Abstract
Phytases are the largest group of feed enzymes increasing the accessibility of organic phosphorus for the animals. Feed phytases are usually sold as dried powder of secreting producers, mainly micellar fungi. We proposed a new technology for producing phytase from Obesumbacterium proteus (OPP) in yeast Yarrowia lipolytica as cytosolic protein (encapsulated OPP), where the capsule (yeast cell) protects the enzyme from unfavourable factors (acid medium and active proteolysis in stomachs) and releases it along with the substrate in the duodenum only. Here we report results of testing the encapsulated OPP on the model of a broiler chicken in comparison to a conventional phytase from Aspergillus ficuum. The encapsulated OPP at a dosage of 30 FYT/kg provided the maximum body weight of the chicken in the end of experiment equal or somewhat higher than in the control group, where the available phosphorus deficit was complemented with a mineral phosphorus supply. In contrast, the conventional soluble phytase at a dosage of 100 or 1000 FYT/kg was not able to compensate for the phosphorus deficit in the diet, although chemical analysis demonstrated much phosphorus in the diet in a non-accessible form. The encapsulated OPP decreased the residual Pi in the chicken faeces by 2.1 times in comparison to the control when added to the diet, whereas the conventional phytases negligibly affected this parameter regardless of the dosage.
1. Introduction
Phytase is one of the main enzymes in the feed and poultry industry [1]. It plays the key role in splitting different kinds of phytate (myo-inositol 1,2,3,4,5,6-hexakisphosphate [2]), which, being the major form of phosphorus and some essential chemical element storage in plants, makes up to 75–80% of total phosphorus in plant seeds [3]. Phytate represents a significant phosphorus share in grains and oilseeds, which cannot be assimilated because of a lack of phytase in the intestines of animals [4]. The available phosphorus demand in animal diets was evaluated as 0.35–0.45% [5]. A diet including corn (~65%) and soybean (~30%) meal possesses 0.13% available phosphorus [6]. Calculation of the data demonstrates that full release of the inorganic phosphate from the phytate in this diet can increase the available phosphorus level up to 0.48% [5].
The efficacy of free phytase decreases due to the following factors: (1) thermal denaturation when the feed is formulated; (2) degradation in the fore stomach and gizzard due to low pH and pepsin; and (3) rapid proteolysis by some proteases along with delayed releasing of the substrate (phytate) from the grain in the duodenum [6]. The Obesumbacterium proteus phytase (OPP) [7] is typical to the members of the family C phytase (PhyC). Its activity reaches its maximum at pH 4.9 and keeps thermal stability up to 60 °C (even at pH 3.0). The phytase in the strain has a maximum activity of ~340 phytase activity units (FYT)/mg. Pichia pastoris is reported to produce the phytase and its modified derivatives with improved thermal stability [8,9,10]. Before, we [11] described another approach to increase the thermal resistance of OPP. The Yarrowia lipolytica yeast produced the enzyme in cytosolic form. The enzyme, encapsulated inside the yeast cell, remained stable upon short heating at 70–80 °C, the temperature of spray drying for feed premixes. Currently, the standard technology for feed enzyme production in a secreted form widely used all over the world permits obtaining pure enzyme of a high activity. The technology is profitable for major manufacturers because of its low prime cost [12]. Nevertheless, it has some disadvantages: the method demands high-quality raw substrate, the waste disposal of the cell mass, power consumption for vacuum concentration, and spray drying of the biomass [13]. The Y. lipolytica yeast is reported to assimilate different kinds of wastes and convert them into a valuable protein. Consequently, the growth of Y. lipolytica recombinant strains, which could produce OPP using waste [14], could let us obtain a feed additive of high thermal stability, grow on fat-containing waste (waste and wastewater of the slaughterhouse, oil and fat industries), and reduce energy consumption for drying. Furthermore, it raises OPP stability in the animal’s stomach and intestine, gradually releasing the phytase as the feed travels in the chyme along the intestine [14].
Animal by-products such as meat, bone meal, and poultry by-product meal are of great significance as feed ingredients in poultry feed, being not very expensive. These animal protein ingredients are good sources of amino acids with high protein levels, total digestible dry matter, and energy. A chicken intestine is rich in protein, but unfortunately it is not used as a protein source in feed additives (e.g., for fish food) [15,16]. Poultry by-products are valuable sources of not only proteins but also lipids. Thus, according to Tabinda and Butt, the lipid amount in a chicken intestine reaches nearly 7.64%, but in the viscera it is from 13.5 to 15.5% [17].
Although the need for the use of microbial phytases in poultry feeding is of no doubt for either poultry keepers or physiologists in the field of animal nutrition, the efficiency of modern technologies for phytases use can be significantly enhanced. The instability of microbial phytases to the acidic pH (pH 2–3) of poultry stomachs in the presence of pepsin is an apparent disadvantage of such technologies. Moreover, the optimum pH values for the phytase classes PhyA (pH 2.2 and pH 5.2) and PhyC (pH 5.5) do not coincide with the pH of the poultry gastrointestinal tract where phytate hydrolysis occurs, namely the duodenal pH (6.4–6.6), the small intestine pH (6.5–7.0), and the colon pH (6.5–7.5) [18]. Consequently, the residual phosphorus in the faeces, even after the phytase action, ranges within 40% to 60% of the initial amount in the feed. A significant increase in the phytase efficacy can be achieved if there are used either phytases with an optimum pH ranging within 6.0–7.5 or encapsulated phytases in the micro-containers that are stable in the poultry stomach but are dissolved in the duodenum.
In this study, we tried to confirm the data on a higher efficacy of the encapsulated OPP compared to the conventional secreted phytase that Danilova et al. reported in their paper [19] using a food-producing animal model (broiler chickens). This is of significance, as the results of the experiment using a mouse model where the encapsulated phytase improved the pivotal zoo technical features, namely absolute body weight throughout the experiment and feed conversion ratio (FCR), cannot be transferred to a food-producing animal model [19]. The recommended dosage of the encapsulated OPP for broiler feeding decreased from 165 FYT/kg to 30 FYT/kg due to the experiment data using a chicken model (Danilova’s unpublished data). This is important, as the phytase activity in the dried cell biomass mixed with residual yeast growth substrate is two to three orders lower than in the highly concentrated conventional phytase preparation produced by drying a cell free culture medium. Moreover, we assayed the residual phosphorus in broilers’ faeces and the content of some other macro and microelements, namely Mg, Ca, K, Zn, and Cu. The reduction of the residual elements in the faeces was considered as independent evidence of the phytase’s ability to suppress the anti-nutritional effect of the grain phytate described before by Mohammadi Ziarat et al. [20].
The influence of the encapsulated and conventional phytases on the broilers’ body weight at the end of the 42-day feeding experiment was considered a cumulative effect of the accelerated phosphorus release from the phytate. This made the phosphorus accessible for the bird organism, suppressing the antinutritional effect of the phytate caused by hindering the absorption of Mg, Ca, K, Zn, Cu, and some other mineral and organic nutrients from the chime [21]. The additive containing encapsulated phytase was produced by using chicken intestine (slaughter house waste) and the fat melted from it as a substrate. The biomass of the non-recombinant (parental) strain Y. lipolytica PO1f was obtained under conditions equivalent to the OPP-producing recombinant strain and used in the diet for a negative control group with regard to equilibrating the nutritional effect of the yeast biomass and residues of the substrate in all groups involved in the experiment.
2. Materials and Methods
2.1. Yeast Strains
The recombinant Y. lipolytica PO1f (pUV3-Op) producing OPP was used as described before [12]. We obtained the parental strain of Y. lipolytica PO1f (MatA, leu2-270, ura3-302, xpr2-322, axp-2) from the CIRM-Levures collection (France). The strain was deposited with the registration number of CLIB-724; it was in ATCC (registration number of MYA-2613).
2.2. Preparing Feed Additives by Cultivation of the Yeast Strain
The Y. lipolytica yeast was raised on a YPD complete solid medium of the following composition (g/L): yeast extract—10, Bacto Peptone—20, glucose—20, and Bacto agar—20. The yeast was cultivated at 28 °C for 48 h. Both the recombinant and parental yeast strains were transferred from one Petri dish (d = 90 mm) to another one three times in the same conditions. Then, the biomass was washed from the Petri dishes (d = 90 mm) with 10 mL of sterile water and used as an inoculum. The culture was raised in batches of 100 mL in a medium of the following composition (g/L): Mg2SO4—0.5, (NH4)2SO4—0.3, KH2PO4—2.0, K2HPO4—0.5, NaCl—0.1, and CaCl2—0.05), with the addition of different kinds of substrates.
The substrate from the waste was prepared as follows: raw chicken intestine with digesta from a slaughterhouse was mixed with distilled water at a 1:1 ratio and cooked at 100 °C for 40 min. The mixture was cooled at room temperature for 2 h, and the melted fat was skimmed with a spoon from the surface and stored at −20 °C in a freezer. The solid phase of the chilled intestine was placed into a plastic vessel and homogenized for 10 min using a hand blender at a rotation of 20,000 rpm and frozen at −20 °C (further referred to as the chicken intestine paste).
The inoculation medium had the following composition (g/L): yeast extract—10, Bacto Peptone—20, fat of the chicken intestine—5, and sophexyl antifoam—0.35 mL/L, at pH 6.1; it was prepared and sterilized in a 10-litre fermenter for an hour (MiniforsInforsAGCH-4103 Bottmingen, Brno, Czech Republic). The inoculum of the recombinant and parental yeast strains was consequently introduced into the fermenter, and cultivation was performed at 28 °C for 21 h at a rotation of 600 rpm and aeration of 3 L/min. The culture medium pH and the partial pressure of oxygen were monitored using a sterilizable Clark-type oxygen sensor with a fast response.
The final cultivation was performed in a 100-litre fermenter with a working volume of 75 litres (LabMakelaa, Baar, Switzerland). The 70 L medium contained yeast extract (Difco, Leeuwarden, The Netherlands)—10 g/L; Bacto Peptone (Difco, Leeuwarden, The Netherlands)—20 g/L; and chicken fat—5 g/L. The defatted chicken intestine paste was placed in the abovementioned volume of medium, and then it was sterilized for 40 min at 120 °C. The initial pH of the cultivation medium was 6.9. The fermentation was performed at 28 °C for 44 h at a rotation of 300 rpm with a sterile air input of 3 L/min. The antifoam solution was prepared using 150 mL sophexyl in 500 mL distilled water, sterilized for 40 min at 120 °C. Following that, 440 mL of the antifoam solution was gradually applied to the medium after 22 h of fermentation. The cultivation medium pH was monitored, and the concentration of dissolved oxygen was measured using the Clark-type electrode with a glass-reinforced TEFLON membrane (Sea&Sun Technology, Trappenkamp, Germany).
After culturing PO1f (pUV3-Op) and PO1f, the cultures were displaced into single vessels (one for each strain). The cell pellets and insoluble residue of the medium were concentrated using flow separation. The biomass was dried using spray drying at 75 °C, with 1 L of the concentrated suspension per hour (Anhydro A/S, Søborg, Denmark). The resulting powder was weighted and assayed for phytase activity. The additive derived from the PO1f (pUV3-Op) strain was used to prepare the diet for the experimental chicken group, and the PO1f-derived powder was used for the diet of the negative control group.
2.3. Assay of the Composition of the Experimental Diets and the Chicken Faeces
All the samples used for element assay were dried at 60 °C until they reached constant weight. Then, 10 g samples were ground in a porcelain mortar, and 0.5 g of sample was picked up for assay. The chemical compositions of the feeds for the experimental groups and the chicken faeces were assayed at Dokuchev Soil Science Institute of the RAS using the method of energy-dispersive X-ray fluorescence EDXRF (Thermo scientific, Waltham, Massachusetts, USA) analysis with the Respect instrument (Tolokonnikov Plant LLC, Moscow, Russia) as it was described in [15].
2.4. Assay of Phytase Activity
The phytase activity in the feed additives was assayed with a colorimetric method to assess the phosphate ion, which is released from phytate as described before [22]. A ten-milligram aliquot of the ground additive was placed into an Eppendorf tube. The sample was mixed with 50 mg of 0.3 mm glass beads and 500 μL of 250 mM sodium acetate buffer at pH 5.5; then, the mixture was kept in an ice-cold bath for 10 min. The swollen cells were homogenised using triple stirring with a vortex for 2 min each. The homogenates were centrifuged at 14,000 g for 10 min.
The suspensions obtained were diluted in a 250 mM sodium acetate buffer with a pH of 5.5, and 5 μL aliquots of the first, second, and fourth dilutions were put into the wells of a flat-bottom immunological plate (Nunc Low-Sorb, Roskilde, Denmark). The medium contained 100 μL of substrate mix (5.1 mM sodium phytate in the same buffer). The plate was closed to prevent exsiccation and exposed to 37 °C heat for an hour. Then, 50 μL of Fiske & Subbarow reagent was applied to each well, and the plate was incubated in the room for 10 min. The absorbance at 415 nm was assayed using a Uniplan plate spectrophotometer (Pikon, Moscow, Russia). The phytase unit was calculated as the amount of the enzyme, which releases 1 µmol of phosphate ions per minute in those conditions.
2.5. Diet Composition for the Experimental Chicken Groups
A complete starter diet PK-5-1 was purchased from Stavropolsky Kombikorm (Stavropolsky Kombikorm, Stavropol, Russia) to feed the chickens beginning from the first day of life. The experimental groups were assembled on the 14th day of the experiment using the method of analogous pairs. The Ladozyme Proxi preparation produced by the Enzyme company (Enzyme, Ladyzhyn, Ukraine) was used as a conventional phytase standard, with a declared activity of 10,000 FYT/g. The recommended dose for the Ladozyme Proxi was 500 FYT/kg (50 g per ton of feed). The basic diet contained (g/kg): corn grain—580, forage wheat—171, sunflower cake—220, fat—40, grass meal—10, chalk—10, and NaCl—5. It was composed of whole grains ground in a mechanical grinder until an average granule size of 0.9 mm was reached, followed by sifting through a sieve. The diets for feeding the experimental groups were produced by applying some other ingredients to the basic diet, as shown in Table 1.

Table 1.
Composition of the diets for the experimental groups of the broilers.
2.6. Birds and Their Keeping
The experimental protocol was approved at the assembly of the Local Ethics Committee of The Vavilov Institute of General Genetics RAS (Protocol No. 1 dated from 02.15.2018). In total, 120 one-day-old old Ross 308 cross broilers were cooped into cages (20 heads in each colony cage for the first 14 days, and then they were reared in mixed-sex groups in floor pens, 20/1394 cm2) in the Skryabin Academy of Veterinary Medicine and Biotechnology vivarium and kept at a temperature of 32 ± 1 °C using a 12 h photoperiod. The chickens had ad libitum access to water. A bird bath per nine heads was provided. The sex of the birds was not distinguished.
For the first 14 days, the chickens were fed using a PK-5-1 complete starter diet from Stavropolsky Kombikorm (Stavropolsky Kombikorm, Stavropol, Russia). The basic feed was used in a crushed form; in the experimental groups, the additives were supplied as powder. Then, every chicken was weighted, and experimental groups of 20 heads each were assembled using the method of analogous pairs. Furthermore, the experiment was performed for 28 days until the 42nd day of the birds’ lives. During this period they were kept on a floor. Every group had ad libitum access to feed. The diet was supplied in excess twice a day to control consumption, and each feed provision was weighed. Before supplying a fresh feed provision, the remaining residue was weighed again, and the amount was subtracted from the initial weight of the feed to determine the feed consumption and calculate the FCR.
2.7. Assay of Body Weight and Statistical Data Analysis
The chickens were weighed weekly on days 14, 21, 28, 35, and 42 of the experiment. Each group was weighed as a whole, and the average bird weight in each group was calculated. The living weight was used as the output parameter. It was expressed as a percentage of the arithmetical mean of the average bird weight in each group compared to the positive control. The FCR was calculated as described in Mohammadi Ziarat et al. [20].
The standard error of the mean (SEM) was calculated as described in [23]. All the data collected were analysed using STATISTICA 10 (2011) (StatSoft, Inc., Tulsa, OK, USA, www.statsoft.com, accessed on 15 September 2022). All the data were presented as group mean values ± standard deviation (mean ± SD), continuous data were compared with ANOVA, and frequency data were compared using a chi-square test. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Feed Additives Production Using the Y. lipolytica Strains
The Y. lipolytica PO1f (pUV3-Op) strain was described before in Isakova et al. [14] and Danilova et al. [19]. However, there were no published data about its cultivation in a fermenter. Therefore, here we demonstrate the results of the encapsulated phytase production in a pilot scale (Table 2). According to Table 2 we can conclude that the recombinant PO1f (pUV3-Op) grows at a slightly lower growth rate than that of the parental Y. lipolytica PO1f. However, both processes of cultivation in a fermenter are similar, and the final biomass yield is only 9.6% lower for the recombinant strain.

Table 2.
Comparison of the cultivation parameters for the Y. lipolytica PO1f and PO1f (pUV3-Op) strains.
3.2. Establishing a Diet for Testing the Impact of the Phytases on Body Weight and FCR
As mentioned in the Introduction, the grain-based diet without bone meal was expected to contain ~0.48% total phosphorus of the dry weight (with 0.13% being available). The available phosphorus deficit in this diet can be assayed as three-fold (~0.35% from the dry weight). The basic feed was divided into batches, and each one was supplied with an additive specific to each experimental group, as described in Materials and Methods. It is worth noting that the diet for the negative control group contained an additive produced by the parental (non-recombinant) Y. lipolytica PO1f strain at a dosage of 1.2% (equal to the highest dosage of the PO1f (pUV3-Op)-derived additive of 30 FYT/kg), whereas the diets containing Ladozyme Proxi commercial phytase contained no yeast-derived additives. Table 3a–c shows the full chemical compositions of the prepared diets.

Table 3.
(a) The elemental analysis of macro- and microelements in the diets for feeding the experimental groups of the broiler chickens. (b) Full diet details for the broiler chickens (the starter). (c) The main diet (the starter one) based on corn.
As can be seen from Table 3a, the total phosphorus amount in all the diets varies from 0.4–0.48%, whereas it is significantly higher in the Ladozyme Proxi (1000 FYT/kg) and positive control groups (0.56 and 0.61%, respectively). Apparently, the diet for the Ladozyme Proxi group contains a large amount of mineral phosphorus and some other elements, namely magnesium and potassium within the filler. The negative control diet contained more Cu than the other diets. Presumably, this microelement was added within the additive based on the parental (non-recombinant) Y. lipolytica PO1f strain.
It is worth noting that the total phosphorus content in the positive control diet made up 0.61% of the dry weight, versus 0.4–0.48% in the other ones. Therefore, the available phosphorus was 0.12–0.2% (0.9–1.5 times) higher than in the basic diet. The chemical composition analysis proved the actual difference between the negative and positive control diets. These diets were used for feeding the broilers in the biological trials. For the first 10 days, 9 chickens out of 119 died (7.5%); throughout the rest of the experiment, the survival rate of Ross 308 cross broiler chickens reached 100%.
3.3. The Influence of the Phytases on Body Weight, Weight Gain, Feed Assimilability, and FCR
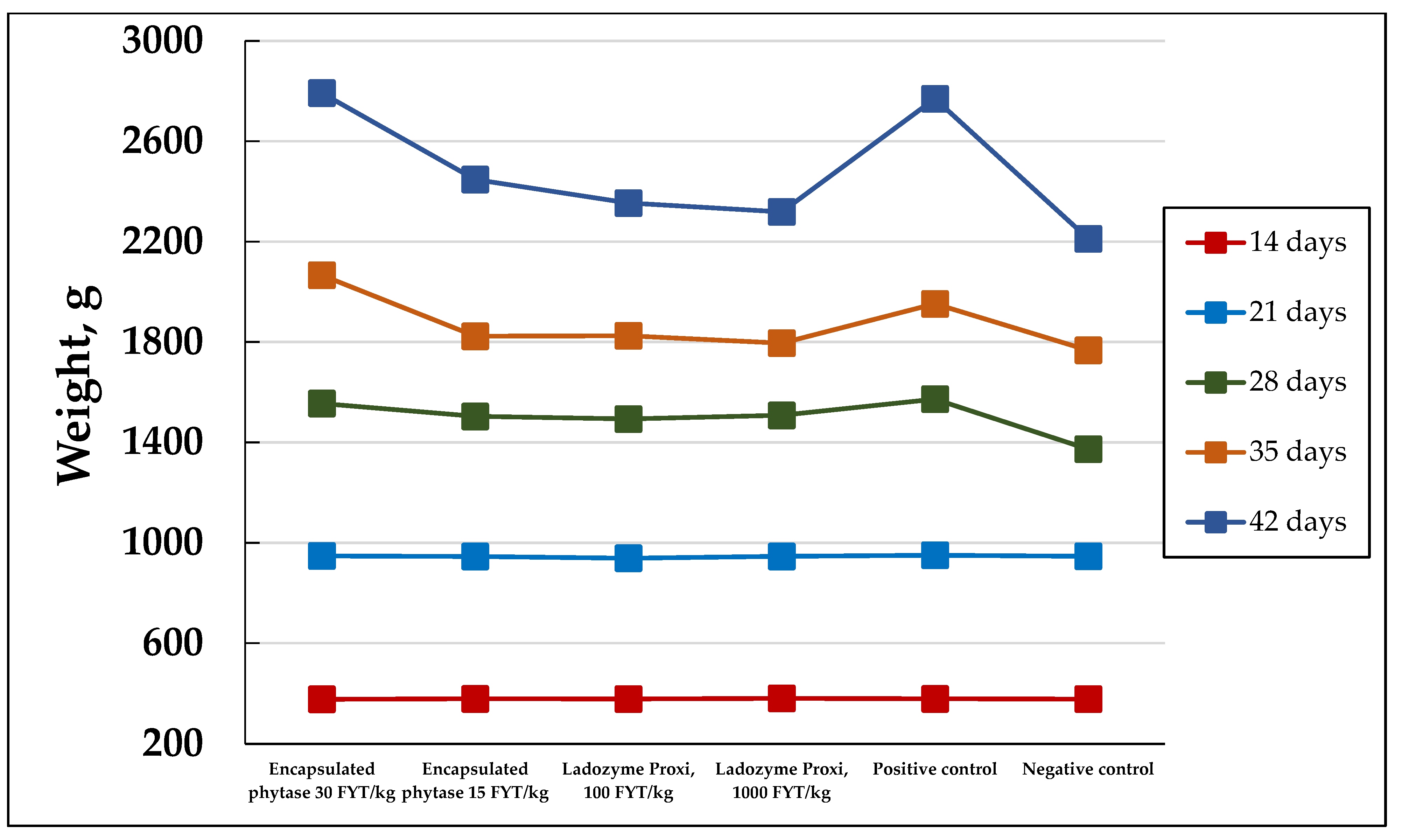
The additives obtained were used for the broilers’ feeds. Some kinds of additives, namely those which were based on the Y. lipolytica PO1f and PO1f (pUV3-Op) strains grown in the waste-containing medium, were added at a maximum dose of 1.2% w/w. This does not exceed the 3% maximal amount recommended for the mixed feed [24]. The specific phytase activity in the dried PO1f (pUV3-Op) additive was 0.25 FYT/g, so the phytase activity in the experimental group diets was 30 and 15 FYT/kg, i.e., 30–60 times less than the recommended one [6,20,25,26]. The Ladozyme Proxi commercial phytase was applied in doses of 100 and 1000 FYT/kg, which were slightly less than and twice as much as, respectively, the recommended dose by the manufacturer (500 FYT/kg). Table 4 and Figure 1 show the dynamics of the body weight of the broilers in the experimental groups.

Table 4.
Weight gain data of the broiler chickens during the experiment.
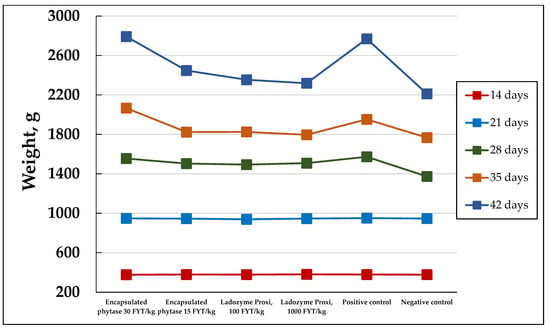
Figure 1.
The dynamics of the body weights of the broiler chickens in the experimental groups.
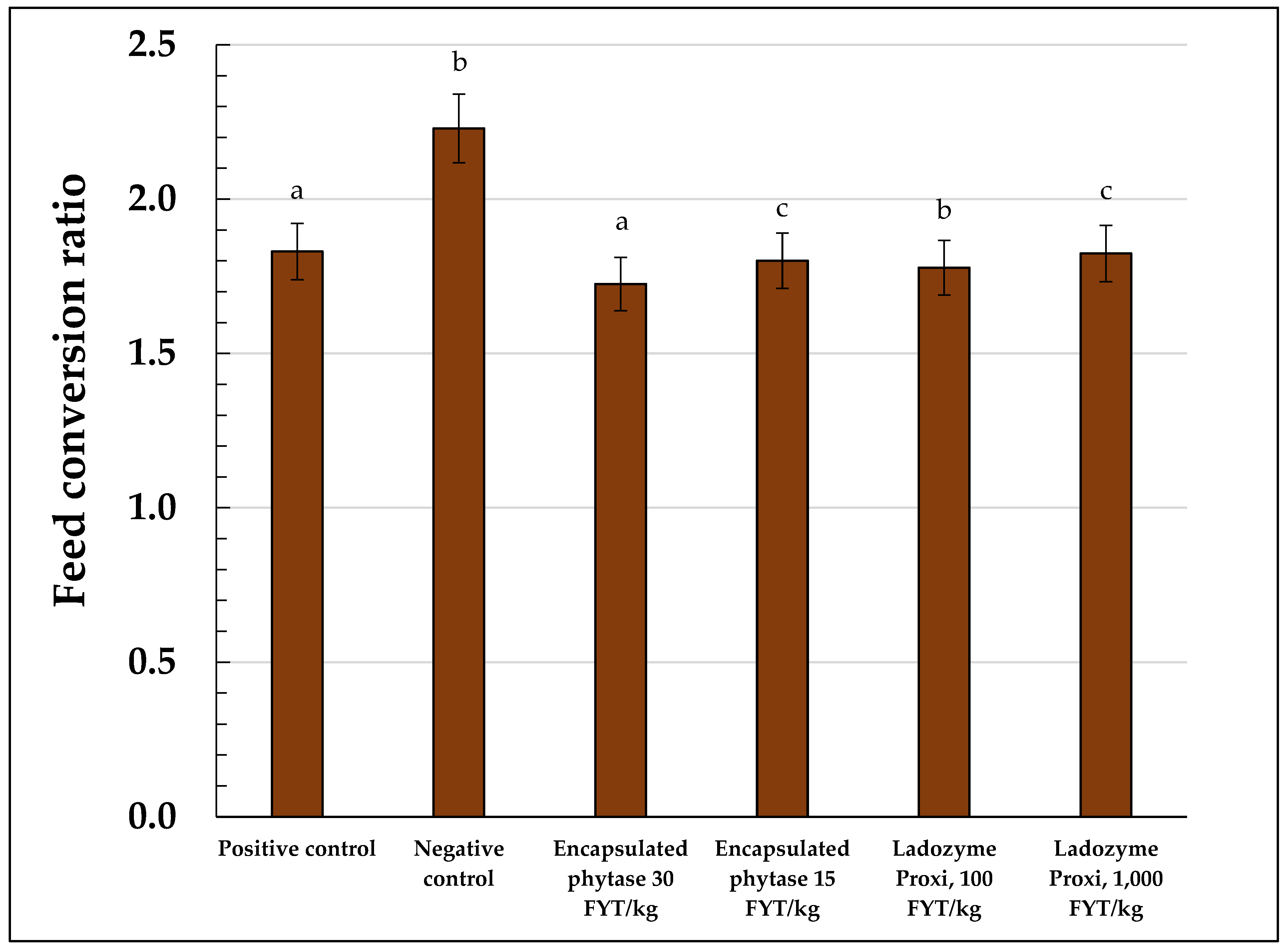
In addition to the average body weight, the FCR was calculated for each experimental group (Table 5, Figure 2). From the time the experimental groups were assembled until the seventh day of the experiment, the FCRs were nearly equal in value in all the groups and varied between 2.0 and 2.1. Then, the FCR in the negative control group (2.1) gradually grew and reached 2.5 on the 42nd day and 2.3 on the 45th day. By contrast, in the positive control group, the FCR decreased and remained around 1.8 throughout the whole experiment. Using the encapsulated OPP at a high dose of 30 FYT/kg increased the FCR up to that in the positive control group. Upon using the conventional phytase as the encapsulated OPP at a low dose of 15 FYT/kg, the FCR significantly changed compared to that in the negative control group. However, the effect was less pronounced compared to that in the group receiving the OPP at a high dose of 30 FYT/kg (Table 5, Figure 2).

Table 5.
The dynamics of the FCRs * of the broilers in the experimental groups.
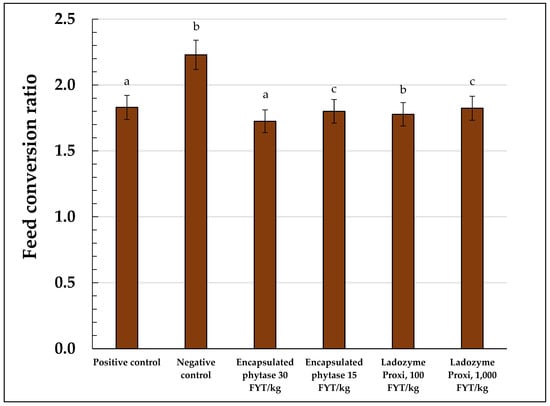
Figure 2.
Feed conversion ratio (FCR) of the broilers in the experimental groups. a—p ≥ 0.05; b—p ≥ 0.02; c—did not differ significantly.
3.4. The Impact of the Phytases on Residual Phosphorus and Macro- and Microelements in the Broilers’ Faeces
Decreasing Pi leakage into ground water is often declared as a substantial reason for the ubiquitous application of phytases, particularly in the Netherlands and other countries of the European Union [27,28]. However, data about the impact of feed phytases on the contents of phosphorus in faeces of productive animals are rare in recent publications. We carried out a regular analysis of the element composition in the diets of all groups and in their faeces. The faeces were collected at the beginning (7th day of the experiment) and at the end of the experiment (28th day of the experiment). About 200 g of faeces was collected from a cage of each broiler group; they were homogenised and dried in air for several days. Then, ~15 g samples were dried in a thermostat at +60 °C; the ~10 g samples were picked up and thoroughly ground in a porcelain mortar with a porcelain pestle. Tablets were formed and used for automated determination of macro- and microelement contents in the samples.
Table 6 shows that using the encapsulated OPP led to the reduction of residual phosphorus by 1.7 times on the 7th day and by 2.1 times on the 28th day of the experiment compared to the negative control. By contrast, using the conventional Ladozyme Proxi phytase led to a 1.5-fold increase in residual phosphorus in the faeces on the 7th and 28th days of the experiment compared to the negative control. Moreover, the residual phosphorus level in the faeces of the group that received encapsulated OPP at 30 FYT/kg was lower by 1.9 and 2.1 times on the 7th and 28th day of the experiment, respectively, than that in the positive control group. The data provide irrefutable proof of the best physiological effect of the encapsulated OPP occurring at a dose of 30 FYT/kg compared to the conventional phytase, even at a dose of 1000 FYT/kg.

Table 6.
The elemental analysis of macro- and microelements in the faeces of the broiler chickens in the experimental groups (n = 11).
The conclusion is confirmed by the fact that the residual contents of essential macroelements, namely Mg, K, and Ca, and the microelements Zn and Cu in the broilers’ faeces are minimal in the encapsulated OPP group with a dose of 30 FYT/kg (Table 6). Their levels are similar to those in the faeces of the negative control group, being significantly lower than that in the positive control and Ladozyme Proxi at 1000 FYT/kg groups. Hard-to-degrade phosphorous organic compounds retain metal ions in the colon and prevent them from being absorbed. However, phytase releases essential macro- and microelements, making them accessible for the chicken organism.
4. Discussions
The model based on weaning mice was proposed to test the impact of the phytases on average daily gain (ADG) [19]. Significant differences in ADG between the positive control (full commercial diet) and the negative one (grain diet without bone meal) on days 5, 10, and 14 of the experiment were shown. Supplying the negative control diet with mineral phosphorus made the ADG in this group indistinguishable from that in the positive control one on days 5, 10, and 14 of the experiment. When testing the commercial Ladozyme Proxi feed additive based on Aspergillus ficuum phytase, which was applied in 30-fold excess of the recommended dose, the experiments showed no difference compared to the negative control; however, the difference was quite significant compared to the positive control on days 10 and 14. The results indicated that the conventional phytase did not affect the ADG when using a mouse model. However, the encapsulated OPP had a reliable and statistically significant effect on ADG. No difference in ADG on days 5 and 10 compared to the positive control group was shown, but a difference for the first threshold on the 14th day was revealed. There was difference between the experimental and negative control groups on days 10 and 14. The lack of difference among the experimental group and both the positive and negative controls on day 5 could be explained by the relatively high average values in all the groups at that time. Taken together, the results obtained confirmed a high influence of the encapsulated OPP on ADG in the suggested mouse model. The feed additive applied upon the culturing the chicken’s fat as the substrate noticeably affected the ADG, even with relatively low phytase activity. The additive supplied at a dose of 165 FYT/kg increased the ADG more than if we used the commercial phytase Ladozyme Proxi, even at a 30-fold overdose.
There is a plethora of experimental results of biological trials of commercial phytase preparations using piglets and broilers [6,20,25,26]. The comparison of the ADG in the diets containing phytase and the phosphate-deficient diets indicates the apparent growth-stimulating effect of the enzymes throughout the whole experiment (Table 5). However, the diet with adequate phosphorus revealed high phytase efficacy only for the animals at an early age—for example, for weaning piglets [26]. As the slaughter time approaches, the efficacy of the mineral phosphorus in the diet is higher than that in the diets with exogenous phytase. Phytase efficacy can be facilitated if the phytase dose increases. Both da Silva et al. [25] and Tsai et al. [6] reported that the highest phytase doses (3000–12,500 phytase activity units, FYT/kg), much higher than the recommended ones, could increase ADG in the experimental group up to the values obtained using an adequate phosphorus diet or even could somewhat surpass those valued. By contrast, the standard phytase dose (250–1000 FYT/kg) has no effect at this life period, although it is quite efficient for young animals. At the late period, in the group with the low-phytase diet, the slaughter weight of barrows was almost the same as those in the group with a phosphorus-deficient diet. The efficacy of low phytase doses in the broilers’ feed is slightly higher than that in the feed of barrows [26].
Our data do not quite agree with the conclusions in the references because we found no substantial influence of the conventional phytase, even if its dose increased from 100 FYT/kg to 1000 FYT/kg. Therefore, the dose of 3000–12,500 recommended by da Silva et al. [25] and Tsai et al. [6] looks unreasonable. The difference in the recommended doses may be caused by different approaches to diet composition. All the referenced authors performed their trials using a well-balanced diet with either a faint or non-existent phosphorus deficit. By contrast, we used an original diet without bone meal based on grain only to make the effect of the phytase more pronounced. The final body weight in the negative control group was 20% less than that in the positive control group, where the phosphorus deficit was compensated with 0.5% disodium hydrophosphate dehydrate; this could confirm the conclusion. This fact shows that the phosphorus deficit was indeed the crucial nutritional factor limiting the chickens’ growth. The application of the encapsulated OPP to the diet increased the chickens’ growth rate to the level of that in the positive control group or even higher. In turn, using conventional phytase at either dose increased the growth rate by 5–6% only compared to the 20% difference between the positive and negative control groups. The data show a qualitatively better effect of the encapsulated phytase compared to the free enzyme, which cannot be compensated by increasing doses. On the other hand, the encapsulated OPP at a dose of 15 FYT/kg was much less efficient than at a dose of 30 FYT/kg and demonstrated efficacy comparable to the conventional phytase from A. ficuum at doses of both 100 and 1000 FYT/kg. This makes us suppose that the encapsulated OPP is effective only within narrow dose range. This shortcoming could be practically important because the molar activity of the encapsulated phytase is low, and a feed yeast overdose may impact chickens unfavourably. It is worth noting that the best FCR values reached in our study (the positive control group and the group ‘OPP at a high dose of 30 FYT/kg’) are substantially higher than the reported ones in the literature [6,25].
The elemental analysis of the chicken faeces from the positive and negative control groups demonstrates that the amount of accessible phosphorus, which is most responsible for difference in the growth rate between these groups, is relatively small compared to the total phosphorus amount in the diet. This conclusion is confirmed by the direct assay of the phosphorus in the negative and positive control group diets. Applying the encapsulated phytase nearly halves the residual phosphorus in the faeces compared to both control groups. In our opinion, this is a key argument in favour of the high efficacy of encapsulated phytase in the intestinal tract of the broilers, unlike that of the free enzyme. Along with the phosphorus release, the encapsulated OPP at a high dose of 30 FYT/kg significantly decreases the residual contents of valuable macro- and microelements, namely Mg, K, Ca, Zn, and Cu. This observation, presumably caused by the anti-nutritional effect of the grain phytase on the colon absorption, explains why the chicken growth rate at a high dose of 30 FYT/kg can be even higher than that in the positive control group. Our data agreed well with the results of Plumstead et al., who during the laying period observed that a decrease in non-phytate phosphorus from 0.37 to 0.09% with added phytase at a dose of 500 FTU/kg led to the reduction of both total manure p and water-soluble p by 42% [29].
Taken together, the obtained results suggest we can suppose that the microencapsulation technology is a highly promising approach to producing feed phytase.
Author Contributions
M.A.D. performed the animal experiments and performed the experiment on the birds; E.Y.E. developed the encapsulated phytase specimen and performed the preparations of the transformant cultures; E.V.T. performed the statistical data analysis; N.V.B. cultured the yeast in the fermenter; A.S.K. and M.S.P. performed data analysis and participated in the writing of the manuscript; Y.I.D. designed the experiments and wrote the final draft of the paper with E.P.I., and E.P.I. conceived the project and participated in all aspects of the study and the writing of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by the Russian Science Foundation (Grant No 22-16-00093 dated from 12 May 2022).
Institutional Review Board Statement
The experimental animal protocol was approved at the assembly of the Local Ethics Committee of The Vavilov Institute of General Genetics RAS (Protocol No. 1 dated from 15 February 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alam, S.; Masood, S.; Zaneb, H.; Rabbani, I.; Khan, R.U.; Shah, M.; Ashraf, S.; Alhidary, I.A. Effect of Bacillus cereus and Phytase on the Expression of Musculoskeletal Strength and Gut Health in Japanese Quail (Coturnix japonica). J. Poult. Sci. 2020, 57, 200–204. [Google Scholar] [CrossRef]
- Kryukov, V.S.; Glebova, I.V.; Zinoviev, S.V. Reevaluation of Phytase Action Mechanism in Animal Nutrition. Biochemistry 2021, 86 (Suppl. 1), S152–S165. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, B.; Raigond, P.; Sahu, C.; Mishra, U.N.; Sharma, S.; Lal, M.K. Phytic acid: Blessing in disguise, a prime compound required for both plant and human nutrition. Food Res. Int. 2021, 142, 110193. [Google Scholar] [CrossRef]
- Morgan, N.K.; Walk, C.L.; Bedford, M.R.; Burton, E.J. Contribution of intestinal- and cereal-derived phytase activity on phytate degradation in young broilers. Poult. Sci. 2015, 94, 1577–1583. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Nutrient Requirements of Swine, 10th ed.; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- Tsai, T.C.; Dove, R.; Bedford, M.R.; Azain, M.J. Effect of phytase on phosphorous balance in 20-kg barrows fed low or adequate phosphorous diets. Anim. Nutr. 2020, 6, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zinin, N.V.; Serkina, A.V.; Gelfand, M.S.; Shevelev, A.B.; Sineoky, S.P. Gene cloning, expression and characterization of novel phytase from Obesumbacterium proteus. FEMS Microbiol. Lett. 2004, 236, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Gordeeva, T.L.; Borshchevskaya, L.N.; Kalinina, A.N.; Sineoky, S.P.; Kashirskaya, M.D.; Voronin, S.P. Increase in Thermal Stability of Phytase from Citrobacter freundii by Site-Directed Saturation Mutagenesis. Biotekhnologiya 2018, 34, 33–42. [Google Scholar] [CrossRef]
- Gordeyeva, T.L.; Borshchevskaya, L.N.; Kalininam, A.N.; Sineokiy, S.P.; Voronin, S.P.; Kashirskaya, M.D. Rekombinantnyy produtsent kormovogo fermenta fitazy na osnove drozhzhey Pichia pastoris. Aktual. Biotekhnologiya 2018, 26, 117. [Google Scholar]
- Gordeeva, T.L.; Borshchevskaya, L.N.; Kalinina, A.N.; Sineokym, S.P.; Voronin, S.P.; Kashirskaya, M.D. Expression and characteristics of phytases from Obesumbacterium proteus in Pichia pastoris Yeast. Biotekhnologiya 2018, 34, 18–25. [Google Scholar] [CrossRef]
- Serdyuk, E.G.; Isakova, E.P.; Gessler, N.N.; Trubnikova, E.V.; Antipov, A.N.; Deryabina, Y.I. Activity of neutral phytase from Obesumbacterium proteus in recombinant strains of Yarrowia lipolytica under cultivation on low-grade vegetable substrate. Appl. Biochem. Microbiol. 2019, 55, 549–555. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Jaiswal, A.K.; Krishna, S.; Pandey, A. Thermostable phytase in feed and fuel industries. Bioresour. Technol. 2019, 278, 400–407. [Google Scholar] [CrossRef]
- Isakova, E.P.; Serdyuk, E.G.; Gessler, N.N.; Trubnikova, E.V.; Biryukova, Y.K.; Epova, E.Y.; Deryabina, Y.I.; Nikolaev, A.V. A new recombinant strain of Yarrowia lipolytica producing encapsulated phytase from Obesumbacterium proteus. Dokl. Biochem. Biophys. 2018, 481, 201–204. [Google Scholar] [CrossRef]
- Tabinda, A.B.; Ghazala, R.; Yasar, A.; Ashraf, M. Utilization of chicken intestine as an alternative protein source in the diet for fingerlings of Cirrhinus mirigala. J. Anim. Plant Sci. 2013, 23, 1603–1608. [Google Scholar]
- Tabinda, A.B.; Butt, A. Replacement of Fish Meal with Poultry By–Product Meal (Chicken Intestine) as a Protein Source in Grass Carp Fry Diet. Pak. J. Zool. 2012, 44, 1373–1381. [Google Scholar]
- Alidadi, H.; Salmani, E.R.; Hamidi, M.R. Assessing fat and aquaculture feed recyclable from chicken wastes of poultry slaughterhouse in Bojnoord, North Khorasan Province, Iran. Arch. Agric. Environ. Sci. 2017, 2, 270–276. [Google Scholar] [CrossRef]
- Ptak, A.; Bedford, M.R.; Świątkiewicz, S.; Żyła, K.; Józefiak, D. Phytase modulates ileal microbiota and enhances growth performance of the broiler chickens. PLoS ONE 2015, 10, e0119770. [Google Scholar] [CrossRef]
- Danilova, M.A.; Epova, E.Y.; Trubnikova, E.V.; Shevelev, A.B. A Feed Additive Containing Encapsulated 6-Phytase within Recombinant Yarrowia lipolytica Cells Produced by Cultivation on Fat-Containing Waste. Appl. Sci. 2022, 12, 3094. [Google Scholar] [CrossRef]
- Ziarat, M.M.; Kermanshahi, H.; Mogaddam, H.N.; Heravi, R.M. Performance of an Escherichia coli phytase expressed in Lactococcus lactis on nutrient retention, bone traits and intestinal morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2020, 104, 909–917. [Google Scholar] [CrossRef]
- Gessler, N.N.; Serdyuk, E.G.; Isakova, E.P.; Deryabina, Y.I. Phytases and the Prospects for Their Application (Review). Appl. Biochem. Microbiol. 2018, 54, 352–360. [Google Scholar] [CrossRef]
- Savichev, A.T.; Sorokin, S.E. Rentgenofluorestsentnyy energodispersionnyy analiz zol’nykh elementov v rasteniyakh. Agrokhimiya 2001, 12, 61–67. [Google Scholar]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Norton, J.D.; Yang, S.P.; Diffley, P. Influence of source and quantity of protein on the development of immunity and resistance to African trypanosomiasis. Infect. Immun. 1986, 51, 455–460. [Google Scholar] [CrossRef]
- Czech, A.; Smolczyk, A.; Grela, E.R.; Kiesz, M. Effect of dietary supplementation with Yarrowia lipolytica or Saccharomyces cerevisiae yeast and probiotic additives on growth performance, basic nutrients digestibility and biochemical blood profile in piglets. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1720–1730. [Google Scholar] [CrossRef]
- da Silva, C.A.; Callegari, M.A.; Dias, C.P.; Bridi, A.M.; Pierozan, C.R.; Foppa, L.; da Silva Martins, C.C.; Dias, F.T.F.; Passos, A.; Hermes, R. Increasing doses of phytase from Citrobacter braakii in diets with reduced inorganic phosphorus and calcium improve growth performance and lean meat of growing and finishing pigs. PLoS ONE 2019, 14, e0217490. [Google Scholar] [CrossRef]
- Srikanthithasan, K.; Macelline, S.P.; Wickramasuriya, S.S.; Tharangani, H.; Li-Ang; Jayasena, D.D.; Heo, J.-M. Effects of adding phytase from Aspergillus niger to a low phosphorus diet on growth performance, tibia characteristics, phosphorus excretion, and meat quality of broilers 35 days after hatching. J. Poult. Sci. 2020, 57, 28–36. [Google Scholar] [CrossRef]
- Moore, P.A., Jr.; Daniel, T.C.; Edwards, D.R. Reducing phosphorus runoff and improving poultry production with alum. Poult. Sci. 1999, 78, 692–698. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994. [Google Scholar]
- Plumstead, P.W.; Romero-Sanchez, H.; Maguire, R.O.; Gernat, A.G.; Brake, J. Effects of phosphorus level and phytase in broiler breeder rearing and laying diets on live performance and phosphorus excretion. Poult. Sci. 2007, 86, 225–231. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).