Influence of the Metal Surface Texture on the Possibility of Controlling the Phase Transition of Water Droplets in the Single-Phase Regime
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Processing
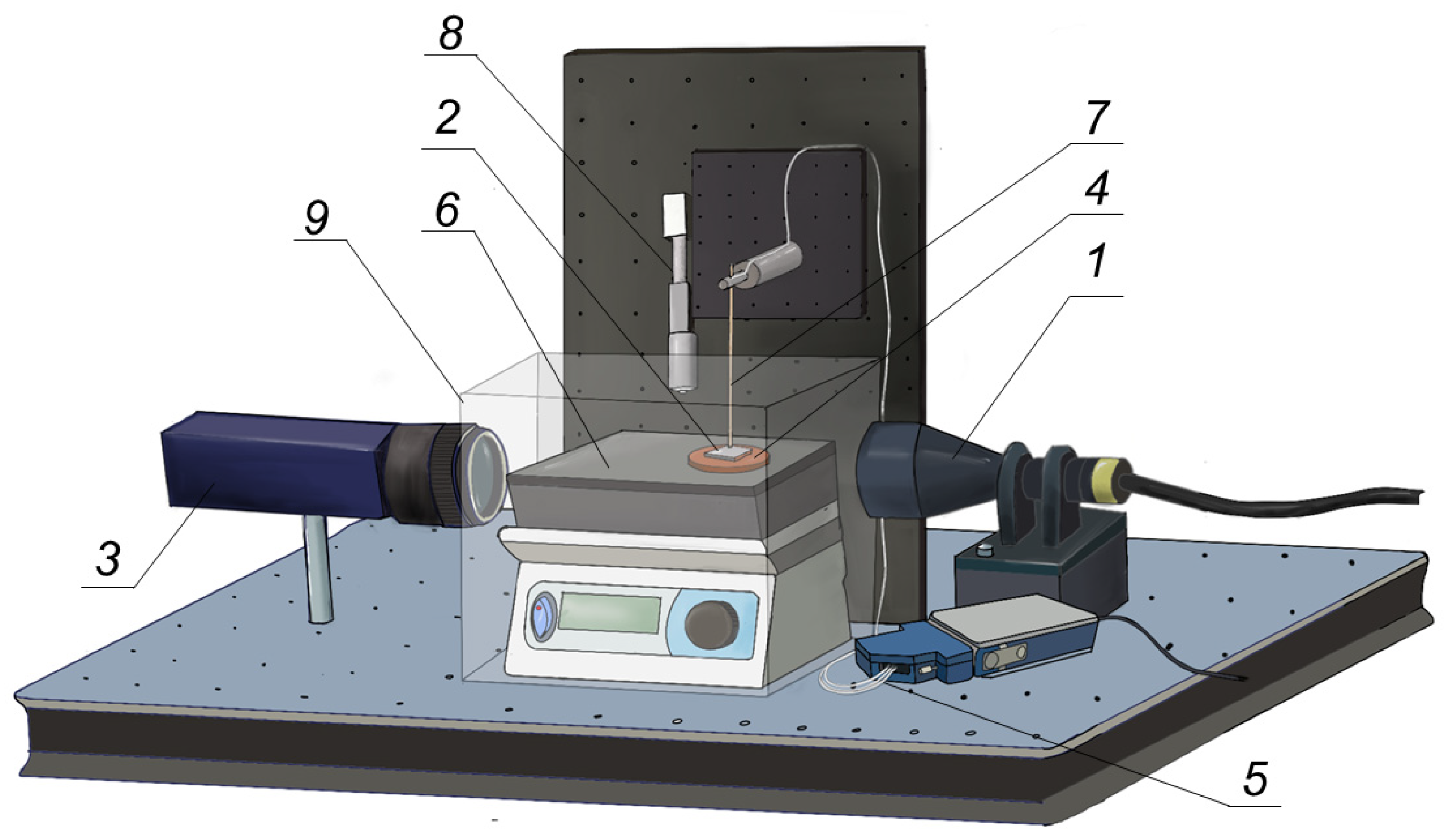
2.2. Equipment and Methods Used for Studying Droplet Evaporation on Metal Surfaces
2.3. Equipment and Methods for Studying the Surfaces of Samples
3. Results and Discussion
3.1. Analysis of the Surfaces of Samples under Study
3.2. Influence of the Metal Surface Texture on the Possibility of Controlling the Phase Transition of water Droplets in the Single-Phase Regime
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CCD | constant contact diameter |
| Nomenclature | |
| d | contact diameter, mm; |
| S | surface area of a droplet, mm2; |
| Sa | arithmetic mean surface deviation, µm; |
| Sdr | developed interfacial area ratio, %; |
| Sz | maximum height, µm; |
| T | temperature, °C; |
| V | volume, μL; |
| Wspecific | specific evaporation rate, kg/(s∙m2); |
| Wtotal | total evaporation rate, kg/s |
| Greek symbols | |
| θ | contact angle, °; |
| Δθ | contact angle hysteresis, °; |
| ρ | liquid density, kg/m3; |
| τ | evaporation droplet time, s; |
| η | average grain size, μm |
References
- Sawan, M.E.; Carbon, M.W. A Review of Spray-Cooling and Bottom-Flooding Work for Lwr Cores. Nucl. Eng. Des. 1975, 32, 191–207. [Google Scholar] [CrossRef]
- Christensen, A.; Graham, S. Thermal Effects in Packaging High Power Light Emitting Diode Arrays. Appl. Therm. Eng. 2009, 29, 364–371. [Google Scholar] [CrossRef]
- Liang, G.; Mudawar, I. Review of Spray Cooling—Part 1: Single-Phase and Nucleate Boiling Regimes, and Critical Heat Flux. Int. J. Heat Mass Transf. 2017, 115, 1174–1205. [Google Scholar] [CrossRef]
- Datrice, N.; Ramirez-San-Juan, J.; Zhang, R.; Meshkinpour, A.; Aguilar, G.; Nelson, J.S.; Kelly, K.M. Cutaneous Effects of Cryogen Spray Cooling on In Vivo Human Skin. Dermatol. Surg. 2006, 32, 1007–1012. [Google Scholar] [CrossRef]
- Smakulski, P.; Pietrowicz, S. A Review of the Capabilities of High Heat Flux Removal by Porous Materials, Microchannels and Spray Cooling Techniques. Appl. Therm. Eng. 2016, 104, 636–646. [Google Scholar] [CrossRef]
- Kandlikar, S.G. Review and Projections of Integrated Cooling Systems for Three-Dimensional Integrated Circuits. J. Electron. Packag. 2014, 136, 024001. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Yang, J.; Tang, C.; Lv, J.; Liu, Y.; Mei, J.; Lau, W.M.; Hui, D. Fabrication of Polydimethylsiloxane Films with Special Surface Wettability by 3D Printing. Compos. Part B Eng. 2017, 129, 58–65. [Google Scholar] [CrossRef]
- Ronshin, F.V.; Chinnov, E.A.; Dementyev, Y.A.; Kabov, O.A. The Bridge Flow Regime in Microchannels. Dokl. Phys. 2021, 66, 229–233. [Google Scholar] [CrossRef]
- Eng Ewe, W.; Fudholi, A.; Sopian, K.; Solomin, E.; Hossein Yazdi, M.; Asim, N.; Fatima, N.; Pikra, G.; Sudibyo, H.; Fatriasari, W.; et al. Jet Impingement Cooling Applications in Solar Energy Technologies: Systematic Literature Review. Therm. Sci. Eng. Prog. 2022, 34, 101445. [Google Scholar] [CrossRef]
- Kabov, O.A.; Gatapova, E.Y.; Semenov, A.A.; Jutley, M.; Ajaev, V.V.; Kirichenko, E.O.; Feoktistov, D.V.; Kuznetsov, G.V.; Zaitsev, D.V. Experimental and Numerical Studies of Evaporation of a Sessile Water Drop on a Heated Conductive Substrate. Interfacial Phenom. Heat Transf. 2018, 6, 421–435. [Google Scholar] [CrossRef]
- Gao, X.; Li, R. Spray Impingement Cooling: The State of the Art. Adv. Cool. Technol. Appl. 2018. [Google Scholar] [CrossRef] [Green Version]
- Hamann, H.F.; Weger, A.; Lacey, J.A.; Hu, Z.; Bose, P.; Cohen, E.; Wakil, J. Hotspot-Limited Microprocessors: Direct Temperature and Power Distribution Measurements. IEEE J. Solid-State Circuits 2007, 42, 56–64. [Google Scholar] [CrossRef]
- Mudawar, I.; Bharathan, D.; Kelly, K.; Narumanchi, S. Two-Phase Spray Cooling of Hybrid Vehicle Electronics. IEEE Trans. Compon. Packag. Technol. 2009, 32, 501–512. [Google Scholar] [CrossRef]
- Lin, L.; Ponnappan, R. Heat Transfer Characteristics of Spray Cooling in a Closed Loop. Int. J. Heat Mass Transf. 2003, 46, 3737–3746. [Google Scholar] [CrossRef]
- Mudawar, I.; Valentine, W.S. Determination of the Local Quench Curve for Spray-Cooled Metallic Surfaces. J. Heat Treat. 1989, 7, 107–121. [Google Scholar] [CrossRef]
- Chen, R.H.; Chow, L.C.; Navedo, J.E. Effects of Spray Characteristics on Critical Heat Flux in Subcooled Water Spray Cooling. Int. J. Heat Mass Transf. 2002, 45, 4033–4043. [Google Scholar] [CrossRef]
- Bernardin, J.D.; Mudawar, I. The Leidenfrost Point: Experimental Study and Assessment of Existing Models. J. Heat Transf. 1999, 121, 894–903. [Google Scholar] [CrossRef]
- Auliano, M.; Auliano, D.; Fernandino, M.; Zhang, P.; Dorao, C.A. Water Droplet Dynamics on a Heated Nanowire Surface. Appl. Phys. Lett. 2018, 113, 253703. [Google Scholar] [CrossRef]
- Duursma, G.; Kennedy, R.; Sefiane, K.; Yu, Y. Leidenfrost Droplets on Microstructured Surfaces. Heat Transf. Eng. 2016, 37, 1190–1200. [Google Scholar] [CrossRef] [Green Version]
- Pais, M.R.; Chow, L.C.; Mahefkey, E.T. Surface Roughness and Its Effects on the Heat Transfer Mechanism in Spray Cooling. J. Heat Transf. 1992, 114, 211–219. [Google Scholar] [CrossRef]
- Sehmbey, M.S.; Chow, L.C.; Hahn, O.J.; Pais, M.R. Spray Cooling of Power Electronics at Cryogenic Temperatures. J. Thermophys. Heat Transf. 2012, 9, 123–128. [Google Scholar] [CrossRef]
- Lester Ortiz, J.E.G. Experiments on Steady-State High Heat Fluxes Using Spray Cooling. Exp. Heat Transf. 2010, 12, 215–233. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Jiang, P.X. Experimental Investigation of Spray Cooling on Flat and Enhanced Surfaces. Appl. Therm. Eng. 2013, 51, 102–111. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Luo, S.Y.; Lee, R.Y.; Liu, H.H. Spray Cooling Heat Transfer on Microstructured Thin Film Enhanced Surfaces. Exp. Therm. Fluid Sci. 2015, 68, 123–134. [Google Scholar] [CrossRef]
- Martínez-Galván, E.; Antón, R.; Ramos, J.C.; Khodabandeh, R. Influence of Surface Roughness on a Spray Cooling System with R134a. Part I: Heat Transfer Measurements. Exp. Therm. Fluid Sci. 2013, 46, 183–190. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Islamova, A.G.; Orlova, E.G.; Ivashutenko, A.S.; Shanenkov, I.I.; Zykov, I.Y.; Feoktistov, D.V. Influence of Roughness on Polar and Dispersed Components of Surface Free Energy and Wettability Properties of Copper and Steel Surfaces. Surf. Coat. Technol. 2021, 422, 127518. [Google Scholar] [CrossRef]
- Olek, S.; Zvirin, Y.; Elias, E. The Relation between the Rewetting Temperature and the Liquid-Solid Contact Angle. Int. J. Heat Mass Transf. 1988, 31, 898–902. [Google Scholar] [CrossRef]
- Schroeder-Richter, D.; Bartsch, G. The Leidenfrost Phenomenon Caused by a Thermo-Mechanical Effect of Transition Boiling: A Revisited Problem of Non-Equilibrium Thermodynamics; ASME: New York, NY, USA, 1990. [Google Scholar]
- Segev, A.; Bankoff, S.G. The Role of Adsorption in Determining the Minimum Film Boiling Temperature. Int. J. Heat Mass Transf. 1980, 23, 637–642. [Google Scholar] [CrossRef]
- Ajaev, V.S.; Kabov, O.A. Levitation and Self-Organization of Droplets. Annu. Rev. Fluid Mech. 2021, 53, 203–225. [Google Scholar] [CrossRef]
- Kim, J.H.; You, S.M.; Choi, S.U.S. Evaporative Spray Cooling of Plain and Microporous Coated Surfaces. Int. J. Heat Mass Transf. 2004, 47, 3307–3315. [Google Scholar] [CrossRef]
- Chien, L.H.; Wu, T.L.; Lee, S.C. A Study of Spray-Impingement Cooling on Smooth and Pin-Finned Surfaces Using FC-72. J. Enhanc. Heat Transf. 2011, 18, 375–387. [Google Scholar] [CrossRef]
- Tran, T.; Staat, H.J.J.; Susarrey-Arce, A.; Foertsch, T.C.; Van Houselt, A.; Gardeniers, H.J.G.E.; Prosperetti, A.; Lohse, D.; Sun, C. Droplet Impact on Superheated Micro-Structured Surfaces. Soft Matter 2013, 9, 3272–3282. [Google Scholar] [CrossRef]
- Geraldi, N.R.; McHale, G.; Xu, B.B.; Wells, G.G.; Dodd, L.E.; Wood, D.; Newton, M.I. Leidenfrost Transition Temperature for Stainless Steel Meshes. Mater. Lett. 2016, 176, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Truong, B.; Buongiorno, J.; Hu, L.W. On the Effect of Surface Roughness Height, Wettability, and Nanoporosity on Leidenfrost Phenomena. Appl. Phys. Lett. 2011, 98, 083121. [Google Scholar] [CrossRef]
- Xu, R.N.; Cao, L.; Wang, G.Y.; Chen, J.N.; Jiang, P.X. Experimental Investigation of Closed Loop Spray Cooling with Micro- and Hybrid Micro-/Nano-Engineered Surfaces. Appl. Therm. Eng. 2020, 180, 115697. [Google Scholar] [CrossRef]
- Berczeli, M.; Weltsch, Z. Enhanced Wetting and Adhesive Properties by Atmospheric Pressure Plasma Surface Treatment Methods and Investigation Processes on the Influencing Parameters on HIPS Polymer. Polymers 2021, 13, 901. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Sun, J.; Li, J.; Xiang, C.; Che, L.; Wang, Z.; Zhou, X. Long-Range Spontaneous Droplet Self-Propulsion on Wettability Gradient Surfaces. Sci. Rep. 2017, 7, 7552. [Google Scholar] [CrossRef] [Green Version]
- Ng, W.H.; Lu, Y.; Liu, H.; Carmalt, C.J.; Parkin, I.P.; Kenyon, A.J. Controlling and Modelling the Wetting Properties of III-V Semiconductor Surfaces Using Re-Entrant Nanostructures. Sci. Rep. 2018, 8, 3544. [Google Scholar] [CrossRef] [Green Version]
- Akbari, R.; Mohammadizadeh, M.R.; Antonini, C.; Guittard, F.; Darmanin, T. Controlling Morphology and Wettability of Intrinsically Superhydrophobic Copper-Based Surfaces by Electrodeposition. Coatings 2022, 12, 1260. [Google Scholar] [CrossRef]
- Lasagni, A.F.; Alamri, S.; Aguilar-Morales, A.I.; Rößler, F.; Voisiat, B.; Kunze, T. Biomimetic Surface Structuring Using Laser Based Interferometric Methods. Appl. Sci. 2018, 8, 1260. [Google Scholar] [CrossRef]
- Ponomarev, K.O.; Kuznetsov, G.V.; Orlova, E.G.; Feoktistov, D.V. Thermosyphon-Assisted Cooling System Working in the Moderate Heat Flux Range. Therm. Sci. Eng. Prog. 2022, 32, 101330. [Google Scholar] [CrossRef]
- Misyura, S.Y.; Feoktistov, D.V.; Morozov, V.S.; Orlova, E.G.; Kuznetsov, G.V.; Islamova, A.G. Effect of Heat Treatment on Corrosion of Laser-Textured Aluminum Alloy Surfaces. J. Mater. Sci. 2021, 56, 12845–12863. [Google Scholar] [CrossRef]
- Gatapova, E.Y.; Semenov, A.A.; Zaitsev, D.V.; Kabov, O.A. Evaporation of a Sessile Water Drop on a Heated Surface with Controlled Wettability. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 776–785. [Google Scholar] [CrossRef]
- Fornasini, P. The Uncertainty in Physical Measurements; Springer: New York, NY, USA, 2008; ISBN 9780333227794. [Google Scholar]
- Feoktistov, D.V.; Glushkov, D.O.; Kuznetsov, G.V.; Orlova, E.G.; Paushkina, K.K. Ignition and Combustion Enhancement of Composite Fuel in Conditions of Droplets Dispersion during Conductive Heating on Steel Surfaces with Different Roughness Parameters. Fuel 2022, 314, 122745. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Misyura, S.Y.; Volkov, R.S.; Morozov, V.S. Marangoni Flow and Free Convection during Crystallization of a Salt Solution Droplet. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 37–46. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Boinovich, L.B.; Emelyanenko, K.A. Spreading of Biologically Relevant Liquids over the Laser Textured Surfaces. J. Colloid Interface Sci. 2020, 567, 224–234. [Google Scholar] [CrossRef]
- Feoktistov, D.; Orlova, E.; Islamova, A. Spreading Modes on Copper and Steel Surfaces. In Proceedings of the EPJ Web of Conferences, Tomsk, Russia, 13–15 October 2016; Volume 110. [Google Scholar]
- Emel’yanenko, A.M.; Boinovich, L.B. Analysis of Wetting as an Efficient Method for Studying the Characteristics of Coatings and Surfaces and the Processes That Occur on Them: A Review. Inorg. Mater. 2011, 47, 1667–1675. [Google Scholar] [CrossRef]
- Emelyanenko, A.M.; Ermolenko, N.V.; Boinovich, L.B. Contact Angle and Wetting Hysteresis Measurements by Digital Image Processing of the Drop on a Vertical Filament. Colloids Surf. A Physicochem. Eng. Asp. 2004, 239, 25–31. [Google Scholar] [CrossRef]
- Czerwiec, T.; Tsareva, S.; Andrieux, A.; Bortolini, G.A.; Bolzan, P.H.; Castanet, G.; Gradeck, M.; Marcos, G. Thermal Management of Metallic Surfaces: Evaporation of Sessile Water Droplets on Polished and Patterned Stainless Steel. IOP Conf. Ser. Mater. Sci. Eng. 2017, 258, 012003. [Google Scholar] [CrossRef]
- De Gennes, P.G. Wetting: Statics and Dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]






| Cu-ETP Copper Surfaces | |||
|---|---|---|---|
| SEM images |  |  |  |
| Roughness | Sa = 0.06 ± 0.005 μm; Sz = 1.1 ± 0.1 μm; Sdr = 0.2 ± 0.02% | Sa = 0.15 ± 0.007 μm; Sz = 2.3 ± 0.15 μm; Sdr = 1.4 ± 0.05% | Sa = 0.22 ± 0.01 μm; Sz = 2.9 ± 0.2 μm; Sdr = 1.8 ± 0.06% |
| Wetting | θ ± Δθ = 66 ± 5° d = 4.1 ± 0.2 mm | θ ± Δθ = 78 ± 5° d = 3.8 ± 0.15 mm | θ ± Δθ = 83 ± 6° d = 3.7 ± 0.12 mm |
| SEM images |  |  |  |
| Roughness | Sa = 0.24 ± 0.01 μm; Sz = 3.2 ± 0.2 μm, Sdr = 2.7 ± 0.1% | Sa = 0.29 ± 0.02 μm; Sz = 5.1 ± 0.3 μm, Sdr = 3.9 ± 0.2% | Sa = 0.35 ± 0.03 μm; Sz = 7.3 ± 0.4 μm, Sdr = 4.6 ± 0.3% |
| Wetting | θ ± Δθ = 85 ± 6° d = 3.6 ± 0.12 mm | θ ± Δθ = 88 ± 4° d = 3.5 ± 0.12 mm | θ ± Δθ = 93 ± 4° d = 3.3 ± 0.1 mm |
| 1.4541 Steel Surfaces | |||
|---|---|---|---|
| SEM images |  |  |  |
| Roughness | Sa = 0.03 ± 0.003 μm; Sz = 0.7 ± 0.05 μm; Sdr = 0.1 ± 0.01% | Sa = 0.07 ± 0.005 μm; Sz = 1.2 ± 0.1 μm; Sdr = 0.3 ± 0.03% | Sa = 0.08 ± 0.006 μm; Sz = 1.3 ± 0.1 μm; Sdr = 0.5 ± 0.04% |
| Wetting | θ ± Δθ = 89 ± 4° d = 3.4 ± 0.11 mm | θ ± Δθ = 78 ± 7° d = 3.8 ± 0.12 mm | θ ± Δθ = 77 ± 6° d = 3.8 ± 0.12 mm |
| SEM images |  |  |  |
| Roughness | Sa = 0.10 ± 0.005 μm; Sz = 1.4 ± 0.1 μm; Sdr = 0.6 ± 0.05% | Sa = 0.12 ± 0.007 μm; Sz = 1.4 ± 0.1 μm; Sdr = 0.7 ± 0.05% | Sa = 0.14 ± 0.01 μm; Sz = 1.7 ± 0.11 μm; Sdr = 0.8 ± 0.06% |
| Wetting | θ ± Δθ = 74 ± 5° d = 3.9 ± 0.12 mm | θ ± Δθ = 73 ± 4° d = 4 ± 0.15 mm | θ ± Δθ = 71 ± 5° d = 4 ± 0.15 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glushkov, D.; Orlova, E.; Islamova, A.; Nikitin, D.; Lyulin, Y.; Feoktistov, D. Influence of the Metal Surface Texture on the Possibility of Controlling the Phase Transition of Water Droplets in the Single-Phase Regime. Appl. Sci. 2022, 12, 12155. https://doi.org/10.3390/app122312155
Glushkov D, Orlova E, Islamova A, Nikitin D, Lyulin Y, Feoktistov D. Influence of the Metal Surface Texture on the Possibility of Controlling the Phase Transition of Water Droplets in the Single-Phase Regime. Applied Sciences. 2022; 12(23):12155. https://doi.org/10.3390/app122312155
Chicago/Turabian StyleGlushkov, Dmitriy, Evgeniya Orlova, Anastasia Islamova, Dmitriy Nikitin, Yuriy Lyulin, and Dmitriy Feoktistov. 2022. "Influence of the Metal Surface Texture on the Possibility of Controlling the Phase Transition of Water Droplets in the Single-Phase Regime" Applied Sciences 12, no. 23: 12155. https://doi.org/10.3390/app122312155





