Lessons for Coastal Applications of IMTA as a Way towards Sustainable Development: A Review
Abstract
:1. Introduction
2. Methods
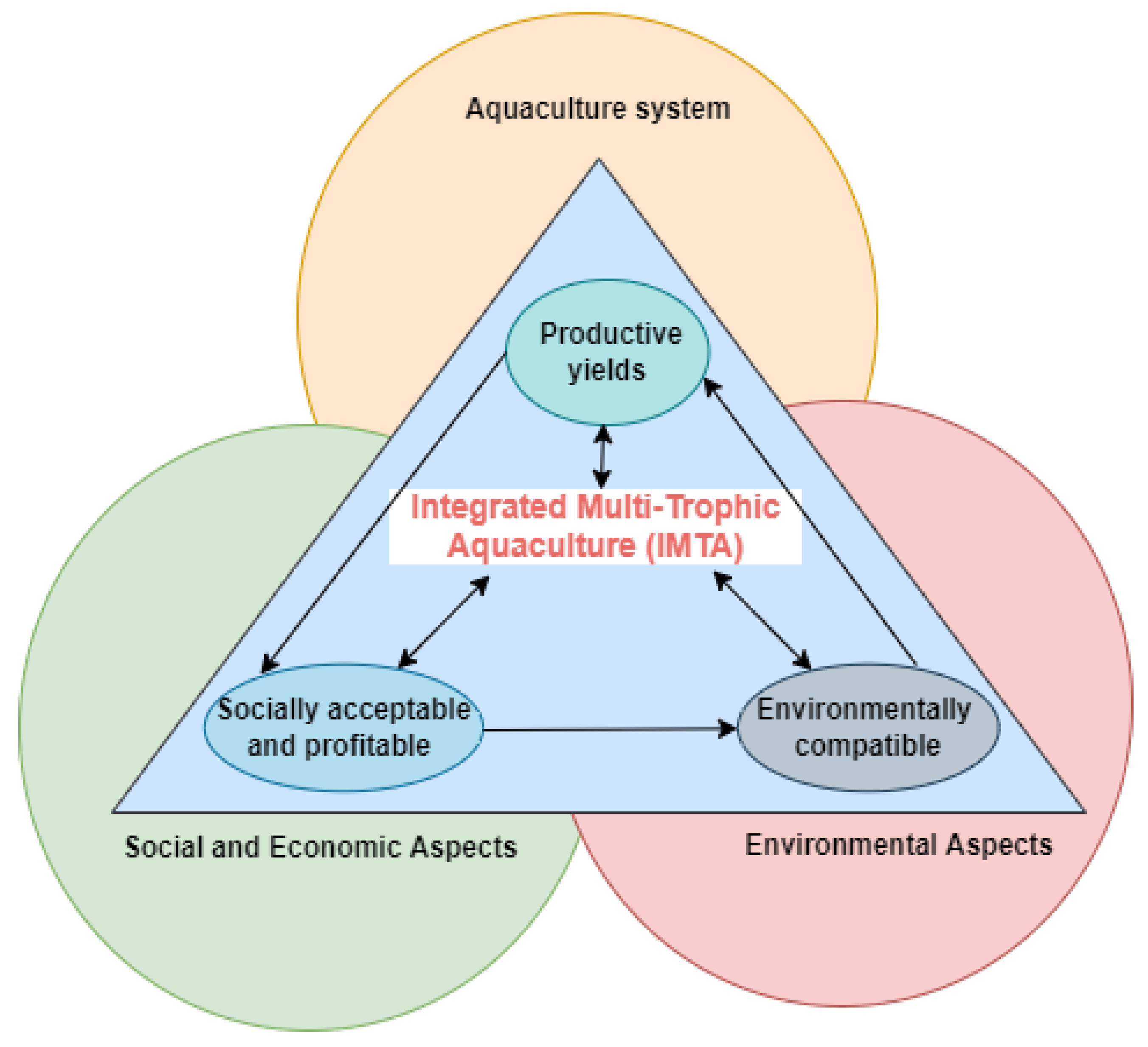
3. Sustainability Potentials of IMTA
3.1. Environmental Sustainability
3.2. Economic Sustainability
3.3. Social Sustainability
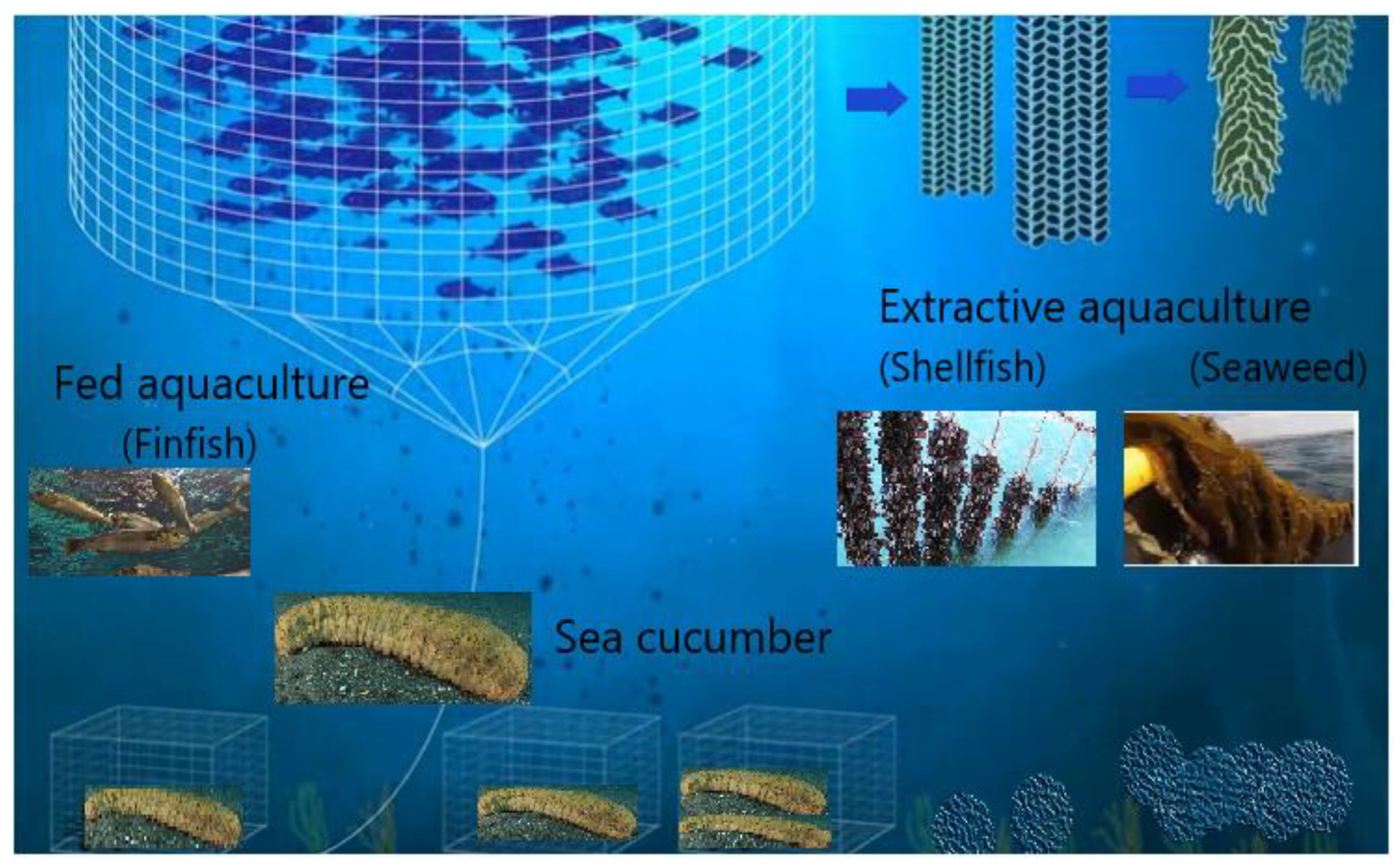
4. Candidate Species for Coastal IMTA
5. Economic Value and Financial Viability of Coastal IMTA
5.1. Species-Specific IMTA
5.2. Region- and Country-Specific IMTA
5.2.1. China
5.2.2. Others Region
6. Bioremediation by Extractive/Additional Species
7. Social Sustainability, Social License, and Aquaculture Governance
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, P.; Zhang, W.; Belton, B.; Little, D.C. Misunderstandings, myths and mantras in aquaculture: Its contribution to world food supplies has been systematically over reported. Mar. Policy 2019, 106, 103547. [Google Scholar] [CrossRef]
- Barange, M.; Bahri, T.; Beveridge, M.C.; Cochrane, K.L.; Funge-Smith, S.; Poulain, F. Impacts of Climate Change on Fisheries and Aquaculture: Synthesis of Current Knowledge, Adaptation and Mitigation Options; FAO: Rome, Italy, 2018. [Google Scholar]
- Hambrey, J. The 2030 Agenda and the Sustainable Development Goals: The Challenge for Aquaculture Development and Management; FAO Fisheries and Aquaculture Circular (C1141): Rome, Italy, 2017. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Thorpe, A.; Reid, C.; Anrooy, R.V.; Brugere, C.; Becker, D. Poverty reduction strategy papers and the fisheries sector: An opportunity forgone? J. Int. Dev. J. Dev. Stud. Assoc. 2006, 18, 489–517. [Google Scholar] [CrossRef]
- Sukhdhane, K.S.; Kripa, V.; Divu, D.; Vase, V.K.; Mojjada, S.K. Integrated multi-trophic aquaculture systems: A solution for sustainability. Aquac. Asia Mag. 2018, 22, 26–29. [Google Scholar]
- UNSD. SDG Indicators Metadata Repository. 2018. Available online: https://unstats.un.org/sdgs/metadata/ (accessed on 27 December 2020).
- Ackefors, H.; Enell, M. Discharge of nutrients from Swedish fish farming to adjacent sea areas. Ambio 1990, 19, 28–35. [Google Scholar]
- Seymour, E.A.; Bergheim, A. Towards a reduction of pollution from intensive aquaculture with reference to the farming of salmonids in Norway. Aquac. Eng. 1991, 10, 73–88. [Google Scholar] [CrossRef]
- Gatlin, D.M.; Barrows, F.T.; Brown, P.; Dabrowski, K.; Gaylord, T.G.; Hardy, R.W.; Heman, E.; Hu, G.; Krogdahl, A.; Nelson, R.; et al. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquaculture. Res. 2007, 38, 551–579. [Google Scholar] [CrossRef]
- Moutinho, S.; Martínez-Llorens, S.; Tomás-Vidal, A.; Jover-Cerdá, M.; Oliva-Teles, A.; Peres, H. Meat and bone meal as partial replacement for fish meal in diets for gilthead seabream (Sparus aurata) juveniles: Growth, feed efficiency, amino acid utilization, and economic efficiency. Aquaculture 2017, 468, 271–277. [Google Scholar] [CrossRef]
- Sarà, G.; Mangano, M.C.; Johnson, M.; Mazzola, A. Integrating multiple stressors in aquaculture to build the blue growth in a changing sea. Hydrobiologia 2018, 809, 5–17. [Google Scholar] [CrossRef]
- Alexander, K.A.; Angel, D.; Freeman, S.; Israel, D.; Johansen, J.; Kletou, D.; Meland, M.; Pecorino, D.; Rebours, C.; Rousou, M.; et al. Improving sustainability of aquaculture in Europe: Stakeholder dialogues on integrated multi-trophic aquaculture (IMTA). Environ. Sci. Policy 2016, 55, 96–106. [Google Scholar] [CrossRef]
- Sarà, G.; Gouhier, T.C.; Brigolin, D.; Porporato, E.M.; Mangano, M.C.; Mirto, S.; Mazzola, A.; Pastres, R. Predicting shifting sustainability trade-offs in marine finfish aquaculture under climate change. Glob. Change Biol. 2018, 24, 3654–3665. [Google Scholar] [CrossRef]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the art and challenges for offshore integrated multi-trophic aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Chopin, T.; Cooper, J.A.; Reid, G.; Cross, S.; Moore, C. Open-water integrated multi-trophic aquaculture: Environmental biomitigation and economic diversification of fed aquaculture by extractive aquaculture. Rev. Aquac. 2012, 4, 209–220. [Google Scholar] [CrossRef]
- Chopin, T.; Robinson, S.M.C.; Troell, M.; Neori, A.; Buschmann, A.; Fang, J.G. Ecological engineering: Multi-trophic integration for sustainable marine aquaculture. Aquaculture 2008, 297, 1–9. [Google Scholar]
- Chopin, T.; Troell, M.; Reid, G.K.; Knowler, D.; Robinson, S. Integrated multi-trophic aquaculture. In Advancing the Aquaculture Agenda: Workshop Proceedings; OECD Publishing: Washington, DC, USA, 2010; pp. 195–217. [Google Scholar]
- Hughes, A.D.; Black, K.D. Going beyond the search for solutions: Understanding trade-offs in European integrated multi-trophic aquaculture development. Aquac. Environ. Interact. 2016, 8, 191–199. [Google Scholar] [CrossRef]
- Chopin, T. Integrated Multi-Trophic Aquaculture. What it is and why you should care… and don’t confuse it with polyculture. North. Aquac. 2006, 12, 4. [Google Scholar]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Troell, M.; Halling, C.; Neori, A.; Chopin, T.; Buschmann, A.H.; Kautsky, N.; Yarish, C. Integrated mariculture: Asking the right questions. Aquaculture 2003, 226, 69–90. [Google Scholar] [CrossRef]
- Ertör, I.; Ortega-Cerdà, M. Political lessons from early warnings: Marine finfish aquaculture conflicts in Europe. Mar. Policy 2015, 51, 202–210. [Google Scholar] [CrossRef]
- Barrington, K.; Chopin, T.; Robinson, S. Integrated multi-trophic aquaculture (IMTA) in marine temperate waters. Integr. Maric. A Glob. Review. FAO Fish. Aquac. Tech. Pap. 2009, 529, 7–46. [Google Scholar]
- Van Osch, S.; Hynes, S.; Freeman, S.; O’Higgins, T. Estimating the public’s preferences for sustainable aquaculture: A country comparison. Sustainability 2019, 11, 569. [Google Scholar] [CrossRef] [Green Version]
- Social Sustainability–Everything You Need to Know. Available online: https://www.open.edu/openlearncreate/mod/page/view.php?id=176455 (accessed on 20 December 2020).
- Harzing, A. Publish or Perish. 2007. Available online: https://harzing.com/resources/publish-or-perish (accessed on 30 August 2021).
- AIT (Asian Institute of Technology). The Promotion of Sustainable Aquaculture; Asian Institute of Technology: Bangkok, Thailand, 1994; 98p. [Google Scholar]
- Yang, L.X. From general principles of civil law to general provisions of civil law: A historical leap in contemporary Chinese civil law. Soc. Sci. China 2019, 2, 85–91. [Google Scholar]
- Chopin, T.; Buschmann, A.H.; Halling, C.; Troell, M.; Kautsky, N.; Neori, A.; Kraemer, G.P.; Zertuche-González, J.A.; Yarish, C.; Neefus, C. Integrating seaweeds into marine aquaculture systems: A key toward sustainability. J. Phycol. 2001, 37, 975–986. [Google Scholar] [CrossRef]
- Shi, H.; Zheng, W.; Zhang, X.; Zhu, M.; Ding, D. Ecological–economic assessment of monoculture and integrated multi-trophic aquaculture in Sanggou Bay of China. Aquaculture 2013, 410, 172–178. [Google Scholar] [CrossRef]
- Whitmarsh, D.J.; Cook, E.J.; Black, K.D. Searching for sustainability in aquaculture: An investigation into the economic prospects for an integrated salmon–mussel production system. Mar. Policy 2006, 30, 293–298. [Google Scholar] [CrossRef]
- Bolton, J.J.; Robertson-Andersson, D.V.; Shuuluka, D.; Kandjengo, L. Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: A SWOT analysis. J. Appl. Phycol. 2009, 21, 575–583. [Google Scholar] [CrossRef]
- Costa-Pierce, B.A. Sustainable ecological aquaculture systems: The need for a new social contract for aquaculture development. Mar. Technol. Soc. J. 2010, 44, 88–112. [Google Scholar] [CrossRef]
- Edwards, P. Traditional Asian aquaculture. In New Technologies in Aquaculture; Burnell, G., Ed.; Chapter 34; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Zhou, Y.; Yang, H.; Liu, S.; Yuan, X.; Mao, Y.; Liu, Y.; Xu, X.; Zhang, F. Feeding and growth on bivalve biodeposits by the deposit feeder Stichopus japonicus Selenka (Echinodermata: Holothuroidea) co-cultured in lantern nets. Aquaculture 2006, 256, 510–520. [Google Scholar] [CrossRef]
- Ridler, N.; Wowchuk, M.; Robinson, B.; Barrington, K.; Chopin, T.; Robinson, S.; Page, F.; Reid, G.; Szemerda, M.; Sewuster, J.; et al. Integrated multi− trophic aquaculture (IMTA): A potential strategic choice for farmers. Aquac. Econ. Manag. 2007, 11, 99–110. [Google Scholar] [CrossRef]
- Dernbach, J.C. Achieving sustainable development: The Centrality and multiple facets of integrated decision making. Glob. Leg. Stud. 2003, 10, 247–284. [Google Scholar] [CrossRef]
- Brodhag, C.; Talière, S. Sustainable development strategies: Tools for policy coherence. Nat. Resour. Forum 2006, 30, 136–145. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Azevedo, C.A.A.; Trigueiro, T.G.; Pereira, D.C.; Carneiro, M.A.A.; Camara, M.R. Bioremediation of aquaculture wastewater using macroalgae and Artemia. Int. Biodeterior. Biodegrad. 2011, 65, 253–257. [Google Scholar] [CrossRef]
- Chávez-Crooker, P.; Obreque-Contreras, J. Bioremediation of aquaculture wastes. Curr. Opin. Biotechnol. 2010, 21, 313–317. [Google Scholar] [CrossRef]
- Abreu, M.H.; Varela, D.A.; Henríquez, L.; Villarroel, A.; Yarish, C.; Sousa-Pinto, I.; Buschmann, A.H. Traditional vs. integrated multi-trophic aquaculture of Gracilaria chilensis CJ Bird, J. McLachlan & EC Oliveira: Productivity and physiological performance. Aquaculture 2009, 293, 211–220. [Google Scholar]
- Matos, J.; Costa, S.; Rodrigues, A.; Pereira, R.; Pinto, I.S. Experimental integrated aquaculture of fish and red seaweeds in Northern Portugal. Aquaculture 2006, 252, 31–42. [Google Scholar] [CrossRef]
- Martínez-Aragón, J.F.; Hernández, I.; Pérez-Lloréns, J.L.; Vázquez, R.; Vergara, J.J. Biofiltering efficiency in removal of dissolved nutrients by three species of estuarine macroalgae cultivated with sea bass (Dicentrarchus labrax) waste waters 1. Phosphate. J. Appl. Phycol. 2002, 14, 365–374. [Google Scholar] [CrossRef]
- Spangenberg, J.H. Economic sustainability of the economy: Concepts and indicators. Int. J. Sustain. Dev. 2005, 8, 47–64. [Google Scholar] [CrossRef]
- Lobo, M.J.; Pietriga, E.; Appert, C. An evaluation of interactive map comparison techniques. In Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems, Seoul, Republic of Korea, 18–23 April 2015; pp. 3573–3582. [Google Scholar]
- Allen, C.; Clouth, S. Green economy, green growth, and low-carbon development–history, definitions and a guide to recent publications. In Division for Sustainable Development; Department of Economic and Social Affairs, United Nations: New York, NY, USA, 2012; pp. 1–63. [Google Scholar]
- Serpa, S.; Ferreira, C.M. Society 5.0 and sustainability digital innovations: A social process. J. Organ. Cult. Commun. Confl. 2019, 23, 1–14. [Google Scholar]
- Robinson, S.; Lander, T.; MacDonald, B.; Barrington, K.; Chopin, T.; Martin, J.D.; Bastarache, S.; Belyea, E.; Haya, K.; Sephton, F.; et al. Development of integrates aquaculture of three trophic levels (finfish, seaweed and shellfish): The AquaNet project in the Bay of Fundy, Canada. The production dynamics of mussels as filter-feeder utilizing enhanced seston fields within a salmon aquaculture site. Beyond Monoculture Abstr. Aquac. Eur. Symp. 2003, 2003, 65–66. [Google Scholar]
- Cook, E.; Black, K.; Sayer, M. In Situ Bio-Filters at Commercial Salmon Farms in Scotland-How Effective are Mussel Lines as Biological Filters?BIOFAQs Workshop, Eilat (October 2002). 2003. Available online: https://pure.uhi.ac.uk/en/publications/in-situ-bio-filters-at-commercial-salmon-farms-in-scotland-how-ef (accessed on 6 August 2021).
- Nobre, A.M.; Robertson-Andersson, D.; Neori, A.; Sankar, K. Ecological–economic assessment of aquaculture options: Comparison between abalone monoculture and integrated multi-trophic aquaculture of abalone and seaweeds. Aquaculture 2010, 306, 116–126. [Google Scholar] [CrossRef]
- Troell, M.; Joyce, A.; Chopin, T.; Neori, A.; Buschmann, A.H.; Fang, J.G. Ecological engineering in aquaculture potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture 2009, 297, 1–9. [Google Scholar] [CrossRef]
- Xiang, J. Recent major advances of biotechnology and sustainable aquaculture in China. Curr. Biotechnol. 2015, 4, 296–310. [Google Scholar] [CrossRef] [Green Version]
- Daly, H.E. UN conferences on environment and development: Retrospect on Stockholm and prospects for Rio. Ecol. Econ. 1992, 5, 9–14. [Google Scholar] [CrossRef]
- Littig, B.; Griessler, E. Social sustainability: A catchword between political pragmatism and social theory. Int. J. Sustain. Dev. 2005, 8, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Saith, A. From universal values to millennium development goals: Lost in translation. Dev. Change 2006, 37, 1167–1199. [Google Scholar] [CrossRef]
- Gray, R. Is accounting for sustainability accounting for sustainability… and how would we know? An exploration of narratives of organizations and the planet. Account. Organ. Soc. 2010, 35, 47–62. [Google Scholar] [CrossRef]
- Guo, F. The spirit and characteristic of the general provisions of civil law. Law Econ. 2017, 3, 5–16. [Google Scholar]
- Glaser, M.; Glaeser, B. The social dimension in the management of social-ecological change. In Integrated Management of Estuaries and Coasts; Elsevier: Munich, Germany, 2012; pp. 5–30. [Google Scholar]
- Glaeser, B.; Glaser, M. Coastal Management Revisited; Cambridge Scholars Publishers: Cambridge, UK, 2022; Chapter 2, in press. [Google Scholar]
- Integrated Multi-Trophic Aquaculture and Precision Aquaculture, Susanne Ricee. Available online: https://diversity.social/social-sustainability/ (accessed on 13 May 2021).
- Benaim, C.A.; Raftis, L. The Social Dimension of Sustainable Development: Guidance and Application. Master’s Thesis, Blekinge Institute of Technology, Karlskrona, Sweden, 2008. [Google Scholar]
- Saner, R.; Yiu, L.; Nguyen, M. Monitoring the SDGs: Digital and social technologies to ensure citizen participation, inclusiveness, and transparency. Dev. Policy Rev. 2019, 38, 483–500. [Google Scholar] [CrossRef]
- FAO. Code of Conduct for Responsible Fisheries; Food and Agriculture Organization of the United Nations: Rome, Italy, 1995; 41p. [Google Scholar]
- Milstein, A. Polyculture in aquaculture. In Animal Breeding Abstracts; CABI Publishing: Wallingford, UK, 2005; Volume 73. [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture 2016: Contributing to Food Security and Nutrition for All; FAO: Rome, Italy, 2016; 200p. [Google Scholar]
- Smith, M.D.; Roheim, C.A.; Crowder, L.B.; Halpern, B.S.; Turnipseed, M.; Anderson, J.L.; Asche, F.; Bourillón, L.; Guttormsen, A.G.; Khan, A.; et al. Sustainability and global seafood. Science 2010, 327, 784–786. [Google Scholar] [CrossRef]
- Stevenson, J.R.; Irz, X. Is aquaculture development an effective tool for poverty alleviation? A review of theory and evidence. Cah. Agric. 2009, 18, 292–299. [Google Scholar] [CrossRef]
- Krause, G.; Billing, S.L.; Dennis, J.; Grant, J.; Fanning, L.; Filgueira, R.; Miller, M.; Agúndez, J.A.P.; Stybel, N.; Stead, S.M.; et al. Visualizing the social in aquaculture: How social dimension components illustrate the effects of aquaculture across geographic scales. Mar. Policy 2020, 118, 103985. [Google Scholar] [CrossRef]
- Neiland, A.E.; Shaw, S.A.; Bailly, D. The social and economic impact of aquaculture: A European review. Aquac. Environ. 1991, 16, 469–482. [Google Scholar]
- Abate, T.G.; Nielsen, R.; Tveterås, R. Stringency of environmental regulation and aquaculture growth: A cross-country analysis. Aquac. Econ. Manag. 2016, 20, 201–221. [Google Scholar] [CrossRef]
- Van Senten, J.; Engle, C.R. The costs of regulations on US baitfish and sportfish producers. J. World Aquac. Soc. 2017, 48, 503–517. [Google Scholar] [CrossRef] [Green Version]
- Gunningham, N.; Kagan, R.A.; Thornton, D. Social license and environmental protection: Why businesses go beyond compliance. Law Soc. Inq. 2004, 29, 307–341. [Google Scholar] [CrossRef]
- Leith, P.; Ogier, E.; Haward, M. Science and social license: Defining environmental sustainability of Atlantic salmon aquaculture in south-eastern Tasmania, Australia. Soc. Epistemol. 2014, 28, 277–296. [Google Scholar] [CrossRef]
- Orchard, S.E.; Stringer, L.C.; Quinn, C.H. Impacts of aquaculture on social networks in the mangrove systems of northern Vietnam. Ocean. Coast. Manag. 2015, 114, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, S.; Estim, A.; Shaleh, S.R.M.; Shapawi, R. Positioning of aquaculture in blue growth and sustainable development goals through new knowledge, ecological perspectives and analytical solutions. Aquac. Indones. 2018, 19, 1–9. [Google Scholar] [CrossRef]
- Paula, S. Improving Bioremediation with Extractive Species in Integrated Aquaculture. Ph.D. Thesis, University of Bremen, Bremen, Germany, 2021. [Google Scholar]
- Lv, Z.M.; Research group. The implementation outline of the “Green Principle” in civil code. China Law Sci. 2018, 1, 7–8. [Google Scholar]
- Piper, L.; de Cosmo, L.M.; Sestino, A.; Giangrande, A.; Stabili, L.; Longo, C.; Guido, G. Perceived social welfare as a driver of green products consumption: Evidences from an integrated multi-trophic aquaculture production. Curr. Res. Environ. Sustain. 2021, 3, 100081. [Google Scholar] [CrossRef]
- VanderZwaag, D.L.; Chao, G. (Eds.) Canadian aquaculture and the principles of sustainable development: Gauging the law and policy tides and charting a course. In Aquaculture Law and Policy; Routledge: London, UK, 2006. [Google Scholar]
- Hanss, D.; Doran, R. Perceived Consumer Effectiveness. In Responsible Consumption and Production; Leal Filho, W., Azul, A.M., Brandli, L., özuyar, P.G., Wall, T., Eds.; Encyclopedia of the UN Sustainable Development Goals; Springer: Cham, Germany, 2020. [Google Scholar]
- Giangrande, A.; Pierri, C.; Arduini, D.; Borghese, J.; Licciano, M.; Trani, R.; Corriero, G.; Basile, G.; Cecere, E.; Petrocelli, A.; et al. An innovative IMTA system: Polychaetes, sponges and macroalgae co-cultured in a Southern Italian in-shore mariculture plant (Ionian Sea). J. Mar. Sci. Eng. 2020, 8, 733. [Google Scholar] [CrossRef]
- Gökalp, M.; Mes, D.; Nederlof, M.; Zhao, H.; de Goeij, J.M.; Osinga, R. The potential roles of sponges in integrated mariculture. Rev. Aquac. 2021, 13, 1159–1171. [Google Scholar] [CrossRef]
- Longo, C.; Cardone, F.; Corriero, G.; Licciano, M.; Pierri, C.; Stabili, L. The co-occurrence of the demosponge Hymeniacidon perlevis and the edible mussel Mytilus galloprovincialis as a new tool for bacterial load mitigation in aquaculture. Environ. Sci. Pollut. Res. 2016, 23, 3736–3746. [Google Scholar] [CrossRef]
- Wu, H.; Huo, Y.; Han, F.; Liu, Y.; He, P. Bioremediation using Gracilaria chouae co-cultured with Sparus macrocephalus to manage the nitrogen and phosphorous balance in an IMTA system in Xiangshan Bay, China. Mar. Pollut. Bull. 2015, 91, 272–279. [Google Scholar] [CrossRef]
- MacDonald, B.A.; Robinson, S.M.; Barrington, K.A. Feeding activity of mussels (Mytilus edulis) held in the field at an integrated multi-trophic aquaculture (IMTA) site (Salmo salar) and exposed to fish food in the laboratory. Aquaculture 2011, 314, 244–251. [Google Scholar] [CrossRef]
- Sarà, G.; Zenone, A.; Tomasello, A. Growth of Mytilus galloprovincialis (mollusca, bivalvia) close to fish farms: A case of integrated multi-trophic aquaculture within the Tyrrhenian Sea. Hydrobiologia 2009, 636, 129–136. [Google Scholar] [CrossRef]
- Yokoyama, H. Growth and food source of the sea cucumber Apostichopus japonicus cultured below fish cages potential for integrated multi-trophic aquaculture. Aquaculture 2013, 372, 28–38. [Google Scholar] [CrossRef]
- Hannah, L.; Pearce, C.M.; Cross, S.F. Growth and survival of California sea cucumbers (Parastichopus californicus) cultivated with sablefish (Anoplopoma fimbria) at an integrated multi-trophic aquaculture site. Aquaculture 2013, 406, 34–42. [Google Scholar] [CrossRef]
- Sun, J.; Hamel, J.F.; Gianasi, B.L.; Graham, M.; Mercier, A. Growth, health and biochemical composition of the sea cucumber Cucumaria frondosa after multi-year holding in effluent waters of land-based salmon culture. Aquac. Environ. Interact. 2020, 12, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Slater, M.J.; Carton, A.G. Effect of sea cucumber (Australostichopus mollis) grazing on coastal sediments impacted by mussel farm deposition. Mar. Pollut. Bull. 2009, 58, 1123–1129. [Google Scholar] [CrossRef]
- Baltadakis, A.; Casserly, J.; Falconer, L.; Sprague, M.; Telfer, T.C. European lobsters utilise Atlantic salmon wastes in coastal integrated multi-trophic aquaculture systems. Aquac. Environ. Interact. 2020, 12, 485–494. [Google Scholar] [CrossRef]
- Stabili, L.; Cecere, E.; Licciano, M.; Petrocelli, A.; Sicuro, B.; Giangrande, A. Integrated multitrophic aquaculture by-products with added value: The polychaete Sabella spallanzanii and the seaweed Chaetomorpha linum as potential dietary ingredients. Mar. Drugs 2019, 17, 677. [Google Scholar] [CrossRef] [Green Version]
- Mandario, M.A.E.; Alava, V.R.; Añasco, N.C. Evaluation of the bioremediation potential of mud polychaete Marphysa sp. in aquaculture pond sediments. Environ. Sci. Pollut. Res. 2019, 26, 29810–29821. [Google Scholar] [CrossRef]
- Shpigel, M.; Ari, T.B.; Shauli, L.; Odintsov, V.; Ben-Ezra, D. Nutrient recovery and sludge management in seabream and grey mullet co-culture in Integrated Multi-Trophic Aquaculture (IMTA). Aquaculture 2016, 464, 316–322. [Google Scholar] [CrossRef]
- Han, Q.; Keesing, J.K.; Liu, D. A review of sea cucumber aquaculture, ranching, and stock enhancement in China. Rev. Fish. Sci. Aquac. 2016, 24, 326–341. [Google Scholar] [CrossRef]
- Paltzat, D.L.; Pearce, C.M.; Barnes, P.A.; McKinley, R.S. Growth and production of California sea cucumbers (Parastichopus californicus, Stimpson) co-cultured with suspended Pacific oysters (Crassostrea gigas, Thunberg). Aquaculture 2008, 275, 124–137. [Google Scholar] [CrossRef]
- Ito, S. Studies on the technological development of the mass production for sea cucumber juvenile, Stichopus japonicus. Bull Saga Prefect Genkai Fish Res Dev Cent. 1995, 4, 1–87. [Google Scholar]
- Kang, K.H.; Kwon, J.Y.; Kim, Y.M. A beneficial coculture: Charm abalone, Haliotis discus hannai and sea cucumber, Stichopus japonicus. Aquaculture 2003, 216, 87–93. [Google Scholar] [CrossRef]
- Zamora, L.N.; Yuan, X.; Carton, A.G.; Slater, M.J. Role of deposit-feeding sea cucumbers in integrated multitrophic aquaculture: Progress, problems, potential and future challenges. Rev. Aquac. 2018, 10, 57–74. [Google Scholar] [CrossRef] [Green Version]
- Handå, A.; Ranheim, A.; Olsen, A.J.; Altin, D.; Reitan, K.I.; Olsen, Y.; Reinertsen, H. Incorporation of salmon fish feed and feces components in mussels (Mytilus edulis): Implications for integrated multi-trophic aquaculture in cool-temperate North Atlantic waters. Aquaculture 2012, 370, 40–53. [Google Scholar] [CrossRef]
- Lander, T.R.; Robinson, S.M.C.; MacDonald, B.A.; Martin, J.D. Characterization of the suspended organic particles released from salmon farms and their potential as a food supply for the suspension feeder, Mytilus edulis in integrated multi-trophic aquaculture (IMTA) systems. Aquaculture 2013, 406, 160–171. [Google Scholar] [CrossRef]
- Wartenberg, R.; Feng, L.; Wu, J.J.; Mak, Y.L.; Chan, L.L.; Telfer, T.C.; Lam, P.K. The impacts of suspended mariculture on coastal zones in China and the scope for integrated multi-trophic aquaculture. Ecosyst. Health Sustain. 2017, 3, 1340268. [Google Scholar] [CrossRef] [Green Version]
- FAO (Food and Agriculture Organization of the United Nations). Sustainable fisheries and aquaculture for food security and nutrition. In A Report by the High-Level Panel of Experts on Food Security and Nutrition; FAO: Rome, Italy, 2014. [Google Scholar]
- Magalhães, R.; Lopes, T.; Martins, N.; Díaz-Rosales, P.; Couto, A.; Pousão-Ferreira, P.; Oliva-Teles, A.; Peres, H. Carbohydrases supplementation increased nutrient utilization in white seabream (Diplodus sargus) juveniles fed high soybean meal diets. Aquaculture 2016, 463, 43–50. [Google Scholar] [CrossRef]
- Wang, X.; Olsen, L.M.; Reitan, K.I.; Olsen, Y. Discharge of nutrient wastes from salmon farms: Environmental effects, and potential for integrated multi-trophic aquaculture. Aquac. Environ. Interact. 2012, 2, 267–283. [Google Scholar] [CrossRef]
- Holdt, S.L.; Edwards, M.D. Cost-effective IMTA: A comparison of the production efficiencies of mussels and seaweed. J. Appl. Phycol. 2014, 26, 933–945. [Google Scholar] [CrossRef] [Green Version]
- Mæhre, H.K.; Malde, M.K.; Eilertsen, K.E.; Elvevoll, E.O. Characterization of protein, lipid and mineral contents in common Norwegian seaweeds and evaluation of their potential as food and feed. J. Sci. Food Agric. 2014, 94, 3281–3290. [Google Scholar] [CrossRef] [PubMed]
- Carras, M.A.; Knowler, D.; Pearce, C.M.; Hamer, A.; Chopin, T.; Weaire, T. A discounted cash-flow analysis of salmon monoculture and Integrated Multi-Trophic Aquaculture in eastern Canada. Aquac. Econ. Manag. 2019, 24, 43–63. [Google Scholar] [CrossRef]
- Knowler, D.; Chopin, T.; Martínez-Espiñeira, R.; Neori, A.; Nobre, A.; Noce, A.; Reid, G. The economics of Integrated Multi-Trophic Aquaculture: Where are we now and where do we need to go? Rev. Aquac. 2020, 12, 1579–1594. [Google Scholar] [CrossRef]
- Fonseca, T.; David, F.S.; Ribeiro, F.A.; Wainberg, A.A.; Valenti, W.C. Technical and economic feasibility of integrating seahorse culture in shrimp/oyster farms. Aquac. Res. 2017, 48, 655–664. [Google Scholar] [CrossRef]
- Petrell, R.J.; Alie, S.Y. Integrated cultivation of salmonids and seaweeds in open systems. In Fifteenth International Seaweed Symposium; Springer: Dordrecht, The Netherlands, 1996; pp. 67–73. [Google Scholar]
- Shuve, H.; Caines, E.; Ridler, N.; Chopin, T.; Reid, G.; Sawhney, M.; Lamontagne, J.; Szemerda, M.; Marvin, R.; Powell, F.; et al. Survey finds consumers support integrated multitrophic aquaculture. Glob. Aquac. Advocate 2009, 12, 22–23. [Google Scholar]
- Zheng, W.; Shi, H.; Chen, S.; Zhu, M. Benefit and cost analysis of mariculture based on ecosystem services. Ecol. Econ. 2009, 68, 1626–1632. [Google Scholar] [CrossRef]
- Giangrande, A.; Gravina, M.F.; Rossi, S.; Longo, C.; Pierri, C. Aquaculture and restoration: Perspectives from mediterranean sea experiences. Water 2021, 13, 991. [Google Scholar] [CrossRef]
- Granada, L.; Sousa, N.; Lopes, S.; Lemos, M.F. Is integrated multitrophic aquaculture the solution to the sectors’ major challenges?—A review. Rev. Aquac. 2015, 8, 283–300. [Google Scholar] [CrossRef]
- Bergamo, G.C.A.; Olier, B.S.; de Sousa, O.M.; Kuhnen, V.V.; Pessoa, M.F.G.; Sanches, E.G. Economic feasibility of mussel (Perna perna) and cobia (Rachycentron canadum) produced in a multi-trophic system. Aquac. Int. 2021, 29, 1909–1924. [Google Scholar] [CrossRef]
- Da Silva, E.G.; Castilho-Barros, L.; Henriques, M.B. Economic feasibility of integrated multi-trophic aquaculture (mussel Perna perna, scallop Nodipecten nodosus and seaweed Kappaphycus alvarezii) in Southeast Brazil: A small-scale aquaculture farm model. Aquaculture 2022, 552, 738031. [Google Scholar] [CrossRef]
- Troell, M.; Halling, C.; Nilsson, A.; Buschmann, A.H.; Kautsky, N.; Kautsky, L. Integrated marine cultivation of Gracilaria chilensis (Gracilariales, Rhodophyta) and salmon cages for reduced environmental impact and increased economic output. Aquaculture 1997, 156, 45–61. [Google Scholar] [CrossRef]
- Tran, N.; Le Cao, Q.; Shikuku, K.M.; Phan, T.P.; Banks, L.K. Profitability and perceived resilience benefits of integrated shrimp-tilapia-seaweed aquaculture in North Central Coast, Vietnam. Mar. Policy 2020, 120, 104153. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Kitazawa, D.; Zhou, J.; Park, S.; Gao, S.; Shen, Y. Bio-mitigation based on integrated multi-trophic aquaculture in temperate coastal waters: Practice, assessment, and challenges. Lat. Am. J. Aquat. Res. 2019, 47, 212–223. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally prefabricated marine biomaterials: Isolation and applications of flat chitinous 3D scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, R.; Machado, M.; Afonso, A.; Fierro-Castro, C.; Reyes-López, F.E.; Tort, L.; Gesto, M.; Conde-Sieira, M.; Míguez, J.M.; Soengas, J.L.; et al. Neuroendocrine and immune responses undertake different fates following tryptophan or methionine dietary treatment: Tales from a teleost model. Front. Immunol. 2017, 8, 1226. [Google Scholar] [CrossRef] [Green Version]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine biomaterials: Biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef]
- Chopin, T. Progression of the integrated multi-trophic aquaculture (IMTA) concept and upscaling of IMTA systems towards commercialization. Aquac. Eur. 2011, 36, 5–12. [Google Scholar]
- Soto, D.; Mena, G. Filter feeding by the freshwater mussel, Diplodon chilensis, as a biocontrol of salmon farming eutrophication. Aquaculture 1999, 171, 65–81. [Google Scholar] [CrossRef]
- Viji, C.S. Studies on Integrated Multi-Trophic Aquaculture in a Tropical Estuarine System in Kerala, India. Ph.D. Thesis, Central Institute of Fisheries Education, Mumbai, India, 2015. 128p. [Google Scholar]
- Viji, C.S.; Chadha, N.K.; Kripa, V.; Prema, D.; Prakash, C.; Sharma, R.; Jenni, B.; Mohamed, K.S. Can oysters control eutrophication in an integrated fish-oyster aquaculture system? J. Mar. Biol. Assoc. India 2014, 56, 67–73. [Google Scholar]
- Freitas, J.R.; Morrondo, J.M.S.; Ugarte, J.C. Saccharina latissima (Laminariales, Ochrophyta) farming in an industrial IMTA system in Galicia (Spain). J. Appl. Phycol. 2016, 28, 377–385. [Google Scholar] [CrossRef]
- Kang, Y.H.; Shin, J.A.; Kim, M.S.; Chung, I.K. A preliminary study of the bioremediation potential of Codium fragile applied to seaweed integrated multi-trophic aquaculture (IMTA) during the summer. J. Appl. Phycol. 2008, 20, 183–190. [Google Scholar] [CrossRef]
- Whitmarsh, D.; Palmieri, M.G. Social acceptability of marine aquaculture: The use of survey-based methods for eliciting public and stakeholder preferences. Mar. Policy 2009, 33, 452–457. [Google Scholar] [CrossRef]
- Nikitina, E. The Role of “Green” Licences in Defining Environmental Controls in Norwegian Salmon Aquaculture. Master’s Thesis, UiT The Arctic University of Norway, Tromsø, Norway, 2015. [Google Scholar]
- Ellis, J.; Tiller, R. Conceptualizing future scenarios of integrated multi-trophic aquaculture (IMTA) in the Norwegian salmon industry. Mar. Policy 2019, 104, 198–209. [Google Scholar] [CrossRef]
- Kleitou, P.; Kletou, D.; David, J. Is Europe ready for integrated multi-trophic aquaculture? A survey on the perspectives of European farmers and scientists with IMTA experience. Aquaculture 2018, 490, 136–148. [Google Scholar] [CrossRef] [Green Version]
- Sarà, G.; Mangano, M.C.; Berlino, M.; Corbari, L.; Lucchese, M.; Milisenda, G.; Terzo, S.; Azaza, M.S.; Babarro, J.M.; Bakiu, R.; et al. The synergistic impacts of anthropogenic stressors and COVID-19 on aquaculture: A current global perspective. Rev. Fish. Sci. Aquac. 2021, 30, 123–135. [Google Scholar] [CrossRef]
- Allsopp, M.; Johnston, P.; Santillo, D. Challenging the Aquaculture Industry on Sustainability; Heldringstraat, O., Ed.; Greenpeace International: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Neumann, B.; Ott, K.; Kenchington, R. Strong sustainability in coastal areas: A conceptual interpretation of SDG 14. Sustainability 2017, 12, 1019–1035. [Google Scholar] [CrossRef] [Green Version]
- Visbeck, M.; Kronfeld-Goharani, U.; Neumann, B.; Rickels, W.; Schmidt, J.; van Doorn, E.; Matz-Lück, N.; Proelss, A. A sustainable development goal for the ocean and coasts: Global ocean challenges benefit from regional initiatives supporting globally coordinated solutions. Mar. Policy 2014, 49, 87–89. [Google Scholar] [CrossRef]
- Newton, A. A systems approach for sustainable development in coastal zones. Ecol. Soc. 2012, 17, 41. [Google Scholar] [CrossRef] [Green Version]
- Zhai, T.; Chang, Y.C. Standing of environmental public-interest litigants in China: Evolution, obstacles and solutions. J. Environ. Law 2018, 30, 369–397. [Google Scholar] [CrossRef]
- Partelow, S.; Schlüter, A.; Armitage, D.; Bavinck, M.; Carlisle, K.; Gruby, R.L.; Hornidge, A.K.; Le Tissier, M.; Pittman, J.; Song, A.M.; et al. Environmental governance theories: A review and application to coastal systems. Ecol. Soc. 2020, 25, 19. [Google Scholar] [CrossRef]
- Partelow, S.; Schlüter, A.; OManlosa, A.; Nagel, B.; Octa Paramita, A. Governing aquaculture commons. Rev. Aquac. 2022, 14, 729–750. [Google Scholar] [CrossRef]
| Region/Countries | Aquaculture Ecosystems | References |
|---|---|---|
| Asia (China, Thailand. Cambodia, Vietnam, Indonesia) | Integrated aquaculture benefits millions of rural people. | [35] |
| Asia (China) | Bioremediation and an increase in total yields of up to fifty percent can be achieved with IMTA, which combines fish, shellfish, and seaweed. | [36] |
| Canada | Cooke Aquaculture, the largest salmon aquaculture company in eastern Canada, embraced integrated multi-trophic aquaculture. | [20,30,37] |
| South Africa | Numerous abalone farms using seaweed to filter effluent water and substitute commercial feed. | [33] |
| Extractive/Additional Species | Fed Species | Reference |
|---|---|---|
| Algae | ||
| Gracilaria chouae | Sparus macrocephalus (black sea bream) | [85] |
| Laminaria saccharina | Salmo salar (Atlantic salmon) | |
| Gracilaria sp. | Feneropenaeus indicus | [6] |
| Gracilaria sp. | fish (not specified) | [83] |
| Kappaphycus alvarezii | Rachycentron canadum (cobia) | [6] |
| Chaetomorpha linum Gracilaria bursa-pastoris | Dicentrarchus labrax (European sea bass) Sparus aurata (sea bream) | [82] |
| Bivalves | ||
| Mytilus edulis (blue mussel) | Salmo salar (Atlantic salmon) | [86] |
| Mytilus galloprovincialis | Dicentrarchus labrax (sea bass) Sparus aurata (sea bream) | [87] |
| Mytilus galloprovincialis | fish (not specified) | [84] |
| Echinoderms | ||
| Apostichopus japonics (sea cucumber) | Red sea bream | [88] |
| Apostichopus japonics (sea cucumber) | fish (not specified) | [83] |
| Parastichopus californicus (sea cucumber) | Anoplopoma fimbria (sable fish) | [89] |
| Cucumaria frondose (sea cucumber) | Salmo salar (Atlantic salmon) | [90] |
| Australostichopus mollis (sea cucumber) | Perna canaliculus (green-lipped mussel) | [91] |
| Decapods | ||
| Homarus gammarus (European lobster) | Salmo salar (Atlantic salmon) | [92] |
| Polychaetes | ||
| Sabella spallanzanii | Dicentrarchus labrax (European sea bass) | [93] |
| Sabella spallanzanii | Dicentrarchus labrax (European sea bass) Sparus aurata (sea bream) | [82] |
| Marphysa sp. (mud polychaete) | fish (not specified) | [94] |
| Sponges | ||
| Hymeniacidon perlevis | fish (not specified) | [84] |
| Sarcotragus spinosulus | Dicentrarchus labrax (European sea bass) Sparus aurata (sea bream) | [82] |
| Halisarca caerulea | fish (not specified) | [83] |
| Fish | ||
| Mugil cephalus (grey mullet) | Sparus aurata (gilthead sea bream) | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.; Senff, P.; Glaser, M. Lessons for Coastal Applications of IMTA as a Way towards Sustainable Development: A Review. Appl. Sci. 2022, 12, 11920. https://doi.org/10.3390/app122311920
Hossain A, Senff P, Glaser M. Lessons for Coastal Applications of IMTA as a Way towards Sustainable Development: A Review. Applied Sciences. 2022; 12(23):11920. https://doi.org/10.3390/app122311920
Chicago/Turabian StyleHossain, Amir, Paula Senff, and Marion Glaser. 2022. "Lessons for Coastal Applications of IMTA as a Way towards Sustainable Development: A Review" Applied Sciences 12, no. 23: 11920. https://doi.org/10.3390/app122311920





