan, A.H. Effects of Low-Intensity Pulsed Ultrasound on Orthodontic Tooth Movement and Orthodontically Induced Inflammatory Root Resorption in Ovariectomized Osteoporotic Rats. Ultrasound Med. Biol. 2016, 42, 808–814. [Google Scholar] [CrossRef]
- Xue, H.; Zheng, J.; Cui, Z.; Bai, X.; Li, G.; Zhang, C.; He, S.; Li, W.; Lajud, S.A.; Duan, Y.; et al. Low-intensity pulsed ultrasound accelerates tooth movement via activation of the BMP-2 signaling pathway. PLoS ONE 2013, 8, e68926. [Google Scholar] [CrossRef]
- Hsu, L.F.; Tsai, M.H.; Shih, A.H.; Chen, Y.C.; Chang, B.E.; Chen, Y.J.; Yao, C.J. 970 nm low-level laser affects bone metabolism in orthodontic tooth movement. J. Photochem. Photobiol. B 2018, 186, 41–50. [Google Scholar] [CrossRef]
- Caccianiga, G.; Crestale, C.; Cozzani, M.; Piras, A.; Mutinelli, S.; Lo Giudice, A.; Cordasco, G. Low-level laser therapy and invisible removal aligners. J. Biol. Regul. Homeost. Agents 2016, 30, 107–113. [Google Scholar]
- Amaro, E.R.S.; Ortiz, F.R.; Dorneles, L.S.; Santos, M.S.; Barrioni, B.R.; Miranda, R.M.; Garlet, G.P.; Teixeira, M.M.; Szawka, R.E.; Silva, T.A.; et al. Estrogen protects dental roots from orthodontic-induced inflammatory resorption. Arch. Oral Biol. 2020, 117, 104820. [Google Scholar] [CrossRef]
- Lund, H.; Gröndahl, K.; Hansen, K.; Gröndahl, H.G. Apical root resorption during orthodontic treatment. A prospective study using cone beam CT. Angle Orthod. 2012, 82, 480–487. [Google Scholar] [CrossRef]
- Jiang, R.P.; McDonald, J.P.; Fu, M.K. Root resorption before and after orthodontic treatment: A clinical study of contributory factors. Eur. J. Orthod. 2010, 32, 693–697. [Google Scholar] [CrossRef]
- Brezniak, N.; Wasserstein, A. Orthodontically induced inflammatory root resorption. Part I: The basic science aspects. Angle Orthod. 2002, 72, 175–179. [Google Scholar] [CrossRef]
- Crossman, J.; Hassan, A.H.; Saleem, A.; Felemban, N.; Aldaghreer, S.; Fawzi, E.; Farid, M.; Abdel-Ghaffar, K.; Gargoum, A.; El-Bialy, T. Effect of gingival fibroblasts and ultrasound on dogs’ root resorption during orthodontic treatment. J. Orthod. Sci. 2017, 6, 28–35. [Google Scholar] [CrossRef]
- Al-Daghreer, S.; Doschak, M.; Sloan, A.J.; Major, P.W.; Heo, G.; Scurtescu, C.; Tsui, Y.Y.; El-Bialy, T. Effect of low-intensity pulsed ultrasound on orthodontically induced root resorption in beagle dogs. Ultrasound Med. Biol. 2014, 40, 1187–1196. [Google Scholar] [CrossRef]
- Gul Amuk, N.; Kurt, G.; Guray, E. Effects of Photobiomodulation and Ultrasound Applications on Orthodontically Induced Inflammatory Root Resorption; Transcriptional Alterations in OPG, RANKL, Cox-2: An Experimental Study in Rats. Photomed. Laser Surg. 2018, 36, 653–659. [Google Scholar] [CrossRef]
- Udagawa, N.; Koide, M.; Nakamura, M.; Nakamichi, Y.; Yamashita, T.; Uehara, S.; Kobayashi, Y.; Furuya, Y.; Yasuda, H.; Fukuda, C.; et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Miner. Metab. 2021, 39, 19–26. [Google Scholar] [CrossRef]
- Nishida, D.; Arai, A.; Zhao, L.; Yang, M.; Nakamichi, Y.; Horibe, K.; Hosoya, A.; Kobayashi, Y.; Udagawa, N.; Mizoguchi, T. RANKL/OPG ratio regulates odontoclastogenesis in damaged dental pulp. Sci. Rep. 2021, 11, 4575. [Google Scholar] [CrossRef]
- Adams, M.S.; Lotz, J.C.; Diederich, C.J. In silico feasibility assessment of extracorporeal delivery of low-intensity pulsed ultrasound to intervertebral discs within the lumbar spine. Phys. Med. Biol. 2020, 65, 215011. [Google Scholar] [CrossRef]
- Tehranchi, A.; Badiee, M.; Younessian, F.; Badiei, M.; Haddadpour, S. Effect of Low-intensity Pulsed Ultrasound on Postorthognathic Surgery Healing Process. Ann. Maxillofac. Surg. 2017, 7, 25–29. [Google Scholar] [CrossRef]
- Qamruddin, I.; Alam, M.K.; Mahroof, V.; Karim, M.; Fida, M.; Khamis, M.F.; Husein, A. Biostimulatory Effects of Low-Intensity Pulsed Ultrasound on Rate of Orthodontic Tooth Movement and Associated Pain, Applied at 3-Week Intervals: A Split-Mouth Study. Pain Res. Manag. 2021, 2021, 6624723. [Google Scholar] [CrossRef]
- Albrektsson, T. Hard tissue implant interface. Aust. Dent. J. 2008, 53 (Suppl. S1), S34–S38. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, X.; Liu, B.; Hu, K.; Zhou, X.; Ding, Y. The effect of low-intensity pulsed ultrasound on the osseointegration of titanium dental implants. Br. J. Oral Maxillofac. Surg. 2012, 50, 244–250. [Google Scholar] [CrossRef]
- Hériveaux, Y.; Audoin, B.; Biateau, C.; Nguyen, V.H.; Haïat, G. Ultrasonic Propagation in a Dental Implant. Ultrasound Med. Biol. 2020, 46, 1464–1473. [Google Scholar] [CrossRef]
- Dorogoy, A.; Haïat, G.; Shemtov-Yona, K.; Rittel, D. Modeling ultrasonic wave propagation in a dental implant—Bone system. J. Mech. Behav. Biomed. Mater. 2020, 103, 103547. [Google Scholar] [CrossRef]
- Iwanabe, Y.; Masaki, C.; Tamura, A.; Tsuka, S.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. The effect of low-intensity pulsed ultrasound on wound healing using scratch assay in epithelial cells. J. Prosthodont. Res. 2016, 60, 308–314. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, Y.; Zhu, Z.; Xiao, W.; Xu, Q.; Li, L.; Li, X.; Chen, W. Effects of Low-Intensity Pulsed Ultrasound on Implant Osseointegration in Ovariectomized Rats. J. Ultrasound. Med. 2016, 35, 747–754. [Google Scholar] [CrossRef]
- Shobara, K.; Ogawa, T.; Shibamoto, A.; Miyashita, M.; Ito, A.; Sitalaksmi, R.M. Osteogenic effect of low-intensity pulsed ultrasound and whole-body vibration on peri-implant bone. An experimental in vivo study. Clin. Oral Implant. Res. 2021, 32, 641–650. [Google Scholar] [CrossRef]
- Ustun, Y.; Erdogan, O.; Kurkcu, M.; Akova, T.; Damlar, I. Effects of low-intensity pulsed ultrasound on dental implant osseointegration: A preliminary report. Eur. J. Dent. 2008, 2, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Chauvel-Picard, J.; Gourmet, R.; Vercherin, P.; Béra, J.C.; Gleizal, A. Stimulation of dental implant osseointegration by low-Intensity pulsed ultrasound: An in vivo preliminary study in a porcine model. J. Prosthodont. Res. 2022, 66, 639–645. [Google Scholar] [CrossRef]
- Jiang, Y.; Yuan, Y.; Xiong, Y.; Wang, B.; Guo, Y.; Gong, P.; Zhang, L. Low-intensity pulsed ultrasound improves osseointegration of dental implant in mice by inducing local neuronal production of αCGRP. Arch. Oral Biol. 2020, 115, 104736. [Google Scholar] [CrossRef] [PubMed]
- Abdulhameed, E.A.; Al-Rawi, N.H.; Uthman, A.T.; Samsudin, A.R. Bone Texture Fractal Dimension Analysis of Ultrasound-Treated Bone around Implant Site: A Double-Blind Clinical Trial. Int. J. Dent. 2018, 2018, 2672659. [Google Scholar] [CrossRef]
- Magkavali-Trikka, P.; Emmanouilidis, G.; Papadopoulos, M.A. Mandibular molar uprighting using orthodontic miniscrew implants: A systematic review. Prog. Orthod. 2018, 19, 1. [Google Scholar] [CrossRef]
- Geshay, D.; Campbell, P.; Tadlock, L.; Schneiderman, E.; Kyung, H.M.; Buschang, P. Stability of immediately loaded 3 mm long miniscrew implants: A feasibility study. Dent. Press J. Orthod. 2021, 26, e2119155. [Google Scholar] [CrossRef]
- Ganzorig, K.; Kuroda, S.; Maeda, Y.; Mansjur, K.; Sato, M.; Nagata, K.; Tanaka, E. Low-intensity pulsed ultrasound enhances bone formation around miniscrew implants. Arch. Oral Biol. 2015, 60, 902–910. [Google Scholar] [CrossRef]
- Miura, K.; Motoyoshi, M.; Inaba, M.; Iwai, H.; Karasawa, Y.; Shimizu, N. A preliminary study of the effects of low-intensity pulsed ultrasound exposure on the stability of orthodontic miniscrews in growing rats. Eur. J. Orthod. 2014, 36, 419–424. [Google Scholar] [CrossRef]
- Acri, T.M.; Shin, K.; Seol, D.; Laird, N.Z.; Song, I.; Geary, S.M.; Chakka, J.L.; Martin, J.A.; Salem, A.K. Tissue Engineering for the Temporomandibular Joint. Adv. Healthc. Mater. 2019, 8, e1801236. [Google Scholar] [CrossRef]
- Jahr, H.; Gunes, S.; Kuhn, A.R.; Nebelung, S.; Pufe, T. Bioreactor-Controlled Physoxia Regulates TGF-β Signaling to Alter Extracellular Matrix Synthesis by Human Chondrocytes. Int. J. Mol. Sci. 2019, 20, 1715. [Google Scholar] [CrossRef]
- Poolman, R.W.; Agoritsas, T.; Siemieniuk, R.A.; Harris, I.A.; Schipper, I.B.; Mollon, B.; Smith, M.; Albin, A.; Nador, S.; Sasges, W.; et al. Low intensity pulsed ultrasound (LIPUS) for bone healing: A clinical practice guideline. Bmj 2017, 356, j576. [Google Scholar] [CrossRef]
- Rothenberg, J.B.; Jayaram, P.; Naqvi, U.; Gober, J.; Malanga, G.A. The Role of Low-Intensity Pulsed Ultrasound on Cartilage Healing in Knee Osteoarthritis: A Review. PM&R 2017, 9, 1268–1277. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—Looking beyond the ‘usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis-A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, J.N.; Gan, Y.H.; Zhou, Y.H. Current understanding of pathogenesis and treatment of TMJ osteoarthritis. J. Dent. Res. 2015, 94, 666–673. [Google Scholar] [CrossRef]
- Tanaka, E.; Liu, Y.; Xia, L.; Ogasawara, N.; Sakamaki, T.; Kano, F.; Hashimoto, N.; Feng, X.; Yamamoto, A. Effectiveness of low-intensity pulsed ultrasound on osteoarthritis of the temporomandibular joint: A review. Ann. Biomed. Eng. 2020, 48, 2158–2170. [Google Scholar] [CrossRef]
- Du, S.; Liang, C.; Sun, Y.; Ma, B.; Gao, W.; Geng, W. The Attenuating Effect of Low-Intensity Pulsed Ultrasound on Hypoxia-Induced Rat Chondrocyte Damage in TMJ Osteoarthritis Based on TMT Labeling Quantitative Proteomic Analysis. Front. Pharmacol. 2021, 12, 752734. [Google Scholar] [CrossRef]
- Yang, T.; Liang, C.; Chen, L.; Li, J.; Geng, W. Low-Intensity Pulsed Ultrasound Alleviates Hypoxia-Induced Chondrocyte Damage in Temporomandibular Disorders by Modulating the Hypoxia-Inducible Factor Pathway. Front. Pharmacol. 2020, 11, 689. [Google Scholar] [CrossRef]
- He, D.; Wang, J.; Li, Y.; Wu, G.; Zhu, G.; Chen, L. Low-intensity pulsed ultrasound promotes aggrecan expression via ZNT-9 in temporomandibular joint chondrocytes. Gene 2021, 768, 145318. [Google Scholar] [CrossRef]
- He, D.; An, Y.; Li, Y.; Wang, J.; Wu, G.; Chen, L.; Zhu, G. RNA sequencing reveals target genes of temporomandibular joint osteoarthritis in rats after the treatment of low-intensity pulsed ultrasound. Gene 2018, 672, 126–136. [Google Scholar] [CrossRef]
- Yi, X.; Liu, J.; Cheng, M.S.; Zhou, Q. Low-intensity pulsed ultrasound inhibits IL-6 in subchondral bone of temporomandibular joint osteoarthritis by suppressing the TGF-β1/Smad3 pathway. Arch. Oral Biol. 2021, 125, 105110. [Google Scholar] [CrossRef]
- Yi, W.; Chen, Q.; Liu, C.; Li, K.; Tao, B.; Tian, G.; Zhou, L.; Li, X.; Shen, J.; Liu, B.; et al. LIPUS inhibits inflammation and catabolism through the NF-κB pathway in human degenerative nucleus pulposus cells. J. Orthop. Surg. Res. 2021, 16, 619. [Google Scholar] [CrossRef] [PubMed]
- Loreto, C.; Filetti, V.; Almeida, L.E.; La Rosa, G.R.M.; Leonardi, R.; Grippaudo, C.; Lo Giudice, A. MMP-7 and MMP-9 are overexpressed in the synovial tissue from severe temporomandibular joint dysfunction. Eur. J. Histochem. 2020, 64, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Ruslin, M.; Hajrah Yusuf, A.S.; Forouzanfar, T.; Greebe, R.B.; Tuinzing, D.B.; Thamrin, S.A.; Boffano, P.; Lo, L.J. One-year stability of the mandibular advancement and counterclockwise rotation for correction of the skeletal class II malocclusion and high mandibular plane angle: Dental and skeletal aspect. Biomed. J. 2022, 45, 206–214. [Google Scholar] [CrossRef]
- Kramer, P.F.; Pereira, L.M.; Ilha, M.C.; Borges, T.S.; Freitas, M.P.M.; Feldens, C.A. Exploring the impact of malocclusion and dentofacial anomalies on the occurrence of traumatic dental injuries in adolescents. Angle Orthod. 2017, 87, 816–823. [Google Scholar] [CrossRef]
- Hadaegh, Y.; Uludag, H.; Dederich, D.; El-Bialy, T.H. The effect of low intensity pulsed ultrasound on mandibular condylar growth in young adult rats. Bone Rep. 2021, 15, 101122. [Google Scholar] [CrossRef]
- Kaur, H.; Uludağ, H.; El-Bialy, T. Effect of nonviral plasmid delivered basic fibroblast growth factor and low intensity pulsed ultrasound on mandibular condylar growth: A preliminary study. BioMed. Res. Int. 2014, 2014, 426710. [Google Scholar] [CrossRef]
- Sasaki, K.; Motoyoshi, M.; Horinuki, E.; Arai, Y.; Shimizu, N. Effect of low-intensity pulsed ultrasound (LIPUS) on mandibular condyle growth in rats analyzed with micro-CT. J. Oral Sci. 2016, 58, 415–422. [Google Scholar] [CrossRef]
- Astrand, J.; Harding, A.K.; Aspenberg, P.; Tägil, M. Systemic zoledronate treatment both prevents resorption of allograft bone and increases the retention of new formed bone during revascularization and remodelling. A bone chamber study in rats. BMC Musculoskelet. Disord. 2006, 7, 63. [Google Scholar] [CrossRef]
- Oyonarte, R.; Zárate, M.; Rodriguez, F. Low-intensity pulsed ultrasound stimulation of condylar growth in rats. Angle Orthod. 2009, 79, 964–970. [Google Scholar] [CrossRef]
- Bohluli, B.; Mohammadi, E.; Oskui, I.Z.; Moharamnejad, N. Treatment of mandibular angle fracture: Revision of the basic principles. Chin. J. Traumatol. 2019, 22, 117–119. [Google Scholar] [CrossRef]
- Wong, S.A.; Hu, D.P.; Slocum, J.; Lam, C.; Nguyen, M.; Miclau, T.; Marcucio, R.S.; Bahney, C.S. Chondrocyte-to-osteoblast transformation in mandibular fracture repair. J. Orthop. Res. 2021, 39, 1622–1632. [Google Scholar] [CrossRef]
- Erdogan, O.; Esen, E.; Ustün, Y.; Kürkçü, M.; Akova, T.; Gönlüşen, G.; Uysal, H.; Cevlik, F. Effects of low-intensity pulsed ultrasound on healing of mandibular fractures: An experimental study in rabbits. J. Oral Maxillofac. Surg. 2006, 64, 180–188. [Google Scholar] [CrossRef]
- Imai, Y.; Hasegawa, T.; Takeda, D.; Akashi, M.; Lee, S.Y.; Niikura, T.; Shibuya, Y.; Kurosaka, M.; Komori, T. The osteogenic activity of human mandibular fracture haematoma-derived cells is stimulated by low-intensity pulsed ultrasound in vitro. Int. J. Oral Maxillofac. Surg. 2014, 43, 367–372. [Google Scholar] [CrossRef]
- Huang, W.; Hasegawa, T.; Imai, Y.; Takeda, D.; Akashi, M.; Komori, T. Low-intensity pulsed ultrasound enhances bone morphogenetic protein expression of human mandibular fracture haematoma-derived cells. Int. J. Oral Maxillofac. Surg. 2015, 44, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.; Panneerselvam, E.; Doss, G.T.; Ponvel, K.; Raja Vb, K. Evaluation of Efficacy of Low Intensity Pulsed Ultrasound in Facilitating Mandibular Fracture Healing-A Blinded Randomized Controlled Clinical Trial. J. Oral Maxillofac. Surg. 2020, 78, e991–e997. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Kumar, S.; Kathiriya, N.; Madan, S.; Shah, A.; Venkataraghavan, K.; Jani, M. An Evaluation of the Effect of Therapeutic Ultrasound on Healing of Mandibular Fracture. Craniomaxillofac. Trauma Reconstr. 2015, 8, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Moussa, D.G.; Aparicio, C. Present and future of tissue engineering scaffolds for dentin-pulp complex regeneration. J. Tissue Eng. Regen. Med. 2019, 13, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Okiji, T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit. Anom. 2016, 56, 144–153. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Yang, Z.; Lu, K.; Zuo, J.; Zhou, Z. Effect of Low-Intensity Pulsed Ultrasound on a Rat Model of Dentin-Dental Pulp Injury and Repair. Ultrasound Med. Biol. 2017, 43, 163–175. [Google Scholar] [CrossRef]
- Li, X.Y.; Ban, G.F.; Al-Shameri, B.; He, X.; Liang, D.Z.; Chen, W.X. High-temperature Requirement Protein A1 Regulates Odontoblastic Differentiation of Dental Pulp Cells via the Transforming Growth Factor Beta 1/Smad Signaling Pathway. J. Endod. 2018, 44, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zuo, J.; Li, Y.; Yang, Z.; Luo, J. [Effect of low intensity pulsed ultrasound on expression of TGF-β1 and Smads during dentin injury and repair]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2017, 42, 1030–1036. [Google Scholar] [CrossRef]
- Zuo, J.; Zhen, J.; Wang, F.; Li, Y.; Zhou, Z. Effect of Low-Intensity Pulsed Ultrasound on the Expression of Calcium Ion Transport-Related Proteins during Tertiary Dentin Formation. Ultrasound Med. Biol. 2018, 44, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghreer, S.; Doschak, M.; Sloan, A.J.; Major, P.W.; Heo, G.; Scurtescu, C.; Tsui, Y.Y.; El-Bialy, T. Short-term effect of low-intensity pulsed ultrasound on an ex-vivo 3-d tooth culture. Ultrasound Med. Biol. 2013, 39, 1066–1074. [Google Scholar] [CrossRef]
- Al-Daghreer, S.; Doschak, M.; Sloan, A.J.; Major, P.W.; Heo, G.; Scurtescu, C.; Tsui, Y.Y.; El-Bialy, T. Long term effect of low intensity pulsed ultrasound on a human tooth slice organ culture. Arch. Oral Biol. 2012, 57, 760–768. [Google Scholar] [CrossRef]
- Lo Giudice, A.; Leonardi, R.; Ronsivalle, V.; Allegrini, S.; Lagravère, M.; Marzo, G.; Isola, G. Evaluation of pulp cavity/chamber changes after tooth-borne and bone-borne rapid maxillary expansions: A CBCT study using surface-based superimposition and deviation analysis. Clin. Oral Investig. 2021, 25, 2237–2247. [Google Scholar] [CrossRef]
- Vitali, F.C.; Cardoso, I.V.; Mello, F.W.; Flores-Mir, C.; Andrada, A.C.; Dutra-Horstmann, K.L.; Duque, T.M. Effect of orthodontic force on dental pulp histomorphology and tissue factor expression. Angle Orthod. 2021, 91, 830–842. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, L.; Wang, M.; Zhou, S.; Lu, Y.; Cui, H.; Racanelli, A.C.; Zhang, L.; Ye, T.; Ding, B.; et al. Targeting fibrosis, mechanisms and cilinical trials. Signal Transduct. Target. Ther. 2022, 7, 206. [Google Scholar] [CrossRef]
- Ikai, H.; Tamura, T.; Watanabe, T.; Itou, M.; Sugaya, A.; Iwabuchi, S.; Mikuni-Takagaki, Y.; Deguchi, S. Low-intensity pulsed ultrasound accelerates periodontal wound healing after flap surgery. J. Periodontal Res. 2008, 43, 212–216. [Google Scholar] [CrossRef]

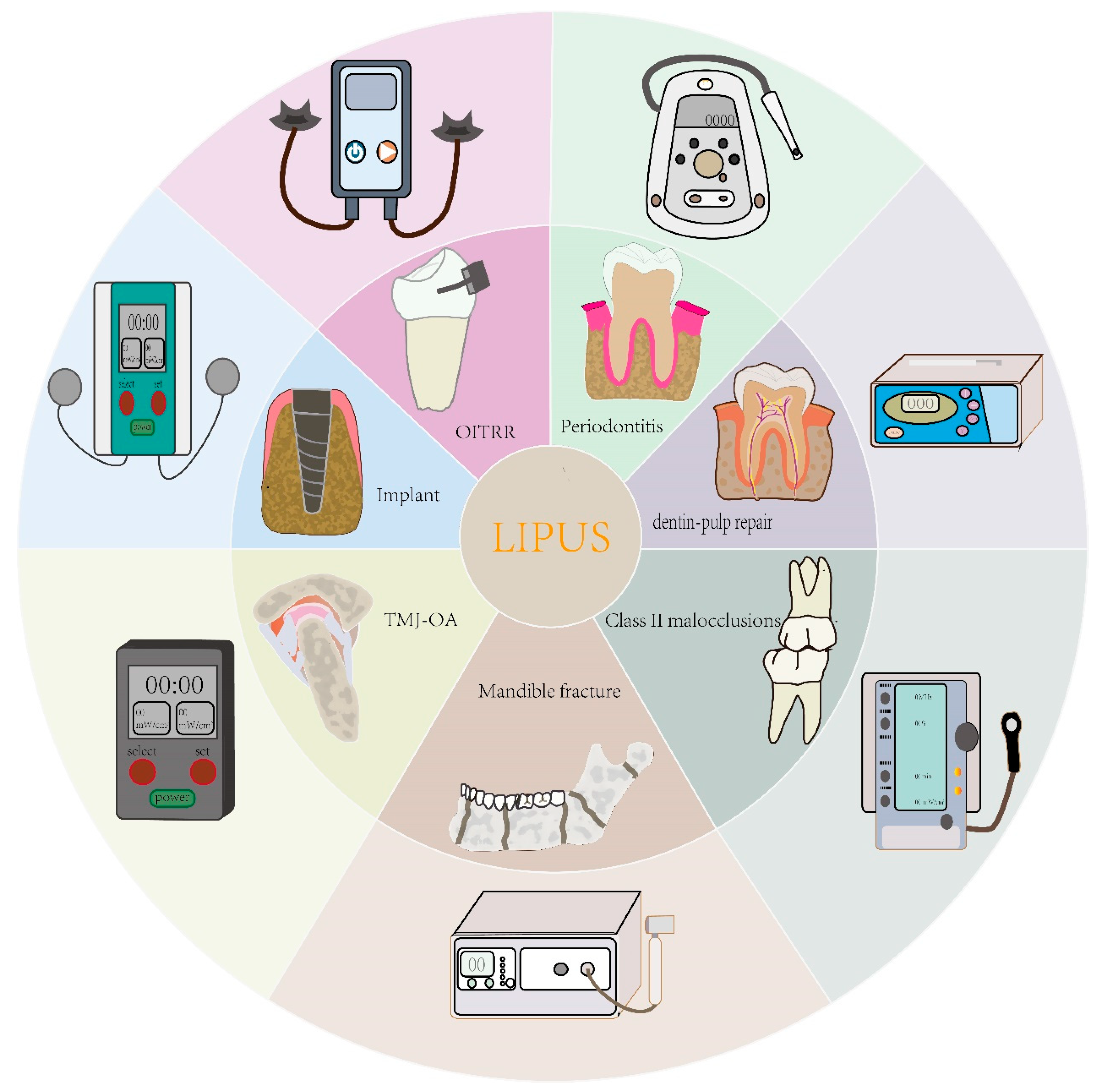

| Field of Employment | Targets | The Parameter of LIPUS | Effect | Reference |
|---|---|---|---|---|
| Periodontal tissue regeneration | osteoblasts, cells in periodontal ligament and gingival epithelium | Pulse frequency: 1.5 MHz Intensity: 30 mW/cm2 Time: 20 min/day for four weeks | periodontal wound healing and bone repair | Ikai, Tamura [129] |
| ECM protein in hPDLSCs | Pulse frequency: 1.5 MHz Intensity: 90 mW/cm2 Time: 30 min/day in a 37 °C water bath | Promote the osteogenic differentiation of hPDLSCs and induce the regeneration of periodontal ligament | Li, Zhou [14] | |
| unfolded protein reaction (UPR) in PDLSCs | Pulse frequency: 1.5 MHz Intensity: 90 mW/cm2 Time: 30 min/day | Enhance the osteogenic ability of PDLSCs and reduce the inflammatory response | Li, Deng [17] | |
| NF-κB pathway | Pulse frequency: 1.5 MHz Intensity: 30, 60, 90 mM/cm2 Time: 15 min/day for 7 days | Facilitate the immunoregulation and osteogenic ability of hPDLSCs | Lin, Wang [26] | |
| TWIST1/SDF-1 signaling pathway | Intensity: 90 mW/cm2 Time: 30 min/day | Promote PDLSCs migration | Wang, Li [29] | |
| p38 MAPK pathway | Pulse frequency: 1 MHz Intensity: 90 mW/cm2 Time: 0 min, 15 min, 30 min, 60 min, 90 min, 120 min, 6 h | Contribute to PDLCs osteogenic differentiation | Ren, Yang [34] | |
| bone morphogenetic protein-smad signaling | Pulse frequency: 1.5 MHz Intensity: 90 mW/cm2 Time: 20 min/day | Accelerate PDLCs osteogenic differentiation | Yang, Ren [36] | |
| miR-182 | Pulse frequency: 1.5 MHz Intensity: 90 mW/cm2 | Enhance PDLCs osteogenic differentiation | Chen, Xiang [39] | |
| HGF | Pulse frequency: 1.5 MHz Intensity: 30 mW/cm2 Time: 5 min/day or 10 min/day for 1~4 weeks | Promote HGF differentiation | Mostafa, Uludağ [43] | |
| OTM | HGF/Runx2/BMP-2 signaling pathway | Pulse frequency: 1.5 MHz Intensity: 30 mW/cm2 Time: 20 min/day | Accelerate OTM and alveolar bone remodeling | Xue, Zheng [59] |
| OITRR | OPG, RANKL, Cox-2 | Pulse frequency: 1.5% ± 5% MHz Intensity: 30% ± 30% mW/cm2 | OITRR inhibition and repair | Gul Amuk, Kurt [68] |
| Dental implant | local neuronal | Pulse frequency: 1 MHz Intensity: 30 mW/cm2 Time: 20 min/day for 14 or 28 days | Facilitate peri-implant osseointegration | Jiang, Yuan [83] |
| TMJ-OA | Sox9, Collagen Ⅱ, Aggrecan, VEGF | Pulse frequency: 1 MHz Intensity: 45 mW/cm2 Time: 20 min/day for 3 days | Promote the recovery of injury chondrocytes | Du, Liang [97] |
| HIF pathway | Pulse frequency: 1 MHz Intensity: 45 mW/cm2 Time: 20 min/day for 4 weeks | Reduce chondrocytes injury | Yang, Liang [98] | |
| ZNT-9 | Pulse frequency: 1 MHz Intensity: 100 mW/cm2 Time: 20 min/day for 5 days a week | Protect chondrocytes | He, Wang [99] | |
| TGF-β1/Smad3 pathway | Pulse frequency: 1 MHz Intensity: 30 mW/cm2 Time: 20 min/day for 6 weeks | Reduce inflammatory response | Yi, Liu [101] | |
| Mandible fracture | BMP | Pulse frequency: 1.5 MHz Intensity: 30 mW/cm2 Time: 20 min/day for 4, 8, 14, 20 days | Promote bone repair | Huang, Hasegawa [115] |
| Dentin-pulp injury | TGF-β1 and Smad 2, 3 | Pulse frequency: 1.5 MHz Intensity: 30 mW/cm2 Time: 20 min/day for 1, 3, 5, 7, 14 days | Dentin-pulp-repair after injury | Wang, Zuo [122] |
| calcium transport-related proteins | Pulse frequency: 1.5 MHz Intensity: 30 mW/cm2 Time: 20 min/day for 1, 3, 7, 14 days | Accelerate the formation of tertiary dentin | Zuo, Zhen [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Guo, Y. Clinical Applications of Low-Intensity Pulsed Ultrasound and Its Underlying Mechanisms in Dentistry. Appl. Sci. 2022, 12, 11898. https://doi.org/10.3390/app122311898
Wei Y, Guo Y. Clinical Applications of Low-Intensity Pulsed Ultrasound and Its Underlying Mechanisms in Dentistry. Applied Sciences. 2022; 12(23):11898. https://doi.org/10.3390/app122311898
Chicago/Turabian StyleWei, Yuzi, and Yongwen Guo. 2022. "Clinical Applications of Low-Intensity Pulsed Ultrasound and Its Underlying Mechanisms in Dentistry" Applied Sciences 12, no. 23: 11898. https://doi.org/10.3390/app122311898
APA StyleWei, Y., & Guo, Y. (2022). Clinical Applications of Low-Intensity Pulsed Ultrasound and Its Underlying Mechanisms in Dentistry. Applied Sciences, 12(23), 11898. https://doi.org/10.3390/app122311898







